Abstract
Study Objectives:
Psoriasis and sleep apnea are associated with significant morbidity and mortality. Although both diseases have been linked with systemic inflammation, studies on their potential bidirectional association are lacking. We investigate the potential association between psoriasis and sleep apnea.
Methods:
All Danish citizens age 18 y or older between January 1, 1997 and December 31, 2011 (n = 5,522,190) were linked at individual level in nationwide registries. Incidence rates (IRs) per 10,000 person-years were calculated and incidence rate ratios (IRRs) adjusted for age, sex, socioeconomic status, smoking history, alcohol abuse, medication, and comorbidity were estimated by Poisson regression.
Results:
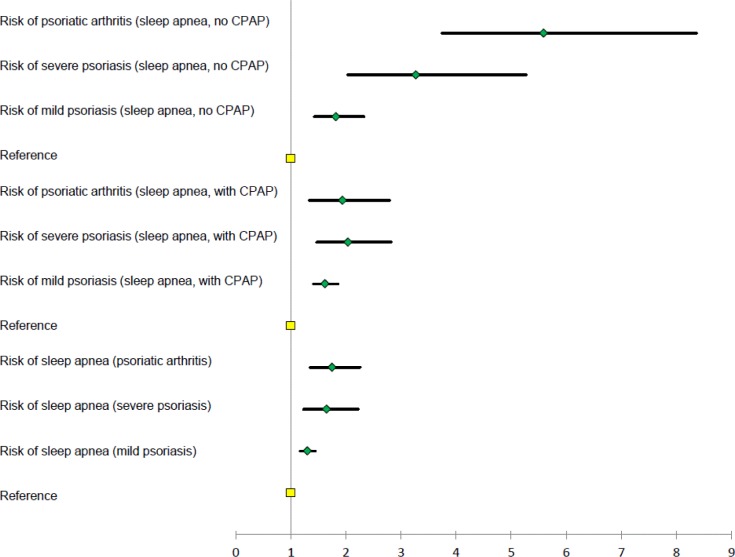
There were 53,290, 6,885, 6,348, and 39,908 incident cases of mild psoriasis, severe psoriasis, psoriatic arthritis, and sleep apnea, respectively. IRRs (95% confidence interval) for sleep apnea were 1.30 (1.17–1.44), 1.65 (1.23–2.22), and 1.75 (1.35–2.26) in subjects with mild and severe psoriasis, and psoriatic arthritis, and IRRs for mild and severe psoriasis, and psoriatic arthritis in sleep apnea without continuous positive airway pressure (CPAP) therapy were 1.62 (1.41–1.86), 2.04 (1.47–2.82), and 1.94 (1.34–2.79), respectively. In patients with sleep apnea and CPAP therapy (i.e., severe sleep apnea) the IRRs were 1.82 (1.43–2.33), 3.27 (2.03–5.27), and 5.59 (3.74–8.37), respectively.
Conclusions:
Psoriasis was associated with increased risk of sleep apnea, and sleep apnea was associated with increased risk of psoriasis. The clinical significance of this bidirectional relationship warrants further study.
Citation:
Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Psoriasis and sleep apnea: a Danish nationwide cohort study. J Clin Sleep Med 2016;12(5):663–671.
Keywords: epidemiology, inflammation, psoriasis, risk, sleep apnea
INTRODUCTION
Psoriasis, in particular in the moderate to severe form, is a common systemic inflammatory disease with increasing prevalence, and is associated with significant morbidity and mortality.1–5 Sleep apnea is a chronic condition in which the upper respiratory airways intermittently collapse during sleep, leading to episodes of hypoxia that can trigger systemic inflammation, sympathetic nervous system activation, metabolic dysregulation, endothelial dysfunction, and increased coagulation.6,7 The prevalence of sleep apnea ranges from 3% to 17%, and risk factors include advanced age, male sex, excess body weight, and race.6,7 Sleep apnea is recognized as an important cause of cardiovascular morbidity and mortality, cognitive dysfunction, impaired work performance, and reduced quality of life.7,8 To date, data on the potential association of sleep apnea and psoriasis are limited and inconsistent.9–12 We therefore examined the effect of psoriasis on the risk of incident sleep apnea and vice versa, in a nationwide cohort of the Danish population.
METHODS
All Danish citizens have free, equal, and universal access to health care. The unique personal identification number, which is assigned to all Danish citizens at immigration or birth, allows for cross linkage of administrative registries, and information on date of birth, sex, and vital statistics is available from the Civil Personal Registry.13 Information on morbidity was obtained from the Danish National Patient Register, in which information on hospital admissions, procedures, and diagnosis has been recorded since 1978.14 Registration of morbidity is according to the International Classification of Diseases (ICD) codes (ICD-8 from 1978 to 1994 and ICD-10) thereafter, and hospital procedures (including hospital-based pharmacological treatment, e.g., with biological therapy) are coded in the Danish National Patient Register as treatment procedure (SKS) codes. Information on all pharmacy-dispensed prescription drugs is recorded in the Danish Registry of Medicinal Products Statistics according to the international Anatomical Therapeutic Chemical (ATC) classification.15
BRIEF SUMMARY
Current Knowledge/Study Rationale: Psoriasis and sleep apnea are inflammatory diseases associated with significant cardiovascular morbidity and mortality, but information on their potential bidirectional association are lacking.
Study Impact: Patients with psoriasis or psoriatic arthritis have a disease severity increased risk of sleep apnea, and patients with sleep apnea have increased risk of psoriasis and psoriatic arthritis.
The cohort comprised all Danish subjects aged 18 y or older, without previous psoriasis or sleep apnea, from January 1, 1997 to December 31, 2011, and followed until migration, death from any cause, December 31, 2011, or the occurrence of an endpoint, whichever came first. Patients with psoriasis were identified when they dispensed their second prescription of topical vita-min D derivatives (ATC D05AX), which is the favored first-line treatment for psoriasis in Denmark, or by their first inpatient or outpatient consultation for psoriasis (ICD-8 696.10, 696.19 and ICD-10 L40) or psoriatic arthritis (ICD-8 696.09 and ICD-10 M070-M073), respectively. Patients were defined as having severe psoriasis if they received systemic treatment consistent with severe disease, i.e. treatment with biological drugs (L04AB01, L04AB02, L04AB04, L04AC05, L04AA21, BOHJ18A1-BOHJ18A3, BOHJ18B3), cyclosporine (L04AD01, BOHJ20), psoralens (D05BA, BNGA1), retinoids (D05BB, BQHB30), or methotrexate (L03BA01, L04AX03, BWHA115), respectively.4 We identified patients with sleep apnea by their first diagnosis of sleep apnea (ICD-10 G473). The diagnostic coding of sleep apnea has recently been validated in the National Patient Registry with a positive predictive value of 82%.8 To define subsequent continuous positive airway pressure (CPAP) therapy, we used codes involving CPAP therapy, CPAP device availability, adjustment of CPAP device, and control of CPAP device (SKS codes BGFC32, ZZ3911, ZZ3912, ZZ3913, ZZ3914, ZZ3915, and ZZ3916, respectively). To ensure adherence to therapy, two successive CPAP-related codes were required, and the second date was used to define the initiation of CPAP therapy.
We described baseline treatment (up to 180 days prior to study inclusion), hypnotic agents and sedatives (ATC N05C), opioids (N02A), and thyroid hormones (H03AA01). Baseline comorbidity for the following diagnoses was described by ICD codes up to 5 y prior to study inclusion: atrial fibrillation, diabetes, stroke, and heart failure, respectively. We identified patients with a history of smoking or alcohol abuse through the use of pharmacotherapy, treatment interventions, or diagnoses related to smoking or alcohol abuse, respectively. Hypertension was defined by either a hospital diagnosis, or if a patient within 90 days received treatment with at least two of the following classes of antihypertensive drugs: α-adrenergic blockers, non-loop diuretics, vasodilators, β-blockers, calcium channel blockers, and renin-angiotensin system inhibitors, as previously validated with a positive predictive value of > 80%.16 Diabetes was defined by either a hospital diagnosis, or use of glucose-lowering drugs. Information on medications, comorbidity, and hospital treatment procedures was continually updated during the follow-up period by use of the respective ICD, SKS, and ATC codes as appropriate. An age-standardized socioeconomic status from 0–4 was calculated for a 5-y period prior to study inclusion. This study was approved by the Danish Data Protection Agency (Ref. 2007-58-0015/int. ref. GEH-2014-018/I-Suite 02736). Approval from an ethics committee is not required for register studies in Denmark. The study was conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations.17
Statistical Analysis
SAS statistical software version 9.4 (SAS Institute Inc. Cary, NC, USA) and STATA software version 11.0 (StataCorp, College Station, TX, USA) were used to summarize incidence rates per 10,000 person-years, and to estimate incidence rate ratios (IRRs) by use of Poisson regression models. All statistical tests were conducted using a level of significance of 0.05, and results reported with 95% confidence intervals (CIs), where applicable. To ensure accurate registration of time at risk, exposure to psoriasis and sleep apnea were included as time-dependent variables, so the patients with psoriasis were only considered to be at risk of sleep apnea from the time that they fulfilled the psoriasis diagnosis and vice versa. IRRs for sleep apnea and psoriasis were calculated as crude, age- and sex-adjusted, and fully adjusted (in which age, sex, socio-economic status, smoking history, alcohol abuse, and comorbidities were considered) variables, respectively. In the fully adjusted IRRs for sleep apnea, we also adjusted for use of concomitant medication. As obesity and diabetes mellitus are common denominators in psoriasis and sleep apnea, we repeated our analyses with exclusion of patients with a diagnosis of obesity (ICD-8 277.99 and ICD-10 E65-E68) or diabetes at baseline or anytime during follow-up.
RESULTS
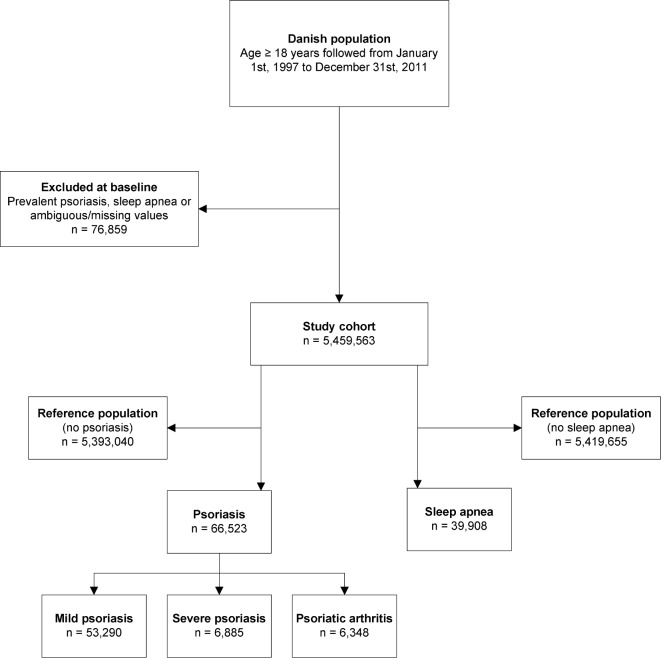
The total study population comprised 5,536,422 individuals, aged 18 y or older between January 1, 1997 and December 31, 2011. We excluded 76,859 individuals with a history of psoriasis, sleep apnea, or missing values (e.g., lost to follow up) prior to inclusion. The final study cohort comprised 5,459,563 Danish citizens with a maximum follow-up of 15 years. During the study 53,290, 6,885, and 6,348 patients were classified with mild and severe psoriasis, and psoriatic arthritis, respectively, and a total of 39,908 patients with sleep apnea were identified. The baseline characteristics of the study population are shown in Table 1, and the study flow chart is shown in Figure 1. The mean age was slightly higher in patients with mild and severe psoriasis compared to the reference population, and psoriatic arthritis was somewhat more common in women. Individuals with sleep apnea were predominantly male (78.9%), and were slightly older compared to the reference population.
Table 1.
Baseline characteristics of the study population.
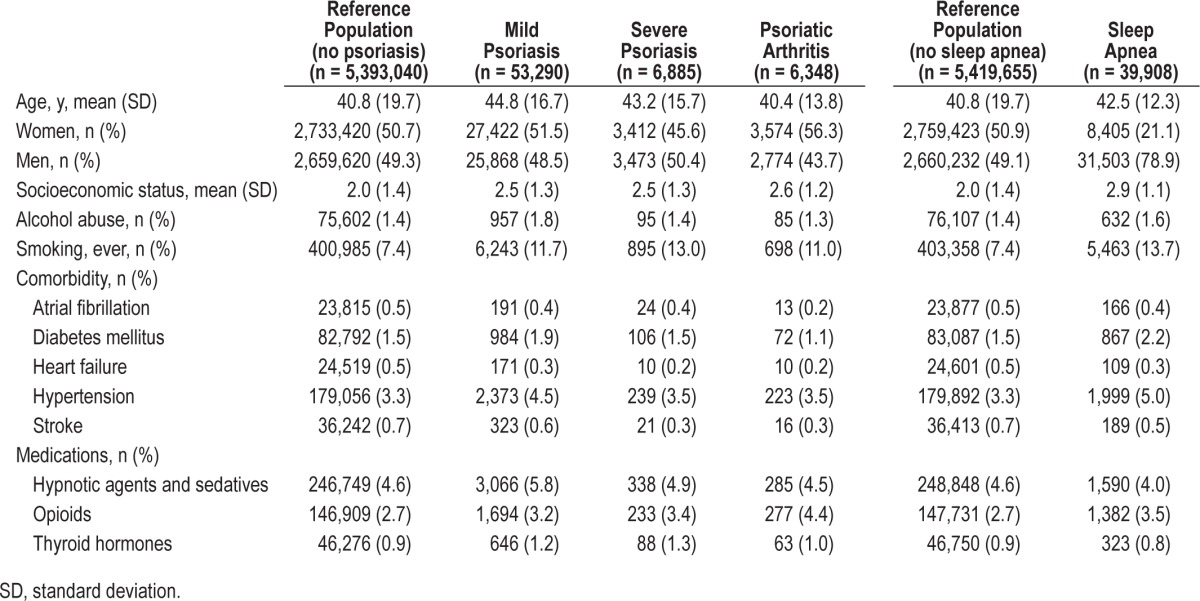
Figure 1. Study flow chart.
Risk of Sleep Apnea in Patients with Psoriasis
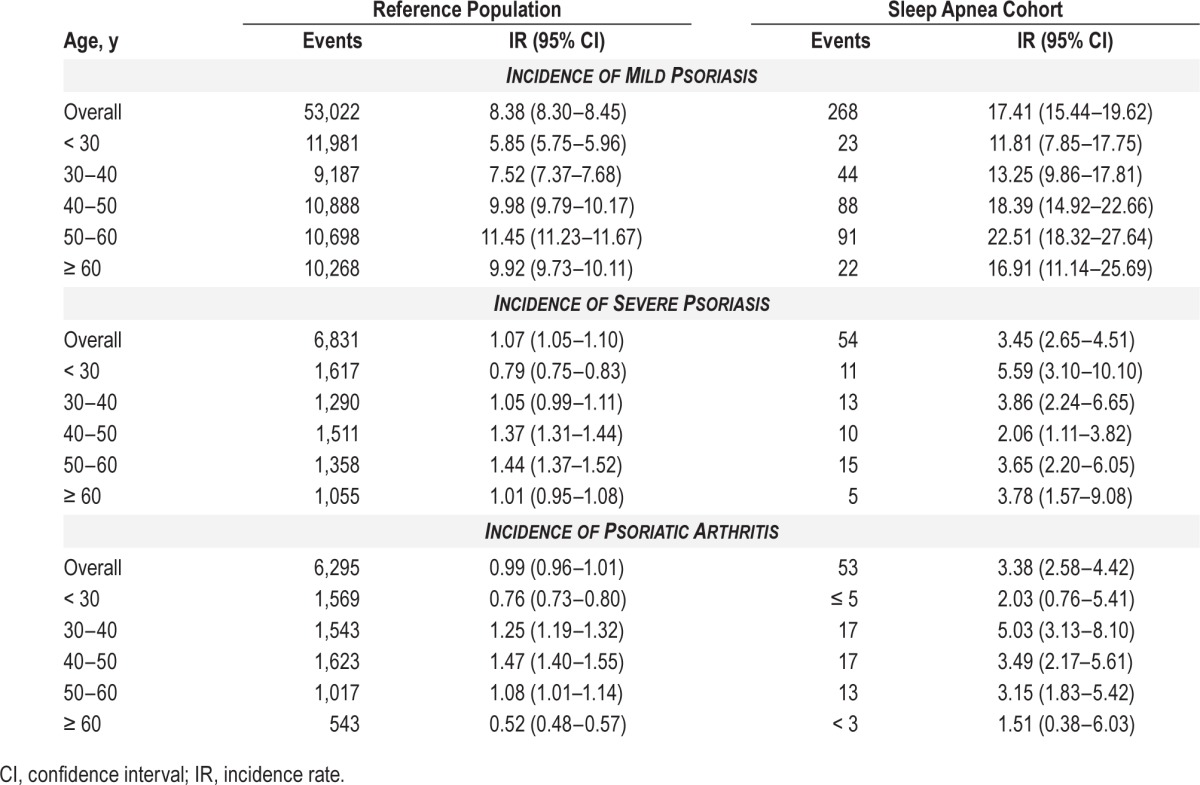
The results showed an increased risk of sleep apnea in patients with psoriasis. As shown in Table 2, the incidence rates of sleep apnea per 10,000 person-years were 6.48 (95% CI 6.41– 6.55), 12.18 (95% CI 10.79–13.53), 18.23 (95% CI 13.82–24.05), and 20.08 (95% CI 15.49–26.04) for the reference population, mild psoriasis, severe psoriasis, and psoriatic arthritis groups, respectively. Stratified by age, the highest incidence of sleep apnea found was in individuals 40 to 50 y of age, both in the reference, mild and severe psoriasis, and psoriatic arthritis groups. Across all age bands, with exception of patients age 60 y or older, the incidence of sleep apnea increased with severity of psoriasis (Table 3).
Table 2.
Incidence rates of sleep apnea per 10,000 person-years in patients with mild and severe psoriasis, and psoriatic arthritis compared to the reference population stratified by age.
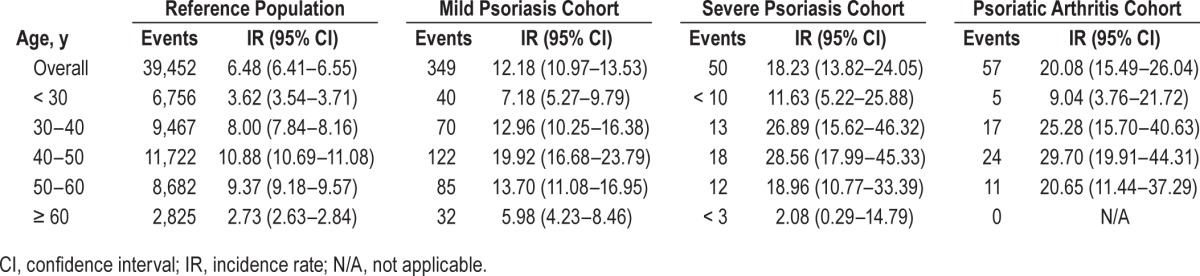
Table 3.
Incidence rates of mild and severe psoriasis, or psoriatic arthritis per 10,000 person-years in patients with sleep apnea compared to the reference population stratified by age.
The crude and age- and sex-adjusted IRRs showed an increased risk of sleep apnea in both patients with mild and severe psoriasis, and psoriatic arthritis (Table 4). Compared with the reference population, the fully adjusted (for age, sex, socioeconomic status, smoking history, alcohol abuse, comorbidity, and medication) multivariate Poisson regression analyses showed a significantly increased risk of sleep apnea in patients with mild psoriasis (IRR 1.30, 95% CI 1.17–1.44), severe psoriasis (IRR 1.65, 95% CI 1.23–2.22), and psoriatic arthritis (IRR 1.75, 95% CI 1.35–2.26), respectively, as shown in Figure 2. After exclusion of patients with a baseline diagnosis of heart failure, atrial fibrillation, or stroke, respectively, the IRRs were 1.37 (95% CI 1.23–1.54), 1.67 (95% CI 1.21–2.30), and 1.96 (95% CI 1.50–2.55), for mild and severe psoriasis, and psoriatic arthritis, respectively. When patients with obesity or diabetes were excluded, the fully adjusted IRRs were 1.36 (95% CI 1.21–1.53), 1.53 (95% CI 1.08– 2.18), and 1.98 (95% CI 1.50–2.61), respectively.
Table 4.
Incidence rate ratios of sleep apnea in patients with psoriasis, and incidence rate ratios of psoriasis in patients with sleep apnea.
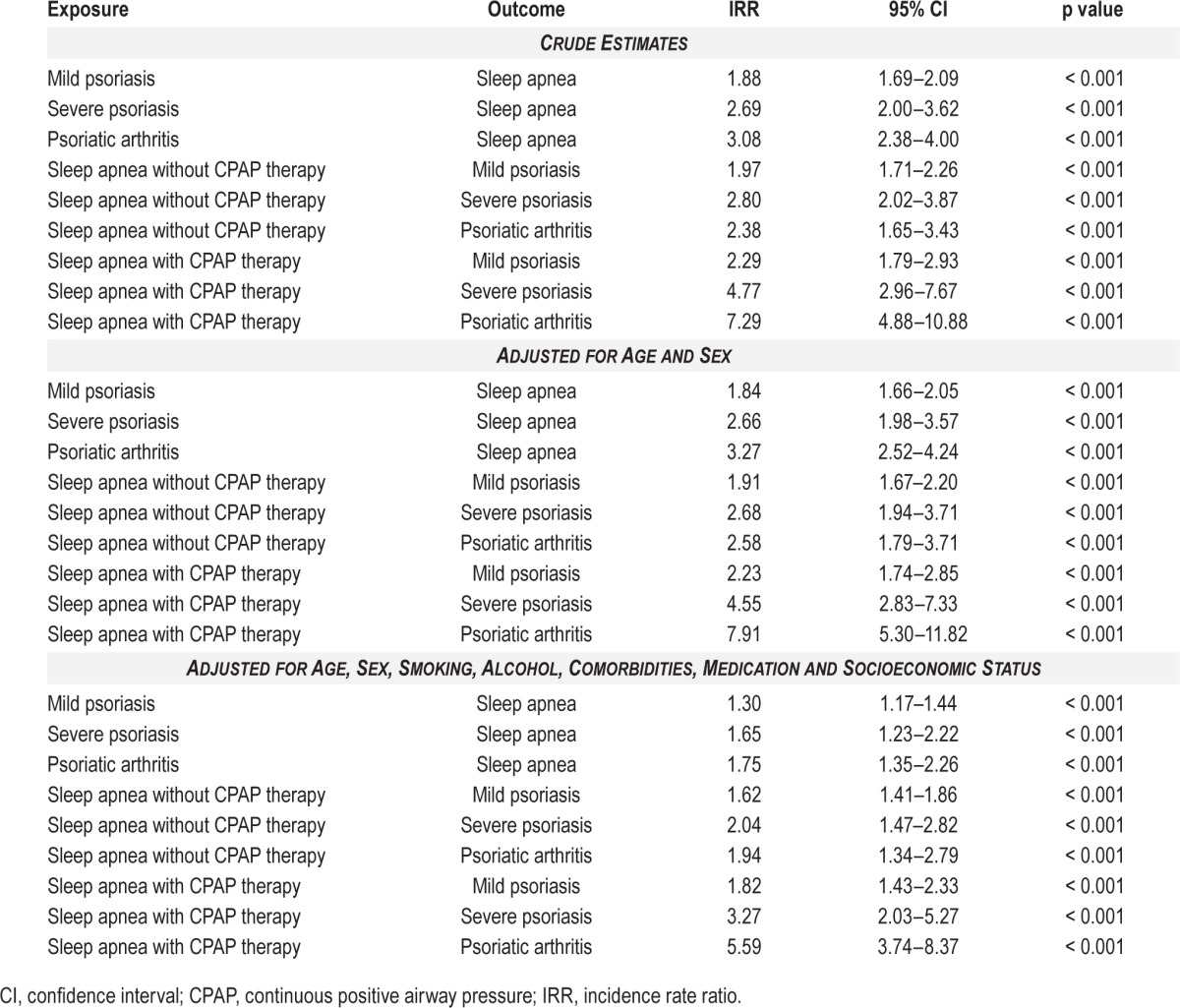
Figure 2. Forest plot of fully adjusted incidence rate ratios of psoriasis and sleep apnea.
CPAP, continuous positive airway pressure.
Risk of Psoriasis in Patients with Sleep Apnea
The incidence rates per 10,000 person-years for mild psoriasis were 8.38 and 17.41, severe psoriasis were 1.07 and 3.45, and incidence rates for psoriatic arthritis were 0.99 and 3.38, in the reference population, and patients with sleep apnea, respectively. The incidence rates of psoriasis were higher among patients with sleep apnea in all age bands.
An increased risk of psoriasis in patients with sleep apnea with and without CPAP therapy was found both in crude, age- and sex-adjusted, and fully (for age, sex, smoking history, alcohol abuse, comorbidities, and socio-economic status) adjusted analyses (Table 4). The increased risk of mild and severe psoriasis, and psoriatic arthritis, in patients with sleep apnea remained statistically significant in the sensitivity analyses where individuals with obesity or diabetes were excluded, with fully adjusted IRRs of 1.62 (95% CI 1.38–1.89), 1.85 (95% CI 1.25–2.74), and 1.98 (95% CI 1.32–2.99), respectively, in patients without CPAP therapy, and IRRs of 1.95 (95% CI 1.48– 2.57), 3.75 (95% CI 2.22–6.34), and 6.84 (95% CI 4.49–10.40), respectively, in patients with CPAP therapy.
DISCUSSION
In this nationwide cohort study, which to our knowledge is the first study to examine simultaneously the potential bidirectional relationship between sleep apnea and psoriasis, we observed a severity-dependent association between the two diseases. The results remained consistent after adjustment for potential confounding factors, as well as in sensitivity analyses.
Only a few studies have previously investigated the risk of sleep apnea in patients with psoriasis and only one study previously examined the risk of psoriasis in patients with sleep apnea.9–12 A study of 35 patients from Greece who had psoriasis found no association between sleep apnea and psoriasis characteristics other than body mass index (BMI) and hypertension.9 Another study of 33 patients with more than 5 y duration of psoriasis found the frequency of sleep apnea to be 54.5%.10 However, these studies were limited by their small number of patients, and neither involved a control group. A third study in 25 patients with psoriasis and 19 patients with chronic bronchitis (which is associated with sleep apnea) matched by age and sex found sleep apnea in 36% of patients with psoriasis, compared to 32% of the patients with chronic bronchitis.11 Yang et al.12 examined the risk of psoriasis in a cohort of 2,258 patients from Taiwan with sleep apnea compared with 11,255 healthy individuals. The study had a maximum follow-up of 3 y, and reported an approximately twofold greater risk (hazard ratio 2.30, 95% CI 1.13–4.69, p < 0.05) of the development of psoriasis or psoriatic arthritis in patients with sleep apnea compared with the healthy individuals. Similar to the latter results, we found a 62% and 82% increased risk of mild psoriasis, and an 82% and 327% increased risk of severe psoriasis in patients with sleep apnea (without and with CPAP therapy, respectively). However, our current data expand these results considerably in a much larger cohort of predominantly Caucasian descent, with up to five times longer follow-up, assessment of patients with CPAP therapy (i.e., more severe sleep apnea), and are an important addition to existing knowledge by suggesting that the association between these two diseases is bidirectional. A recent study on 71,598 US women examined the association between sleep disordered breathing, i.e., sleep apnea, and the risk of incident psoriasis or psoriatic arthritis using a questionnaire.18 Although an increased risk of psoriasis was reported, these authors did not find an increased risk of psoriatic arthritis in women with sleep apnea, contrary to our findings, which may be due to the large male predominance in our sleep apnea cohort (78.9%), or the difference in outcome measure between the two studies (hospital diagnosis of psoriatic arthritis versus questionnaire).
The bidirectional relationship between psoriasis and sleep apnea independent of measured confounders suggest the existence of shared pathogenic pathways and systemic inflammation would appear to provide an obvious mechanistic link. The activities of the proinflammatory transcription factor in the interleukin (IL)-17 signaling pathway and nuclear factor (NF)-κB, and systemic concentrations of downstream inflammatory cytokines such as tumor necrosis factor (TNF)-α and IL-6, respectively, are significantly increased in patients with sleep apnea compared to obese controls; and in patients with psoriasis, circulating levels of TNF-α, IL-6, and IL-17 have been positively correlated with disease severity.19–24 Furthermore, studies in patients with sleep apnea have demonstrated a decrease in TNF-α, IL-6, and other NF-κB–dependent cytokines after treatment with CPAP therapy.25,26 Although the elevated levels of inflammatory mediators in patients with sleep apnea were previously thought to be exclusively the result of the condition itself, this dogma has been challenged in recent years.27–34 For example, a placebo-controlled, double-blind pilot study examined the effect on treatment with a TNF-α inhibitor in patients with sleep apnea absent of other inflammatory conditions (e.g., psoriasis or rheumatoid arthritis) and found that neutralization of TNF-α activity was associated with a significant reduction of sleep apnea.27 Although one study failed to find a reduced risk of sleep apnea in patients with psoriasis after inhibition of TNF-α with a monoclonal antibody, studies with other TNF-α inhibitors have reported improvements on symptoms of sleep apnea in both patients with psoriasis and rheumatoid arthritis.28–31 Indeed, in line with these findings, evidence is accumulating that high levels of systemic inflammatory molecules are part of the pathogenic mechanisms that lead to sleep apnea.27,32–34
Sleep apnea has been associated with cardiovascular disease (CVD) and a recent study of 4.5 million individuals in Denmark reported that compared with the general population, patients with sleep apnea had 71% and 50% increased risk of myocardial infarction and ischemic stroke, respectively, with patients younger than 50 years being at particularly high risk.35 In addition, patients with psoriasis have increased risk of CVD, and evidence suggests that young subjects with severe skin disease and/or psoriatic arthritis are at highest risk.35,36 Furthermore, in a study of patients with atrial fibrillation, we have found that psoriasis was associated with increased risk of stroke independent of traditional risk factors.37 Recently, the same relationship was suggested for patients with sleep apnea, which indicates that both psoriasis and sleep apnea are clinical risk factors for CVD that are not adequately captured by current risk score algorithms.38 Also, a reduced risk of CVD in patients with sleep apnea following treatment with CPAP therapy has been reported in some, but not all, studies.8,39,40 Currently, it is unknown if sleep apnea carries an additional risk of CVD in patients with psoriasis, and currently CPAP therapy is reserved for patients with more severe sleep apnea, suggesting that the observed higher risk of psoriasis and psoriatic arthritis in these patients may be attributed to increased sleep apnea severity. However, in view of our current finding of a bidirectional relationship between psoriasis and sleep apnea, it is tempting to speculate that in patients with both diseases, CPAP may also exert antipsoriatic effects.
Studies in recent years have demonstrated an increase in the prevalence of psoriasis, but explanations for this phenomenon have not been firmly established.1,2 However, the observed association between sleep apnea and psoriasis may contribute to this development and obesity is a driver of both these diseases. Indeed, the global prevalence of obesity is also on the rise and obesity is associated with sleep apnea, for which weight loss is first-line recommended therapy.41–43 Patients with psoriasis have a higher BMI compared with their healthy peers, and BMI is directly correlated with psoriasis severity.44 Moreover, diabetes has been strongly linked to both sleep apnea and psoriasis.45,46 Obese patients exhibit chronic subclinical inflammation, and obesity is both a predisposing factor and a potential consequence of the increased systemic inflammatory load in chronic inflammatory diseases.47 In our study, however, sensitivity analyses with exclusion of patients with obesity or diabetes showed that the risk of mild and severe psoriasis, and psoriatic arthritis, respectively, remained elevated in patients with sleep apnea, and vice versa. Therefore, the results indicate that confounding by obesity is unlikely to fully explain the observed association and it is likely that the observed risk is driven, in part, by systemic inflammatory mechanisms resulting from interplay between all three conditions.
Certain limitations of the study should be taken into consideration. Information on risk factors for sleep apnea, such as hypothyroidism, was not available, but we attempted to adjust for this by use of a surrogate, i.e., use of thyroid hormones. In addition, the sensitivity of the registry diagnosis of obesity is limited because many obese subjects are not referred to hospitals. Importantly, the population in Denmark is primarily of Caucasian origin, and extrapolation of the results to other ethnic groups should therefore be done with caution. Important strengths of the study include the use of validated outcome measures, the use of nationwide registries of hospitalization data and prescription claims from all pharmacies in Denmark, whereby bias due to sex, age, concurrent medication, comorbidity, and socioeconomic status are practically eradicated. Also, the length and accuracy of follow-up, and the large number of individuals provide a high degree of credibility to our findings, and baseline exclusion of patients with psoriasis and sleep apnea ensured accurate risk-time allocation and enabled evaluation of the temporal relationship between the two conditions.
In conclusion, we found a disease severity–dependent bidirectional association between psoriasis and sleep apnea. The clinical significance of this bidirectional relationship warrants further study.
DISCLOSURE STATEMENT
This work was supported by a grant from Pfizer. Drs. Khalid and Hansen are supported by an unrestricted grant from the LEO Foundation. Dr. Gislason is supported by an unrestricted research scholarship from the Novo Nordisk Foundation. Dr. Egeberg is supported by a grant from Pfizer, and is a former employee of Pfizer. Dr. Mallbris is currently employed by Eli Lilly and Company. Dr. Skov has indicated no financial conflicts of interest. This research was performed independently through the authors' academic university affiliations. Pfizer, Eli Lilly, the Novo Nordisk Foundation, and the LEO Foundation had no influence on data collection, no access to the data, and no influence on the decision to submit.
ACKNOWLEDGMENTS
Author Contributions: Drs. Egeberg and Khalid had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Egeberg, Khalid, Gislason, Mallbris, Skov, and Hansen. Acquisition, analysis, and interpretation of data: Egeberg, Khalid, Gislason, Mallbris, Skov, and Hansen. Drafting of the manuscript: Egeberg and Hansen. Critical revision of the manuscript for important intellectual content: Egeberg, Khalid, Gislason, Mallbris, Skov, and Hansen. Statistical analysis: Egeberg and Khalid. Obtained funding: Egeberg. Administrative, technical, or material support: Gislason. Study supervision: Egeberg, Khalid, Gislason, Mallbris, Skov, and Hansen.
ABBREVIATIONS
- ATC
Anatomical Therapeutic Chemical
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- CVD
cardiovascular disease
- ICD
International Classification of Diseases
- IL
interleukin
- IR
incidence rates
- IRR
incidence rate ratio
- NF-κB
nuclear factor kappa B
- SKS
sundhedsvæsnets klassifikationssystem (translated: the Health Service Classification System)
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- TNF-α
tumor necrosis factor alpha
REFERENCES
- 1.Danielsen K, Olsen AO, Wilsgaard T, Furberg AS. Is the prevalence of psoriasis increasing? A 30-year follow-up of a population-based cohort. Br J Dermatol. 2013;168:1303–10. doi: 10.1111/bjd.12230. [DOI] [PubMed] [Google Scholar]
- 2.Icen M, Crowson CS, McEvoy MT, Dann FJ, Gabriel SE, Maradit Kremers H. Trends in incidence of adult-onset psoriasis over three decades: a population-based study. J Am Acad Dermatol. 2009;60:394–401. doi: 10.1016/j.jaad.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong AW, Schupp C, Bebo B. Psoriasis comorbidities: results from the National Psoriasis Foundation survey 2003 to 2011. Dermatology. 2012;225:121–6. doi: 10.1159/000342180. [DOI] [PubMed] [Google Scholar]
- 4.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–41. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 5.Yeung H, Takeshita J, Mehta NN, et al. Psoriasis severity and the prevalence of major medical comorbidity: a population-based study. JAMA Dermatol. 2013;149:1173–9. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badran M, Ayas N, Laher I. Insights into obstructive sleep apnea research. Sleep Med. 2014;15:485–95. doi: 10.1016/j.sleep.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 7.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamberts M, Nielsen OW, Lip GY, et al. Cardiovascular risk in patients with sleep apnoea with or without continuous positive airway pressure therapy: follow-up of 4.5 million Danish adults. J Intern Med. 2014;276:659–66. doi: 10.1111/joim.12302. [DOI] [PubMed] [Google Scholar]
- 9.Papadavid E, Vlami K, Dalamaga M, et al. Sleep apnea as a comorbidity in obese psoriasis patients: a cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J Eur Acad Dermatol Venereol. 2013;27:820–6. doi: 10.1111/j.1468-3083.2012.04580.x. [DOI] [PubMed] [Google Scholar]
- 10.Karaca S, Fidan F, Erkan F, et al. Might psoriasis be a risk factor for obstructive sleep apnea syndrome? Sleep Breath. 2013;17:275–80. doi: 10.1007/s11325-012-0686-2. [DOI] [PubMed] [Google Scholar]
- 11.Buslau M, Benotmane K. Cardiovascular complications of psoriasis: does obstructive sleep apnoea play a role? Acta Derm Venereol. 1999;79:234. doi: 10.1080/000155599750011075. [DOI] [PubMed] [Google Scholar]
- 12.Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea: a longitudinal population-based study. Sleep Med. 2012;13:285–9. doi: 10.1016/j.sleep.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–9. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 14.Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Danish Med Bull. 1999;46:263–8. [PubMed] [Google Scholar]
- 15.Gaist D, Sorensen HT, Hallas J. The Danish prescription registries. Danish Med Bull. 1997;44:445–8. [PubMed] [Google Scholar]
- 16.Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. Br Med J. 2011;342:d124. doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 18.Cohen JM, Jackson CL, Li TY, Wu S, Qureshi AA. Sleep disordered breathing and the risk of psoriasis among US women. Arch Dermatol Res. 2015;307:433–8. doi: 10.1007/s00403-015-1536-4. [DOI] [PubMed] [Google Scholar]
- 19.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 20.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:277–81. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu C, Wu L, Li X. IL-17 family: cytokines, receptors and signaling. Cytokine. 2013;64:477–85. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–9. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 23.Bonifati C, Carducci M, Cordiali Fei P, et al. Correlated increases of tumour necrosis factor-alpha, interleukin-6 and granulocyte monocyte-colony stimulating factor levels in suction blister fluids and sera of psoriatic patients--relationships with disease severity. Clin Exper Dermatol. 1994;19:383–7. doi: 10.1111/j.1365-2230.1994.tb02687.x. [DOI] [PubMed] [Google Scholar]
- 24.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273–9. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–34. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 26.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 27.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 28.Maari C, Bolduc C, Nigen S, Marchessault P, Bissonnette R. Effect of adalimumab on sleep parameters in patients with psoriasis and obstructive sleep apnea: a randomized controlled trial. J Dermatolog Treat. 2014;25:57–60. doi: 10.3109/09546634.2012.713458. [DOI] [PubMed] [Google Scholar]
- 29.Zamarron C, Maceiras F, Mera A, Gomez-Reino JJ. Effect of the first infliximab infusion on sleep and alertness in patients with active rheumatoid arthritis. Ann Rheum Dis. 2004;63:88–90. doi: 10.1136/ard.2003.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor-Gjevre RM, Gjevre JA, Nair BV, Skomro RP, Lim HJ. Improved sleep efficiency after anti-tumor necrosis factor alpha therapy in rheumatoid arthritis patients. Ther Adv Musculoskel Dis. 2011;3:227–33. doi: 10.1177/1759720X11416862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thaci D, Galimberti R, Amaya-Guerra M, et al. Improvement in aspects of sleep with etanercept and optional adjunctive topical therapy in patients with moderate-to-severe psoriasis: results from the PRISTINE trial. J Eur Acad Dermatol Venereol. 2014;28:900–6. doi: 10.1111/jdv.12207. [DOI] [PubMed] [Google Scholar]
- 32.Entzian P, Linnemann K, Schlaak M, Zabel P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med. 1996;153:1080–6. doi: 10.1164/ajrccm.153.3.8630548. [DOI] [PubMed] [Google Scholar]
- 33.Riha RL, Brander P, Vennelle M, et al. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur Respir J. 2005;26:673–8. doi: 10.1183/09031936.05.00130804. [DOI] [PubMed] [Google Scholar]
- 34.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–7. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 35.Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147–57. doi: 10.1111/j.1365-2796.2010.02310.x. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2:e000062. doi: 10.1161/JAHA.113.000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahlehoff O, Gislason G, Lamberts M, et al. Risk of thromboembolism and fatal stroke in patients with psoriasis and nonvalvular atrial fibrillation: a Danish nationwide cohort study. J Intern Med. 2015;277:447–55. doi: 10.1111/joim.12272. [DOI] [PubMed] [Google Scholar]
- 38.Yaranov DM SA, Usantii N, Butler A, Petrini JR, Mendez J, Warshofsky MK. Effect of obstructive sleep apnea on frequency of stroke in patients with atrial fibrillation. Am J Cardiol. 2015;115:461–5. doi: 10.1016/j.amjcard.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012;186:909–16. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 40.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 41.Strobel RJ, Rosen RC. Obesity and weight loss in obstructive sleep apnea: a critical review. Sleep. 1996;19:104–15. doi: 10.1093/sleep/19.2.104. [DOI] [PubMed] [Google Scholar]
- 42.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yatsuya H, Li Y, Hilawe EH, et al. Global trend in overweight and obesity and its association with cardiovascular disease incidence. Circ J. 2014;78:2807–18. doi: 10.1253/circj.cj-14-0850. [DOI] [PubMed] [Google Scholar]
- 44.Duarte GV, Oliveira Mde F, Cardoso TM, et al. Association between obesity measured by different parameters and severity of psoriasis. Int J Dermatol. 2013;52:177–81. doi: 10.1111/j.1365-4632.2011.05270.x. [DOI] [PubMed] [Google Scholar]
- 45.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84–91. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 47.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]