Abstract
Using data from the large-scale HONEST (Home blood pressure measurement with Olmesartan Naive patients to Establish Standard Target blood pressure) study, we investigated the characteristics of the effects of olmesartan-based treatment on morning hypertension in Asian hypertensive patients. Specifically, we investigated the relationship between baseline blood pressure (BP) and BP reduction after 16 weeks by linear regression analyses; determinants of BP reduction were also investigated. For both morning home BP (MHBP) and clinic BP (CBP), reduced systolic BP (SBP) after 16 weeks was associated with baseline SBP (P<0.001). The slope of the regression lines was similar for morning home SBP (MHSBP) (−0.744) and clinic SBP (−0.735). Although sex, concomitant diabetes mellitus and concomitant hepatic disease significantly influence the relationship between BP reduction and baseline BP for MHSBP, none were deemed clinically relevant. In conclusion, olmesartan-based treatment robustly reduced baseline high MHBP, similar to CBP, and the effect was associated with baseline BP but unaffected by patient background factors.
Keywords: angiotensin receptor antagonists, Asia, blood pressure monitoring, olmesartan medoxomil
Introduction
Because hypertension increases cardiovascular risk, managing blood pressure (BP) is important in hypertensive patients.1 To determine their prognosis, home BP (HBP) is considered more useful than clinic BP (CBP),2, 3, 4, 5, 6 and morning systolic BP (SBP) is the strongest predictor of stroke.7
Generally, the effects of an antihypertensive drug are weakest in the early morning, just before patients take their tablets.8 At this time, sharp increases in BP in response to increased renin–angiotensin system (RAS) and sympathetic nervous system activity may cause cerebrovascular and cardiovascular events.7, 9 Therefore, the ideal antihypertensive drug would have a stable, favorable effect on morning home BP (MHBP) in addition to CBP.
The effects of antihypertensive drugs are influenced by various factors, including salt intake,10, 11 and East Asians, including the Japanese, have high salt intake.12 Furthermore, Japanese people are more likely than those in western countries to have polymorphisms in the candidate genes associated with increased salt sensitivity.13 In East Asian countries, dihydropyridine calcium channel blockers (CCBs) are most frequently used, partly because their antihypertensive effect is unaffected by salt sensitivity.14 However, RAS inhibitors are recommended as first-line therapy for high-risk hypertensive patients with diabetes or chronic kidney disease in the guidelines of the Japanese Society of Hypertension (JSH 2014).15
The Home BP measurement with Olmesartan Naive patients to Establish Standard Target blood pressure (HONEST) study is a prospective observational study that followed >20 000 patients who received angiotensin receptor blocker (ARB) (olmesartan)-based antihypertensive treatment for 2 years; time from start of treatment to first occurrence of cardiovascular events is the primary end point.16 We hypothesized that the antihypertensive effect of a RAS inhibitor, olmesartan, may differ according to patient characteristics (for example, individual differences in sex, age, obesity medical history and comorbidity) and type of BP (MHBP/CBP) in Japanese patients, who are likely to have salt-sensitive hypertension associated with high salt intake. In the present analysis, we used data for morning home systolic BP (MHSBP) and clinic systolic BP (CSBP) at 16 weeks from the HONEST study to investigate the characteristics of ARB-based BP-lowering effects.
Methods
Study design and patients
The HONEST study is a 2-year large-scale prospective observational study. Its protocol and main results have been reported.16, 17 Briefly, olmesartan-naive patients with essential hypertension (physician-reported, no specific BP range) measured their MHBP on ⩾2 days using their own electrical devices and had their CBP measured in the 28 days before they began taking olmesartan. Patients with a history of recent cardiovascular events and planned cardiovascular interventions were excluded. Patients received olmesartan (10 or 20 mg per day) at the discretion of the participating physicians. Prior antihypertensive treatment (with the exception of prior use of olmesartan) and combination antihypertensive drug treatment during the study were allowed. Participating physicians reported patient characteristics, CBP and HBP, clinic and home pulse rate, laboratory test values and the incidence of cardiovascular events and adverse events during the study period. In the present analysis, we used data from the HONEST study for patients who received olmesartan over the first 16 weeks.
All patients provided written informed consent. The study protocol was approved by the Ethical Committee of Daiichi Sankyo and by the research ethics committee of participating institutions, at their discretion. The study protocol was in accordance with the pharmaceutical affairs laws of Japan. Approval was obtained from the Ministry of Health, Labour and Welfare (MHLW) of Japan. The study was undertaken in registered medical institutions in compliance with Japan's Good Postmarketing Study Practice and the internal regulations at each institution. The study is registered at http://www.umin.ac.jp/ctr/index.htm (UMIN000002567).
HBP measurements
Patients who already had a sphygmomanometer based on the cuff-oscillometric principle were registered. All such devices available in Japan have been validated and approved by the MHLW. The devices also comply with either Association for the Advancement of Medical Instrumentation18 or European standards.19 In the HONEST study, patients were asked at the time of obtaining informed consent to measure their HBP twice in the morning (within 1 h of waking, after urination, before the morning dose, before breakfast and after 1–2 min of resting in a sitting position) and twice at bedtime (after 1–2 min of rest in a sitting position), according to the Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009).20 Because the present analysis aimed to evaluate the effects of antihypertensive treatment on morning hypertension, we evaluated MHBP. Additionally, for consistency with the previous study,21 we used only the first morning measurement of HBP at baseline and after 4 and 16 weeks. In this analysis, HBP measurements were obtained before informed consent was sought; patients had measured their BP using their own methods. HBP at each measurement point was defined as an averaged value over 2 days.
CBP measurements
Because the HONEST study was performed in the setting of daily medical practice, CBP and pulse rates were measured according to the usual methods of each institution; no recommendations or training were provided with respect to CBP measurement, which was carried out at baseline and after 4 and 16 weeks. For each measurement point, one measurement was reported.
Statistical analysis
To compare the predicted reduction between HBP and CBP according to baseline BP, we performed multiple linear regression analyses using change in SBP at 16 weeks as a dependent variable and baseline SBP as an independent variable with other covariates (sex, age, history of cardiac disease, alcohol drinking habit, diabetes mellitus, hepatic disease and dyslipidemia). The regression lines on the scatter plots (change of SBP on the ordinate vs. baseline SBP on the abscissa) were based on the coefficients for baseline SBP obtained in multiple regression analyses. When drawing the regression lines, other covariates were assigned the most frequent value for each category.
To determine the extent of BP reduction in relation to baseline BP, we classified patients into three groups according to baseline SBP (130–149, 150–169 and 170–189 mm Hg), and the other covariates were assigned their most frequent values. Using predicted BP reduction (ΔSBP) for each baseline BP group, we calculated the percentage of patients in the following ranges for difference from ΔSBP: ±0–5, ±6–10, ±11–15 and ±16–20 mm Hg from ΔSBP.
In addition, to identify determinants of antihypertensive effects that differed by baseline BP, we calculated P-values for interactions between each candidate factor and baseline SBP using a separate multiple linear regression model that included baseline SBP, a candidate factor, the interaction term between baseline SBP and the candidate factor and other candidate factors as covariates. The candidate factors were selected from sex, age, body mass index, disease duration, history of drug allergies, concomitant treatment, history of cerebrovascular disease, history of cardiovascular disease, concomitant dyslipidemia, concomitant diabetes, concomitant heart disease, concomitant kidney disease, concomitant liver disease, concomitant cerebrovascular disease, smoking habit, alcohol drinking habit, family history, chronic kidney disease, pulse and baseline SBP using backward elimination, with a criterion of P<0.05 for retention.
Furthermore, we evaluated changes in mean SBP after olmesartan-based treatment with use of concomitant drugs (none, CCB, diuretics, CCB plus diuretics and CCB plus β-blockers). All statistical analyses were two-sided, and P<0.05 was considered significant. Continuous variables and categorical variables are expressed as mean±s.d. and proportion, respectively. SAS release 9.2 software (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Patient enrollment
A total of 22 373 patients from 3039 medical institutions across Japan were registered between October 2009 and September 2010. Case report forms for 22 162 patients, which included data from baseline to ⩾16 weeks, were collected. This analysis included data from 21 341 patients. The data were fixed in April 2012.
Patient characteristics
Table 1 shows the baseline characteristics of the 21 341 patients whose data were included in the effectiveness analysis. At baseline, MHSBP and diastolic BP (DBP) were 151.6±16.4 mm Hg and 87.1±11.8 mm Hg, respectively. Morning home pulse was 70.8±10.0 beats per min. CSBP and clinic DBP were 153.6±19.0 and 87.1±13.4 mm Hg, respectively. Clinic pulse was 74.1±11.2 beats per min.
Table 1. Baseline patient characteristics (effectiveness analysis population, n=21 341).
| Characteristic | Mean±s.d. or % |
|---|---|
| Male/female | 49.5/50.5 |
| Age (years) | 64.8±11.9 |
| Body mass index (kg m−2) | 24.31±3.70 |
| Risk factors | |
| History of cerebrovascular or cardiovascular disease | 10.5 |
| Cerebrovascular disease | 6.6 |
| Cardiovascular disease | 4.5 |
| Complications | 63.2 |
| Dyslipidemia | 44.4 |
| Diabetes mellitus | 20.4 |
| Cardiac disease | 9.3 |
| Chronic kidney disease | 20.1 |
| Current smokers | 12.3 |
| Regular alcohol drinkers | 16.1 |
| Measurement of home BP | |
| Before medication | 91.4 |
| After medication | 2.5 |
| Not specified | 6.1 |
| Previous antihypertensive drug use | 50.3 |
| CCB | 36.0 |
| ARB | 21.3 |
| β-Blocker | 6.3 |
| Diuretic | 5.8 |
| ACE inhibitor | 3.7 |
| α-Blocker | 2.1 |
| Other antihypertensive drugs | 0.4 |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BP, blood pressure; CCB, calcium channel blocker.
Antihypertensive drugs
Use of antihypertensive drugs in the HONEST study has been described.21 Briefly, the starting dose of olmesartan was >5–⩽10 mg per day (mainly 10 mg per day) in 25.5% (n=5450) and >10–⩽20 mg per day (mainly 20 mg per day) in 66.5% (n=14,193). The dose after 16 weeks was >5–⩽10 mg per day (mainly 10 mg per day) in 20.9% (n=4455) and >10–⩽20 mg per day (mainly 20 mg per day) in 65.5% (n=13 982). Of the antihypertensive drugs that had previously been used, CCBs were most common, followed by ARBs. The percentage of patients receiving concomitant antihypertensive drugs was 38.8% at baseline and 44.9% at 16 weeks, with more than half of patients remaining on olmesartan monotherapy. At baseline, the percentages of the concomitant antihypertensive drugs being administered were as follows: CCBs, 33.9% (n=7245); β-blockers, 6.0% (n=1276); diuretics, 4.5% (n=961); α-blockers, 2.0% (n=436); ACE inhibitors, 1.4% (n=309); ARBs, 0.7% (n=160); and other antihypertensive drugs, 0.3% (n=74). The number of antihypertensive drugs used, including olmesartan, changed from 1.5±0.7 at baseline to 1.6±0.8 at 16 weeks. Table 2 shows patients' antihypertensive treatment by baseline BP.
Table 2. Antihypertensive treatment by baseline BP levelsa.
|
Baseline morning home systolic BP |
Baseline clinic systolic BP |
|||||
|---|---|---|---|---|---|---|
| 130–149 mm Hg | 150–169 mm Hg | 170–189 mm Hg | 130–149 mm Hg | 150–169 mm Hg | 170–189 mm Hg | |
| Number of patients | 8083 | 8734 | 2382 | 6739 | 8867 | 3094 |
| Baseline dose of olmesartan (mg per day, mean±s.d.) | 18.1±7.3 | 18.1±6.7 | 18.9±6.6 | 18.2±7.3 | 18.1±6.9 | 18.3±6.5 |
| Receiving concomitant antihypertensive drugs (excluding olmesartan) | 3661 (45.3) | 2878 (33.0) | 709 (29.8) | 3163 (46.9) | 2982 (33.6) | 877 (28.3) |
| Total number of antihypertensive drugs used (including olmesartan, mean±s.d.) | 1.6±0.8 | 1.4±0.7 | 1.4±0.7 | 1.6±0.8 | 1.4±0.7 | 1.3±0.6 |
Abbreviation: BP, blood pressure.
Number of patients (%), unless otherwise indicated.
Change in BP
MHBP changed from 151.6/87.1 mm Hg at baseline to 138.3/80.7 mm Hg at 4 weeks and to 135.0/78.8 mm Hg at 16 weeks (P<0.0001 for both). CBP changed from 153.6/87.1 mm Hg at baseline to 139.0/79.5 mm Hg at 4 weeks and to 135.5/77.5 mm Hg at 16 weeks (P<0.0001 for both). Evening HBP changed from 144.3/82.8 mm Hg at baseline to 132.3/76.2 mm Hg at 4 weeks and to 129.7/74.7 mm Hg at 16 weeks (P<0.0001 for both).21
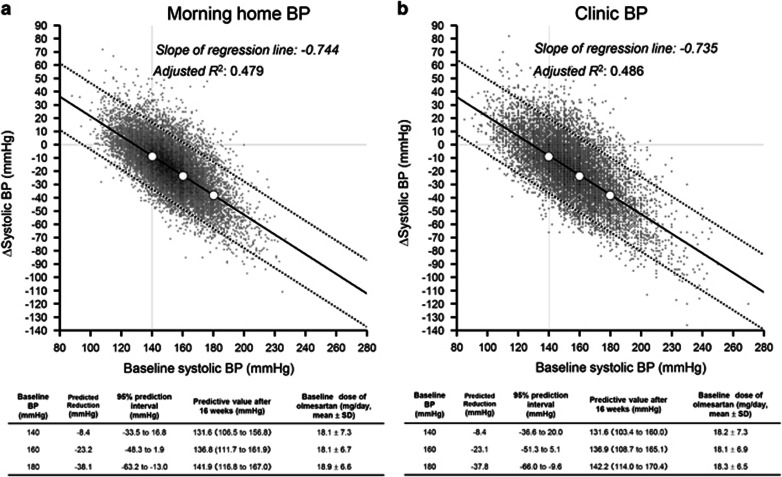
Regression lines for the reduction in MHSBP and CSBP from baseline
Multiple linear regression analyses using baseline SBP as an independent variable and change in SBP (the value at 16 weeks minus the baseline value: ΔSBP) as a dependent variable showed that the SBP reduction after 16 weeks was associated with the baseline SBP for both MHBP and CBP (both P<0.001). The slope of the regression line was also similar for MHSBP (−0.744) and CSBP (−0.735) (Figure 1). For example, when the baseline MHSBP values were 140, 160 and 180 mm Hg, the predicted values for ΔSBP were −8.4, −23.2 and −38.1 mm Hg, respectively. The results for CSBP were similar; when the baseline CSBP values were 140, 160 and 180 mm Hg, the predicted values of ΔSBP were −8.4, −23.1 and −37.8 mm Hg, respectively.
Figure 1.
Changes in systolic blood pressure (BP) from baseline after 16 weeks of olmesartan-based treatment. The figure shows a multiple regression line and scatter plots. The reduction in both (a) morning home systolic BP and (b) clinic systolic BP increased significantly in patients with higher BP at baseline, and there was essentially the same reduction in BP for morning home systolic BP and clinic systolic BP (−0.744 vs. −0.735 per baseline systolic BP). Analysis adjusted for sex, age, history of cardiac disease, alcohol drinking habit, diabetes mellitus, hepatic disease and dyslipidemia by assigning the most frequent values of each category. Δsystolic BP, the value at 16 weeks of olmesartan-based treatment minus the baseline value. Adjusted R2, coefficient of determination.
The coefficients of determination (adjusted R2) obtained by multiple linear regression analysis were similar for MHSBP and CSBP (0.479 and 0.486, respectively) and were not substantially different from those determined by simple regression (0.462 and 0.481, respectively). Furthermore, after adjustment for baseline dose of olmesartan and total number of antihypertensive drugs used in addition to other covariates, the slope of the regression line and the adjusted R2 value were, respectively, −0.738 and 0.485 for MHSBP vs. −0.731 and 0.490 for CSBP, values that were also not substantially different from those determined by simple regression. Additionally, we conducted multiple linear regression analyses by excluding patients in whom (1) another ARB or an ACE inhibitor was switched to olmesartan or (2) olmesartan was added to an ACE inhibitor or another ARB. The results were essentially not different; the slope of the regression line and the adjusted R2 value were, respectively, −0.751 and 0.468 for MHSBP vs. −0.751 and 0.489 for CSBP.
Distribution of patients by range of difference from predicted BP reduction
Table 3 shows the distribution of patients by the ranges of difference from predicted BP reduction (ΔSBP). For both MHBP and CBP, the percentages of patients tended to be greater the closer ΔSBP was to its predicted value. The patient distribution was not substantially different between MHBP and CBP; ΔSBP ±0–5 mm Hg, 24–31% ΔSBP ±6–10 mm Hg, 20–25% ΔSBP ±11–15 mm Hg, 15–17% and ΔSBP ±16–20 mm Hg, 10–12% of patients. Most patients (approximately 90%) had baseline BP 130−190 mm Hg. We conducted additional analyses that included patients with baseline BP outside this range (that is, <130 and >190 mm Hg). The results showed a similar trend; the percentages of patients were greater as ΔSBP was closer to its predicted value (data not shown).
Table 3. Distribution of patients by difference from predicted blood pressure (BP) reduction (ΔSBP), according to baseline morning home systolic BP and baseline clinic systolic BPa.
|
Baseline morning home systolic BP |
Baseline clinic systolic BP |
|||||
|---|---|---|---|---|---|---|
| 130–149 mm Hg | 150–169 mm Hg | 170–189 mm Hg | 130–149 mm Hg | 150–169 mm Hg | 170–189 mm Hg | |
| Number of patients | 8083 | 8734 | 2382 | 6739 | 8867 | 3094 |
| Predicted ΔSBP (mm Hg)b | −8.4 | −23.2 | −38.1 | −8.4 | −23.1 | −37.8 |
| Difference from predicted ΔSBP | ||||||
| ΔSBP±0–5 mm Hg | 2470 (30.6) | 2378 (27.2) | 573 (24.1) | 1971 (29.2) | 2432 (27.4) | 737 (23.8) |
| ΔSBP±6–10 mm Hg | 1957 (24.2) | 2061 (23.6) | 470 (19.7) | 1674 (24.8) | 2089 (23.6) | 620 (20.0) |
| ΔSBP±11–15 mm Hg | 1313 (16.2) | 1467 (16.8) | 362 (15.2) | 1106 (16.4) | 1461 (16.5) | 538 (17.4) |
| ΔSBP±16–20 mm Hg | 780 (9.6) | 906 (10.4) | 272 (11.4) | 709 (10.5) | 926 (10.4) | 363 (11.7) |
Number of patients (%), unless otherwise indicated.
Middle BP values for each baseline BP group (that is, 140,160 and 180 mm Hg) were used to calculate the predicted ΔSBP.
Determinants of antihypertensive effects by baseline BP
The determinants of antihypertensive effects that statistically significantly differed by baseline BP were sex, comorbidity of diabetes mellitus and comorbidity of hepatic disease for MHSBP and age, history of cardiac disease, comorbidity of diabetes mellitus and comorbidity of dyslipidemia for CSBP (Table 4). However, differences in the predicted values for BP reduction for these factors were statistically significant but small for MHSBP and CSBP.
Table 4. Determinants for the antihypertensive effect of olmesartan on morning home systolic blood pressure (BP) and clinic BPa.
|
Morning home systolic BP at baseline |
Clinic systolic BP at baseline |
|||||||
|---|---|---|---|---|---|---|---|---|
| 140 mm Hg | 160 mm Hg | 180 mm Hg | Interaction Pb | 140 mm Hg | 160 mm Hg | 180 mm Hg | Interaction Pb | |
| Sex | <0.001* | |||||||
| Male | –8.4 (–33.1 to 16.3) | –22.8 (–47.5 to 1.9) | –37.1 (–61.8 to –12.4) | |||||
| Female | –9.2 (–34.9 to 16.5) | –24.5 (–50.2 to 1.2) | –39.9 (–65.6 to –14.2) | |||||
| Difference | –0.7 | –1.8 | –2.8 | NS | ||||
| Age | 0.022* | |||||||
| ⩾65 years | −8.0 (−36.8 to 20.9) | −22.4 (−51.3 to 6.4) | −36.9 (−65.8 to −8.1) | |||||
| <65 years | −9.4 (−37.1 to 18.3) | −24.3 (−52.1 to 3.4) | −39.3 (−67.0 to −11.5) | |||||
| Difference | NS | −1.5 | −1.9 | −2.4 | ||||
| History of cardiac disease | 0.002* | |||||||
| No | −7.9 (−36.2 to 20.3) | −22.7 (−50.9 to 5.6) | −37.4 (−65.7 to −9.2) | |||||
| Yes | −7.6 (−37.8 to 22.7) | −20.7 (−50.9 to 9.6) | −33.7 (−64.1 to −3.4) | |||||
| Difference | NS | 0.4 | 2.0 | 3.7 | ||||
| Alcohol drinking habit | 0.082 | |||||||
| Not daily | –9.4 (–34.7 to 15.9) | –24.4 (–49.7 to 0.9) | –39.4 (–64.7 to –14.1) | |||||
| Daily | –9.3 (–34.2 to 15.7) | –23.7 (–48.6 to 1.2) | –38.1 (–63.1 to –13.2) | |||||
| Difference | 0.1 | 0.7 | 1.3 | NS | ||||
| Comorbidity of diabetes mellitus | <0.001* | <0.001* | ||||||
| No | –9.4 (–34.3 to 15.5) | –24.5 (–49.4 to 0.4) | –39.6 (–64.5 to –14.7) | −7.6 (−35.4 to 20.2) | −22.5 (−50.3 to 5.2) | −37.5 (−65.2 to −9.7) | ||
| Yes | –7.9 (–34.3 to 18.6) | –21.9 (–48.3 to 4.6) | –35.9 (–62.4 to –9.4) | −6.8 (−37.2 to 23.6) | −20.4 (−50.8 to 10.0) | −34.1 (−64.5 to −3.7) | ||
| Difference | 1.5 | 2.6 | 3.7 | 0.8 | 2.1 | 3.4 | ||
| Comorbidity of dyslipidemia | 0.023* | |||||||
| No | −7.7 (−35.8 to 20.4) | −22.6 (−50.7 to 5.5) | −37.5 (−65.6 to −9.4) | |||||
| Yes | −8.3 (−37.0 to 20.3) | −22.7 (−51.4 to 5.9) | −37.1 (−65.8 to −8.5) | |||||
| Difference | NS | −0.6 | −0.1 | 0.4 | ||||
| Comorbidity of hepatic disease | 0.043* | |||||||
| No | –9.4 (–34.6 to 15.7) | –24.4 (–49.6 to 0.8) | –39.3 (–64.5 to –14.2) | |||||
| Yes | –9.7 (–35.5 to 16.1) | –23.7 (–49.5 to 2.2) | –37.7 (–63.5 to –11.8) | |||||
| Difference | −0.2 | 0.7 | 1.7 | NS | ||||
Abbreviation: NS, not significant.
Data expressed as predictive value (95% prediction interval). *P<0.05.
Interaction P-values are for the interaction term between each factor and baseline systolic BP using a multiple regression model for morning home systolic BP (left) or clinic systolic BP (right).
Antihypertensive effect of olmesartan as monotherapy and combination therapy for MHSBP and CSBP
Regardless of the use or type of concomitant antihypertensive drugs or baseline BP, mean MHSBP had decreased nearly to the target of ⩽135 mm Hg after 16 weeks of olmesartan-based treatment (Figure 2a). Mean CSBP decreased to <140 mm Hg by 4 weeks of treatment (Figure 2b). Patients receiving olmesartan monotherapy did not require a higher dose of olmesartan at baseline and week 16 compared with those who received combination therapy (data not shown). Supplementary Table shows the percentages of patients who achieved the target BP according to their age and complications (as defined in the JSH 200920) at week 16 in the treatment groups shown in Figure 2.
Figure 2.
Changes in blood pressure (BP) from baseline after olmesartan-based treatment by previous antihypertensive drugs used (excluding patients who switched antihypertensive treatment). Mean morning home systolic BP had nearly reached the target level by 16 weeks in most patients (a), and mean clinic systolic BP had reached the target level by 4 weeks (b), regardless of baseline BP or the use or type of previous antihypertensive drugs. Dotted lines, target BP. β, β-blocker, CCB, calcium channel blocker; D, diuretics; OLM, olmesartan. Gray bars represent the mean value (error bar: s.d.).
Discussion
In the large-scale real-world observational HONEST study, which involved >20 000 Japanese hypertensive patients, we demonstrated that olmesartan-based treatment robustly and comparably reduced self-measured MHBP to a similar degree as CBP, indicating a persistent 24-h BP-lowering effect. Although HBP is often lower than CBP by approximately 6–8 mm Hg,4, 22 baseline MHBP and CBP were similar in the present study. Another study in Japan that enrolled patients who were receiving antihypertensive treatment had similar results.23 In the present study, approximately 50% of the BP-lowering effect depended on baseline BP, and this BP-lowering effect was unaffected by patient characteristics or concomitant antihypertensive drugs of a different class. The results suggest that potent RAS inhibition of olmesartan24, 25 could eliminate differences among individuals in hypertensive Asian patients.
Antihypertensive effect of olmesartan on MHSBP and CSBP
In the present study, we evaluated the antihypertensive effect of olmesartan-based treatment on MHSBP and CSBP by using the slope of the regression lines, calculating the predicted values for BP reduction and comparing the distribution of patients by range of difference from predicted BP reduction. The results were similar between MHSBP and CSBP. The finding that R2 was 0.462 for MHSBP and 0.481 for CSBP indicates that nearly 50% of the antihypertensive effect noted in this study was explained by baseline BP itself. For both MHSBP and CSBP, the antihypertensive effect of olmesartan-based treatment was stronger in patients with higher baseline BP. After adjusting for baseline dose of olmesartan and total number of antihypertensive drugs, the slope of the regression lines and adjusted R2 were not different from those before adjustment. Moreover, both MHSBP and CSBP in most patients were ±0–5 mm Hg from predicted BP reduction. Furthermore, the predicted reductions in MHSBP and CSBP were essentially the same. These results suggest that the contribution of baseline BP to the antihypertensive effect of olmesartan after 16 weeks is relatively high.
In our previous report,21 we compared the results with those of another study (the At-HOME study26) for which the observation period was 16 weeks. Based on those results, we chose 16 weeks as the observation period for the present study.
An increased antihypertensive effect in patients with higher BP at baseline has been reported for CCBs27, 28, 29 but not ARBs.27, 30 A meta-analysis that compared the effects of dihydropyridine CCBs against mainly RAS inhibitors in East Asians showed a correlation between baseline BP and BP reduction by dihydropyridine CCBs, whereas the correlation for RAS inhibitors was weak.14
We did not determine the percentage of patients with salt-sensitive hypertension in this study; however, because the study involved a large number of Japanese patients (>20 000), it is assumed that a certain number of patients with salt-sensitive hypertension would have been included.13 In such populations, olmesartan-based treatment reduced BP from baseline. We also did not measure sodium excretion by calculating sodium/creatinine ratios in the morning urine samples, which may have provided some perspective on the relationship between dietary salt and the antihypertensive effect of olmesartan.
Furthermore, to compare the results with those in previous studies, we performed simple linear regression analysis; the slope of the regression lines and the adjusted R2 were −0.741 and 0.462, respectively, for MHBP and −0.740 and 0.481, respectively, for CBP. Although direct comparison is difficult, the slope of the regression lines and the R2 values in this analysis were greater than those reported for amlodipine (−0.5727 and 0.3597, respectively)31 and valsartan (R2, 0.04).27 Olmesartan binds strongly with the angiotensin II type 1 receptor.32 In the previous analysis from the HONEST study, we reported that olmesartan-based treatment was effective in hypertensive patients with increased pulse, especially in those with concomitant chronic kidney disease; both conditions are associated with increased sympathetic nerve activity.33 Based on these mechanisms, we consider that olmesartan-based treatment may exert an antihypertensive effect according to baseline BP. Furthermore, the finding that olmesartan-based treatment reduces both MHSBP and CSBP suggests the sustainability of its antihypertensive effect.34 Moreover, the effects of olmesartan on MHBP and CBP are reported to be consistent whether it is administered in the morning or in the evening.34 This may be a reason that the potential difference in the time of day at which MHSBP and CSBP were measured (prior to or after daily olmesartan administration) did not affect the results.
Determinants of the antihypertensive effect of olmesartan by baseline BP
In Table 4, we analyzed determinants for the antihypertensive effect of olmesartan on BP by classifying baseline BP into 140, 160 and 180 mm Hg, based on the hypertension diagnostic criteria for CBP defined in guidelines (classification by 20 mm Hg). Setting these as the middle values, we used the range of ±10 mm Hg of those values (that is, 130–149 mm Hg, 150–169 mm Hg and 170–189 mm Hg) for the analyses shown in Tables 2 and 3. We used the same ranges for CBP and HBP to investigate whether CBP and HBP would show similar decreasing trend (we confirmed that the mean values of CBP and HBP were similar at baseline.).
The determinants of antihypertensive effect that differed by baseline BP were sex, comorbidity of diabetes mellitus and comorbidity of hepatic disease for MHSBP and age, history of cardiac disease, comorbidity of diabetes mellitus and comorbidity of dyslipidemia for CSBP.
In a previous study that investigated the antihypertensive effect of combined losartan–hydrochlorothiazide,35 there was a difference in the BP-lowering effect of ⩾4 mm Hg in female or elderly patients compared with other patient groups at the baseline BP of approximately 160 mm Hg. By contrast, in the present study, although there was an interaction between baseline BP and sex or age, the difference in the BP-lowering effect at baseline BP of approximately 160 mm Hg was small—that is, there was no clinically significant difference in the antihypertensive effect of olmesartan by patient background factors.
Influence of concomitant antihypertensive drugs
Regardless of baseline BP and concomitant antihypertensive drugs, the addition of olmesartan reduced both MHSBP and CSBP to near-target levels. Generally, combination therapy that targets different mechanisms is thought to yield greater effects than an increased dose of a single antihypertensive drug.36
Previous studies showed no difference in the effects of olmesartan when used in combination with diuretics or CCBs.37, 38 In the present study, olmesartan alone and in combination with diuretics or CCBs showed similar antihypertensive effects in real-world clinical practice, even though patients receiving olmesartan monotherapy did not require a higher dose of olmesartan from baseline compared with those who received combination therapy and their concomitant antihypertensive drugs may have varied.
In conclusion, olmesartan-based treatment robustly reduced baseline high MHBP, similar to CBP, and the effect is associated with baseline BP and unaffected by patient background factors.
Study limitations
The HONEST study was designed to represent the real world of clinical practice, so patients were not blinded to treatment and there was no control group. Therefore, the possibility of regression toward the mean cannot be excluded. Furthermore, the effects of olmesartan shown in this study can be achieved by other antihypertensive drugs. Nevertheless, we believe that the results are useful because they reflect real-world clinical practice.
Although HBP was measured twice in the morning and twice at bedtime in the HONEST study, the present analysis focused on the MHBP and used only the first MHBP measurement (averaged value over 2 days) based on the previous report,21 in which the first morning measurement of HBP was used to compare the results in another study.26
Acknowledgments
We thank the numerous study investigators, fellows, nurses and research coordinators who participated in the HONEST study. The study was supported by funding for data collection and statistical analysis by Daiichi Sankyo (Tokyo, Japan). Statistical analyses were carried out by EPS Corporation (Tokyo, Japan) under the direction of the sponsor and the authors. Editorial assistance was provided by Macmillan Medical Communications (Tokyo, Japan) and funded by Daiichi Sankyo.
Author contributions
The sponsor Daiichi Sankyo was involved in the design, conduct, analysis and reporting of the study. All authors contributed to writing or critically reviewing the manuscript, and all approved the final version for submission.
Footnotes
Supplementary Information accompanies the paper on Hypertension Research website (http://www.nature.com/hr)
Some of the findings described in this manuscript were presented at the 36th Annual Scientific Meeting of the Japanese Society of Hypertension (Osaka, Japan; 24–26 October 2013).
Dr Kario, Dr Saito, Dr Kushiro, Dr Teramukai and Dr Shimada have received honoraria from Daiichi Sankyo. Ms Yaginuma, Mr Mori, Mr Okuda and Dr Kobayashi are employees of Daiichi Sankyo.
Supplementary Material
References
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913. [DOI] [PubMed] [Google Scholar]
- Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation 2005; 111: 1777–1783. [DOI] [PubMed] [Google Scholar]
- Hara A, Tanaka K, Ohkubo T, Kondo T, Kikuya M, Metoki H, Hashimoto T, Satoh M, Inoue R, Asayama K, Obara T, Hirose T, Izumi S, Satoh H, Imai Y. Ambulatory versus home versus clinic blood pressure: the association with subclinical cerebrovascular diseases: the Ohasama Study. Hypertension 2012; 59: 22–28. [DOI] [PubMed] [Google Scholar]
- Niiranen TJ, Hänninen MR, Johansson J, Reunanen A, Jula AM. Home-measured blood pressure is a stronger predictor of cardiovascular risk than office blood pressure: the Finn-Home study. Hypertension 2010; 55: 1346–1351. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Shimada K, Kario K, , Japan Morning Surge-Home Blood Pressure Study Investigators Group. Nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension 2012; 60: 921–928. [DOI] [PubMed] [Google Scholar]
- Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003; 107: 1401–1406. [DOI] [PubMed] [Google Scholar]
- Kario K, Ishikawa J, Pickering TG, Hoshide S, Eguchi K, Morinari M, Hoshide Y, Kuroda T, Shimada K. Morning hypertension: the strongest independent risk factor for stroke in elderly hypertensive patients. Hypertens Res 2006; 29: 581–587. [DOI] [PubMed] [Google Scholar]
- Kario K, White WB. Early morning hypertension: what does it contribute to overall cardiovascular risk assessment? J Am Soc Hypertens 2008; 2: 397–402. [DOI] [PubMed] [Google Scholar]
- Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56: 765–773. [DOI] [PubMed] [Google Scholar]
- Morgan TO, Anderson AI, MacInnis RJ. ACE inhibitors, beta-blockers, calcium blockers, and diuretics for the control of systolic hypertension. Am J Hypertens 2001; 14: 241–247. [DOI] [PubMed] [Google Scholar]
- Mori H, Ukai H, Yamamoto H, Saitou S, Hirao K, Yamauchi M, Umemura S. Current status of antihypertensive prescription and associated blood pressure control in Japan. Hypertens Res 2006; 29: 143–151. [DOI] [PubMed] [Google Scholar]
- Zhou BF, Stamler J, Dennis B, Moag-Stahlberg A, Okuda N, Robertson C, Zhao L, Chan Q, Elliott P, INTERMAP Research Group. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens 2003; 17: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res 2003; 26: 521–525. [DOI] [PubMed] [Google Scholar]
- Wang JG, Kario K, Lau T, Wei YQ, Park CG, Kim CH, Huang J, Zhang W, Li Y, Yan P, Hu D, Asian Pacific Heart Association. Use of dihydropyridine calcium channel blockers in the management of hypertension in Eastern Asians: a scientific statement from the Asian Pacific Heart Association. Hypertens Res 2011; 34: 423–430. [DOI] [PubMed] [Google Scholar]
- Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S, Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014 37: 253–390. [Google Scholar]
- Saito I, Kario K, Kushiro T, Teramukai S, Zenimura N, Hiramatsu K, Kobayashi F, Shimada K. Rationale, study design, baseline characteristics and blood pressure at 16 weeks in the HONEST Study. Hypertens Res 2013; 36: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kario K, Saito I, Kushiro T, Teramukai S, Ishikawa Y, Mori Y, Kobayashi F, Shimada K. Home blood pressure and cardiovascular outcomes in patients during antihypertensive therapy primary results of HONEST, a large-scale prospective, real-world observational study. Hypertension 2014; 64: 989–996. [DOI] [PubMed] [Google Scholar]
- Association for the Advancement of Medical Instrumentation. American National Standard. Electronic or Automated Sphygmomanometers NSI/AAMI SP 10-1992. AAMI: Arlington, VA, USA, 1993, pp 40.
- European Committee for Standardization. Non-invasive Sphygmomanometers Part 3. Supplementary Requirements for Electromechanical Blood Pressure Measuring Systems. British Standard BS EN 1060-3: 1997. European Standard EN 1060-3: 1997. ESC: Brussels, 1997.
- Ogihara T, Kikuchi K, Matsuoka H, Fujita T, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ito S, Iwao H, Kario K, Kawano Y, Kim-Mitsuyama S, Kimura G, Matsubara H, Matsuura H, Naruse M, Saito I, Shimada K, Shimamoto K, Suzuki H, Takishita S, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Ueshima H, Umemura S, Ishimitsu T, Rakugi H, Japanese Society of Hypertension Committee. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res 2009; 32: 3–107.19300436 [Google Scholar]
- Kario K, Saito I, Kushiro T, Teramukai S, Ishikawa Y, Hiramatsu K, Kobayashi F, Shimada K. Effect of the angiotensin II receptor antagonist olmesartan on morning home blood pressure in hypertension: HONEST study at 16 weeks. J Hum Hypertens 2013; 27: 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Kikuya M, Ito S, Satoh H, Hisamichi S. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens 1998; 16: 971–975. [DOI] [PubMed] [Google Scholar]
- Asayama K, Ohkubo T, Metoki H, Obara T, Inoue R, Kikuya M, Thijs L, Staessen JA, Imai Y, , Hypertension Objective Treatment Based on Measurement by Electrical Devices of Blood Pressure (HOMED-BP). Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res 2012; 35: 1102–1110.22895063 [Google Scholar]
- Mire DE, Silfani TN, Pugsley MK. A review of the structural and functional features of olmesartan medoxomil, an angiotensin receptor blocker. J Cardiovasc Pharmacol 2005; 46: 585–593. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Sada T, Ikeda M, Fukuda N, Miyamoto M, Yanagisawa H, Koike H. Pharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonist. Eur J Pharmacol 1995; 285: 181–188. [DOI] [PubMed] [Google Scholar]
- Kario K, Sato Y, Shirayama M, Takahashi M, Shiosakai K, Hiramatsu K, Komiya M, Shimada K. Inhibitory effects of azelnidipine tablets on morning hypertension. Drugs R D 2013; 13: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi K, Kario K, Hoshide Y, Hoshide S, Ishikawa J, Morinari M, Ishikawa S, Shimada K. Comparison of valsartan and amlodipine on ambulatory and morning blood pressure in hypertensive patients. Am J Hypertens 2004; 17: 112–117. [DOI] [PubMed] [Google Scholar]
- Eguchi K, Tomizawa H, Ishikawa J, Hoshide S, Fukuda T, Numao T, Shimada K, Kario K. Effects of new calcium channel blocker, azelnidipine, and amlodipine on baroreflex sensitivity and ambulatory blood pressure. J Cardiovasc Pharmacol 2007; 49: 394–400. [DOI] [PubMed] [Google Scholar]
- Kario K, Ando S, Kido H, Nariyama J, Takiuchi S, Yagi T, Shimizu T, Eguchi K, Ohno M, Kinoshita O, Yamada T. The effects of the L/N-type calcium channel blocker (cilnidipine) on sympathetic hyperactive morning hypertension: results from ACHIEVE-ONE. J Clin Hypertens (Greenwich) 2013; 15: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabia MJ, Abdilla N, Oltra R, Fernandez C, Redon J. Antihypertensive activity of angiotensin II AT1 receptor antagonists: a systematic review of studies with 24h ambulatory blood pressure monitoring. J Hypertens 2007; 25: 1327–1336. [DOI] [PubMed] [Google Scholar]
- Kario K, Odawara M, Kimura K, Node K. Nearly half of uncontrolled hypertensive patients could be controlled by high-dose titration of amlodipine in the clinical setting: the ACHIEVE Study. Curr Hypertens Rev 2011; 7: 102–110. [Google Scholar]
- Miura S, Fujino M, Hanzawa H, Kiya Y, Imaizumi S, Matsuo Y, Tomita S, Uehara Y, Karnik SS, Yanagisawa H, Koike H, Komuro I, Saku K. Molecular mechanism underlying inverse agonist of angiotensin II type 1 receptor. J Biol Chem 2006; 281: 19288–19295. [DOI] [PubMed] [Google Scholar]
- Kario K, Saito I, Kushiro T, Teramukai S, Mori Y, Hiramatsu K, Kobayashi F, Shimada K. Enhanced blood pressure-lowering effect of olmesartan in hypertensive patients with chronic kidney disease-associated sympathetic hyperactivity: HONEST study. J Clin Hypertens (Greenwich) 2013; 15: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Yamamoto H, Ukai S, Yuasa S, Nakajima K, Mikawa T, Niizuma M, Hirao K, Umemura S, COMPATIBLE Study Group. Comparison of effects of angiotensin II receptor blocker on morning home blood pressure and cardiorenal protection between morning administration and evening administration in hypertensive patients: the COMPATIBLE study. Hypertens Res 2013; 36: 202–207. [DOI] [PubMed] [Google Scholar]
- Maeda K, Adachi M, Kinoshita A, Koh N, Miura Y, Murohara T. Efficacy and safety of the losartan–hydrochlorothiazide combination tablet in patients with hypertension uncontrolled by angiotensin II receptor antagonist therapy: the Aichi Research on Combination therapy for Hypertension (ARCH) Study. Intern Med 2012; 51: 1167–1175. [DOI] [PubMed] [Google Scholar]
- Bilo G, Koch W, Hoshide S, Parati G. Efficacy of olmesartan/amlodipine combination therapy in reducing ambulatory blood pressure in moderate-to-severe hypertensive patients not controlled by amlodipine alone. Hypertens Res 2014; 37: 836–844. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Eguchi K, O'Rourke MF, Ishikawa J, Miyashita H, Shimada K, Kario K. Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients. Hypertension 2009; 54: 716–723. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Saruta T, Rakugi H, Saito I, Shimamoto K, Matsuoka H, Shimada K, Ito S, Horiuchi M, Imaizumi T, Takishita S, Higaki J, Katayama S, Kimura G, Umemura S, Ura N, Hayashi K, Odawara M, Tanahashi N, Ishimitsu T, Kashihara N, Morita S, Teramukai S, COLM Investigators. Combinations of olmesartan and a calcium channel blocker or a diuretic in elderly hypertensive patients: a randomized, controlled trial. J Hypertens 2014; 32: 2054–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.