Abstract
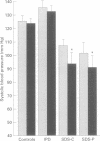
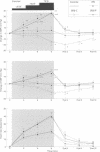
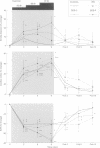
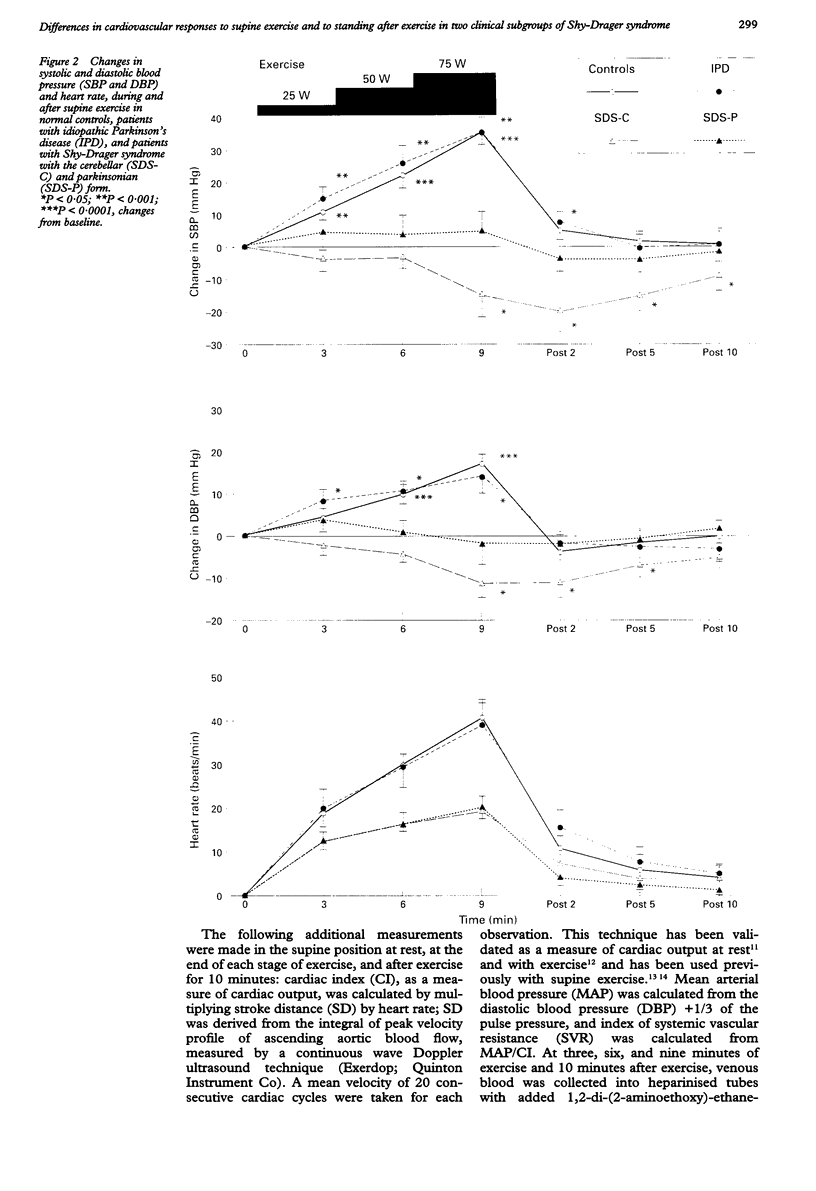
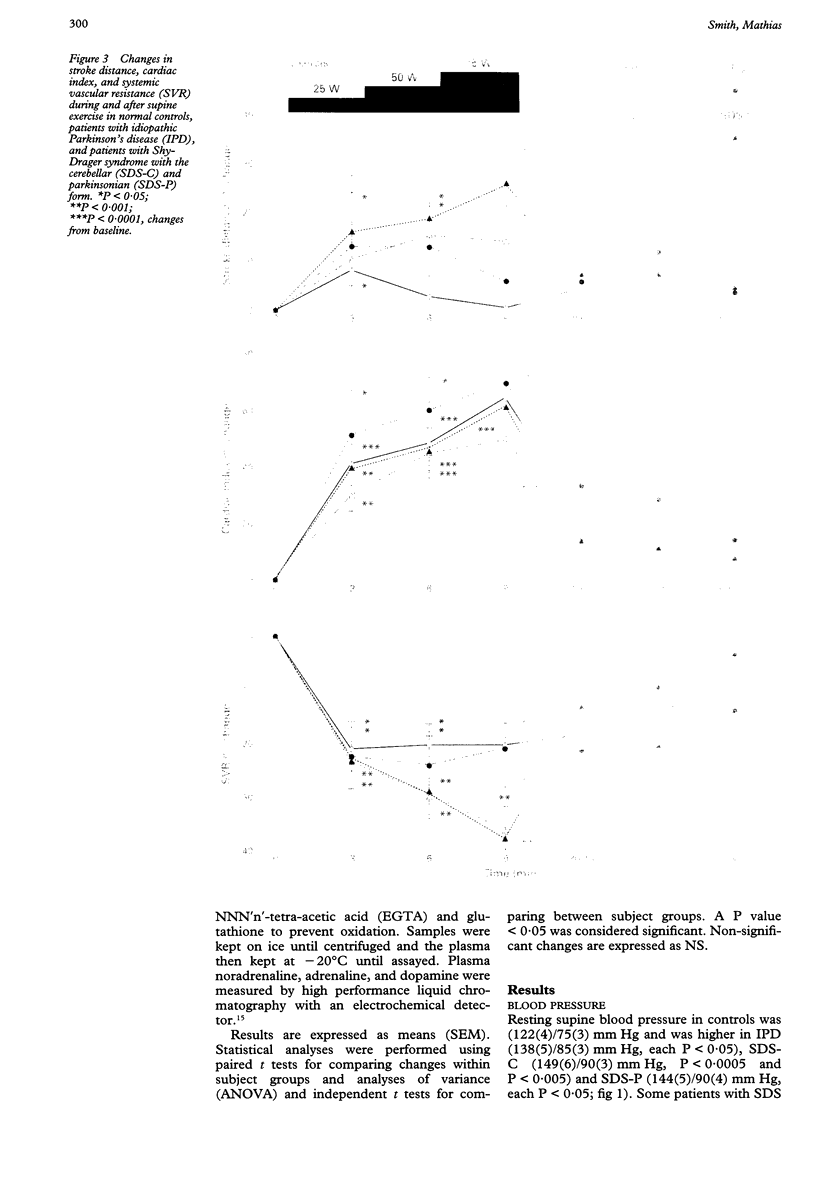
BACKGROUND: In chronic autonomic failure of varying aetiologies, there are differences in the cardiovascular responses to supine leg exercise and to standing after exercise. Whether this occurs between the different subgroups with Shy-Drager syndrome (SDS) is unknown. METHODS: Fourteen patients with the cerebellar form (SDS-C) and 11 patients with parkinsonian features (SDS-P) were studied. RESULTS: Both groups had a similar degree of autonomic failure and postural hypotension. Their responses were compared with nine patients with idiopathic Parkinson's disease (IPD) and 15 normal subjects (controls), all with normal autonomic function. With supine exercise, blood pressure and heart rate rose similarly in controls and patients with IPD and there was no fall in blood pressure on standing after exercise. In both SDS groups there were abnormal responses to exercise: blood pressure fell in SDS-C, but did not fall or rise in SDS-P. Heart rate increased similarly in both SDS groups, calculated systemic vascular resistance fell similarly, but cardiac index rose more in SDS-P than SDS-C. Resting plasma noradrenaline concentrations were subnormal in both forms of SDS, and did not increase with exercise. Postural hypotension was enhanced after exercise to the same extent in SDS-C and SDS-P. CONCLUSIONS: The greater cardiovascular abnormalities in response to exercise in SDS-C suggests that cerebellar or brain stem autonomic pathways are impaired to a greater extent in SDS-C than in SDS-P. Pooling SDS subgroups, therefore, may obscure pathophysiological differences to certain stimuli. Clinically when postural hypotension is being assessed, separation of the subgroups may not be essential, as they responded similarly.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. L., Guyenet P. G. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res. 1985 Mar;56(3):359–369. doi: 10.1161/01.res.56.3.359. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Williams J. L., Lutherer L. O. Cerebellar lesions alter autonomic responses to transient isovolaemic changes in arterial pressure in anaesthetized cats. Clin Auton Res. 1994 Oct;4(5):263–272. doi: 10.1007/BF01827432. [DOI] [PubMed] [Google Scholar]
- Eriksen M., Waaler B. A., Walløe L., Wesche J. Dynamics and dimensions of cardiac output changes in humans at the onset and at the end of moderate rhythmic exercise. J Physiol. 1990 Jul;426:423–437. doi: 10.1113/jphysiol.1990.sp018147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M. M., Yahr M. D. Parkinsonism: onset, progression and mortality. Neurology. 1967 May;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Huntsman L. L., Stewart D. K., Barnes S. R., Franklin S. B., Colocousis J. S., Hessel E. A. Noninvasive Doppler determination of cardiac output in man. Clinical validation. Circulation. 1983 Mar;67(3):593–602. doi: 10.1161/01.cir.67.3.593. [DOI] [PubMed] [Google Scholar]
- Loeppky J. A., Greene E. R., Hoekenga D. E., Caprihan A., Luft U. C. Beat-by-beat stroke volume assessment by pulsed Doppler in upright and supine exercise. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jun;50(6):1173–1182. doi: 10.1152/jappl.1981.50.6.1173. [DOI] [PubMed] [Google Scholar]
- MARSHALL R. J., SCHIRGER A., SHEPHERD J. T. Blood pressure during supine exercise in idiopathic orthostatic hypotension. Circulation. 1961 Jul;24:76–81. doi: 10.1161/01.cir.24.1.76. [DOI] [PubMed] [Google Scholar]
- May C. N., Ham I. W., Heslop K. E., Stone F. A., Mathias C. J. Intravenous morphine causes hypertension, hyperglycaemia and increases sympatho-adrenal outflow in conscious rabbits. Clin Sci (Lond) 1988 Jul;75(1):71–77. doi: 10.1042/cs0750071. [DOI] [PubMed] [Google Scholar]
- Mehta N., Iyawe V. I., Cummin A. R., Bayley S., Saunders K. B., Bennett E. D. Validation of a Doppler technique for beat-to-beat measurement of cardiac output. Clin Sci (Lond) 1985 Oct;69(4):377–382. doi: 10.1042/cs0690377. [DOI] [PubMed] [Google Scholar]
- Nystrom E., Reid K. H., Bennett R., Couture L., Edmonds H. L., Jr A comparison of two automated indirect arterial blood pressure meters: with recordings from a radial arterial catheter in anesthetized surgical patients. Anesthesiology. 1985 Apr;62(4):526–530. doi: 10.1097/00000542-198504000-00030. [DOI] [PubMed] [Google Scholar]
- Smith G. D., Watson L. P., Pavitt D. V., Mathias C. J. Abnormal cardiovascular and catecholamine responses to supine exercise in human subjects with sympathetic dysfunction. J Physiol. 1995 Apr 1;484(Pt 1):255–265. doi: 10.1113/jphysiol.1995.sp020663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning G. K., Ben-Shlomo Y., Magalhães M., Daniel S. E., Quinn N. P. Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1995 Feb;58(2):160–166. doi: 10.1136/jnnp.58.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]