Abstract
Study Objectives:
We examined cross-sectional and prospective associations between sleep debt and adiposity measures, as well as homeostatic model assessment-insulin resistance (HOMA-IR) in early type 2 diabetes.
Methods:
Prospective data analysis from participants of a randomized controlled trial based on an intensive lifestyle intervention (usual care, diet, or diet and physical activity). Data were collected at baseline, 6 months, and 12 months post-intervention. The study was performed across five secondary care centers in the United Kingdom. Patients (n = 593) with a recent diagnosis of type 2 diabetes were recruited. Objective height and weight were ascertained for obesity status (body mass index [BMI]; ≥ 30 kg/m2), waist circumference (cm) for central adiposity, and fasting blood samples drawn to examine insulin resistance (IR). Seven-day sleep diaries were used to calculate weekday sleep debt at baseline, calculated as average weekend sleep duration minus average weekday sleep duration.
Results:
At baseline, compared to those without weekday sleep debt, those with weekday sleep debt were 72% more likely to be obese (OR = 1.72 [95% CI:1.03–2.88]). At six months, weekday sleep debt was significantly associated with obesity and IR after adjustment, OR = 1.90 (95% CI:1.10–3.30), OR = 2.07 (95% CI:1.02–4.22), respectively. A further increase at 12 months was observed for sleep debt with obesity and IR: OR = 2.10 (95% CI:1.14–3.87), OR = 3.16 (95% CI:1.38–7.24), respectively. For every 30 minutes of weekday sleep debt, the risk of obesity and IR at 12 months increased by 18% and 41%, respectively.
Conclusions:
Sleep debt resulted in long-term metabolic disruption, which may promote the progression of type 2 diabetes in newly diagnosed patients. Sleep hygiene/education could be an important factor for future interventions to target early diabetes.
Citation:
Arora T, Chen MZ, Cooper AR, Andrews RC, Taheri S. The impact of sleep debt on excess adiposity and insulin sensitivity in patients with early type 2 diabetes mellitus. J Clin Sleep Med 2016;12(5):673–680.
Keywords: insulin resistance, type 2 diabetes mellitus, sleep debt, obesity, body mass index, waist circumference, central adiposity
INTRODUCTION
The International Diabetes Federation recently estimated that, globally, over 382 million individuals had diabetes in 2013 (an estimated 46.3% undiagnosed) and that this number is expected to rise to 592 million by 2035.1 The majority of affected individuals (over 90%) have type 2 diabetes, which is closely related to excess adiposity and its associated lifestyle factors such as poor diet and low physical activity. Sleep (duration and quality) may be another important factor associated with both obesity and type 2 diabetes, as suggested by both population and experimental studies.2–6 It has been hypothesized that social and work demands increasingly result in shorter sleep duration than required physiologically.7 This sleep loss, accumulated into a sleep debt during weekdays, may be partially repaid by extra sleep on weekends. Sleep debt is particularly evident in shift-workers and has been linked with metabolic disruption including increased secretion of cortisol, and higher C-reactive protein, triglycerides and low-density lipoprotein cholesterol levels.8,9 Persistent sleep debt may therefore play a role in promoting the onset/progression of chronic metabolic disease. While insufficient sleep, which results in sleep debt, and sleep disruption are associated with a potential increased risk for diabetes,10 little is known about the impact of weekday sleep debt once diabetes is present. We therefore investigated the relationship between sleep debt, and obesity, central adiposity, and insulin resistance in a well-characterized group of patients with early type 2 diabetes. We hypothesized that there would be a relationship between weekday sleep debt and four key features of type 2 diabetes mellitus: obesity, central adiposity, insulin resistance, and glycemic control.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep loss has been attributed to diabetes onset and progression, but little is known about the chronic effect of weekday sleep debt, which is common in contemporary society, upon diabetes outcomes in those with a recent diagnosis. Our study was performed to assess the long-term impact of weekday sleep debt upon multiple common, yet modifiable, diabetes outcomes (obesity, insulin sensitivity and central adiposity) in newly diagnosed type 2 diabetes mellitus patients.
Study Impact: Our findings highlight the importance of improving sleep in those with a new diagnosis of type 2 diabetes mellitus. Avoiding accumulation of sleep debt on weekdays may be a key component for minimizing adverse outcomes associated with diabetes mellitus (obesity, insulin resistance, and excess central adiposity), which could be incorporated into the future clinical management of this patient population.
METHODS
Data were from the Early ACTivity In Diabetes (Early ACTID) randomized controlled trial (Trial register ISRCTN92162869), which has been previously described.11 Briefly, participants aged 30–80 years with newly diagnosed type 2 diabetes mellitus (diagnosed within 5–8 months prior to participation) were recruited from primary care and randomly allocated to receive either lifestyle intervention (dietary modification or dietary modification plus additional physical activity) or usual care. Participants were excluded if they fulfilled one or more of the following criteria: HbA1c concentration > 10%, blood pressure > 180/100 mm Hg, LDL cholesterol concentration > 4 mmol/L, body mass index (BMI) < 25 kg/m2, body weight > 180 kg, use of weight-loss drugs, taking a sulphonylurea at the maximum dose, unstable angina, a myocardial infarction 3 months prior to participation, inability to increase physical activity, pregnancy/planning pregnancy. A total of 593 participants were recruited and underwent a detailed assessment, including medical history and a physical examination. Participants were randomized according to computer-generated allocation and were assigned using a 2:5:5 ratio (usual care: intensive diet intervention: intensive diet intervention plus activity). A 7-day sleep diary and a sleep questionnaire, previously employed in a well-established sleep cohort,12 were administered prior to commencement of the intervention. Follow-up data were obtained at 6 and 12 months post-intervention for all participants. The study received ethical approval from the Bath Hospital Research Ethics Committee and all participants provided written informed consent (05/Q 2001/5).
Sleep Measures
Weekday sleep duration was derived from the 7-day sleep diary by calculating the difference between the estimated time of sleep onset and sleep offset (wake). Average weekday sleep duration was then calculated by adding sleep duration from Sunday through to Thursday and dividing by 5.13 Similarly, average weekend sleep duration was derived in the same manner by adding sleep duration for Friday and Saturday and dividing by 2.13 We then derived weekday sleep debt (average weekend sleep duration minus average weekday sleep duration). Participants were also asked to complete 7-day sleep diaries at 6 and 12 months post-intervention.
From the sleep questionnaire (see supplemental material), we acquired information about sleep problems (physician diagnosed OSA/snoring/other/none). We also collected data regarding shift-work with response options of yes/no to the question “I have a job that involves shift-work or night work” and considered this as a potential confounder in our analyses.
Obesity and Central Adiposity
Height (cm) was obtained objectively at baseline and body weight (kg) was ascertained and recorded for all participants at baseline and then at 6 and 12 months post-intervention. These measures were used to derive BMI. Overweight/obesity status was considered as BMI ≥ 30 kg/m2. Waist circumference (cm) was measured in participants at each of the 3 visits (baseline and 6 and 12 months post-intervention). Participants were requested to stand with feet shoulder-width apart, and a measuring tape was passed horizontally around the center of the iliac crest to obtain the measurement, which was conducted and recorded by a trained nurse. As a measure of central adiposity, we used sex-specific cut points based on the National Institute of Health, where > 88 cm (women) and > 102 cm (men) were considered as excess central adiposity.14
Diabetes Outcomes
Participants presented to the research center at the 3 time points (baseline, 6 months, and 12 months post-intervention) following an overnight fast. A blood sample was drawn for assessment of glucose and insulin using standard assays to determine insulin resistance, using a standardized technique (homeostatic model assessment-insulin resistance)15 at baseline, 6 months, and 12 months post-intervention. Furthermore, we also measured hemoglobin A1c percent (HbA1c %) from the overnight fasted blood sample as a measure of diabetes control.
Other Measures
We obtained demographic information (age, gender), which we considered as potential confounders in our multivariate analyses. Physical activity levels were assessed across a 7-day period using a waist-worn accelerometer (GT1M; ActiGraph LLC, Pensacola, FL, USA). Participants were instructed to wear the device on awakening and remove at bedtime. Data were downloaded from the device using the manufacturer's software and the average counts per minute (cpm) were derived from all days that the monitor was worn.16 We also ascertained information relating to medication use at each of the 3 time points, including lipid-reducing medication (yes/ no), blood pressure lowering medication (yes/no) and number of medications for diabetes mellitus (DM). None of the participants were on insulin on recruitment into the study, and there was no significant change in DM medication 6 months post-intervention. The proportion of participants taking DM medication 12 months post-intervention increased in all conditions, and 2 participants became insulin-treated. We considered medication use of any type as a potential confounder in our analyses.
Statistical Analysis
All statistical analyses were performed using Stata version 13 (Texas, USA). Continuous data were checked for normality of distribution by visual inspection, prior to conducting statistical tests. First, we conducted a series of independent t-tests, χ2 or Wilcoxon signed ranks test, as appropriate, to compare those with zero or negative weekday sleep debt to those with positive weekday sleep debt in a number of baseline characteristics. Secondly, we investigated the potential relationships, using multivariate logistic regression, comparing those with baseline weekday sleep debt to those with zero/negative weekday sleep debt (referent). Four dependent variables were considered in our analyses. Firstly, we dichotomized BMI to determine obesity status where < 30 kg/m2 (overweight/non-obese) was considered as the referent and compared with obesity (BMI ≥ 30 kg/m2). Secondly, we considered excess central adiposity using sex-specific cut points for waist circumference based on the National Institute of Health recommendations.14 Thirdly, we dichotomized HOMA-IR comparing ≤ 2.5 as the referent, with > 2.5 indicating insulin resistance.17,18 Finally, we dichotomized HbA1c% using standardized cut points (< 6.5% as the referent versus ≥ 6.5%). Each of the outcome variables was assessed at baseline, 6 months, and 12 months post-intervention. In our series of multivariate logistic regression analyses, we present Model 1 (univariate) and then first adjusted for age and gender, as appropriate (Model 2); we then further adjusted for medications (lipids, blood pressure, diabetes mellitus), sleep dysfunction, shift-work, physical activity (counts per minute), change from baseline to study time point assessed in BMI, waist circumference HOMA-IR and HbA1c (as appropriate), as well as intervention type (usual care [referent], diet, diet and physical activity), at 6 and 12 months post-intervention only. We further adjusted for BMI in Model 3 for the outcomes: waist circumference, insulin resistance, and HbA1c. To further examine the effect of weekday sleep debt, we then repeated our multivariate logistic regression, to assess the effect size for every 30 minutes of weekday sleep debt in relation to obesity, central adiposity, insulin resistance, and HbA1c. The odds ratios (OR) and 95% confidence intervals (95% CIs) are reported for all analyses. We performed a one-way ANOVA with post hoc Bonferroni tests to assess potential mean differences between weekday and weekend sleep duration at each of the 3 time points assessed according to randomized condition (usual care, diet, or diet and physical activity intervention).
RESULTS
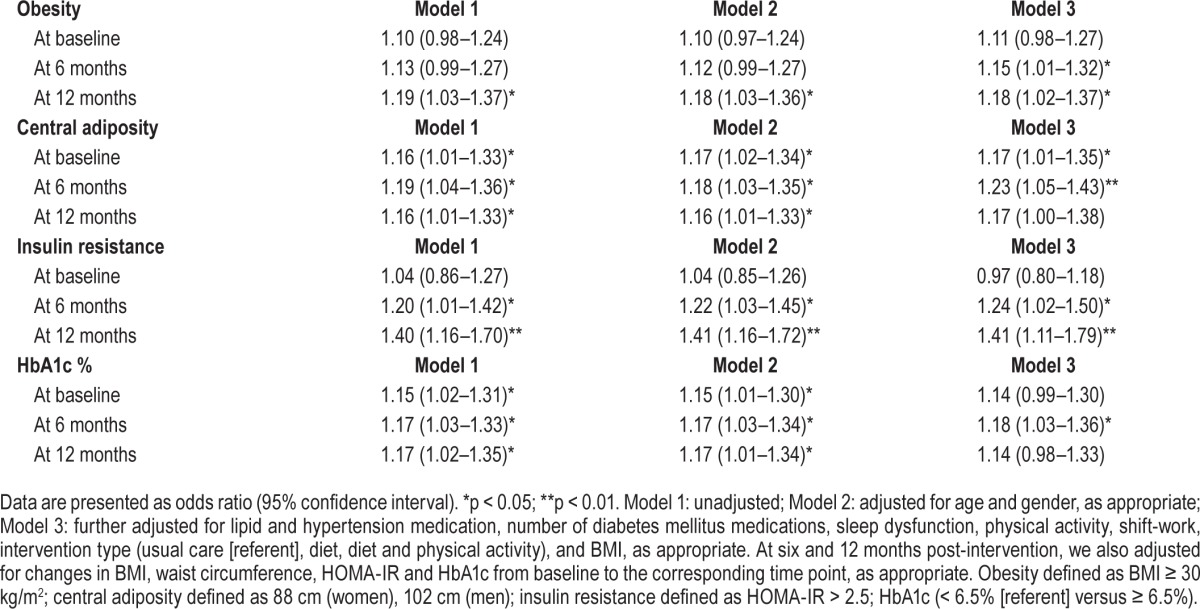
Complete information on all variables of interest was available in 365 newly diagnosed type 2 diabetes mellitus patients. The baseline characteristics of the sample, according to the presence/absence of weekday sleep debt, are presented in Table 1. Of the total sample, 61% (n = 221) reported positive weekday sleep debt, where the mean was 46 minutes (95% CI: 40, 53). On average those without weekday sleep debt reported −40 minutes' discrepancy between weekend and weekday sleep duration (95% CI: −46, −34). Briefly, compared to those with no weekday sleep debt, those with a weekday sleep deficit had significantly higher BMI (p = 0.014), body weight (p = 0.031), weekday and weekend sleep duration (p = 0.002 and p < 0.001), sleep dysfunction (p < 0.001), waist circumference (p = 0.003), and HOMA-IR (p = 0.005). There was no significant difference in the number of diabetes mellitus medications across the 3 time points assessed (p = 0.109) for the total sample. Differences in the percentage of participants with obesity, excess central adiposity, insulin resistance and HbA1c ≥ 6.5% according to the presence or absence of weekday sleep debt are presented in Figure 1A–1D, respectively.
Table 1.
Baseline characteristics of 365 newly diagnosed type 2 diabetes mellitus patients in the ACTID study, according to weekday sleep debt status.
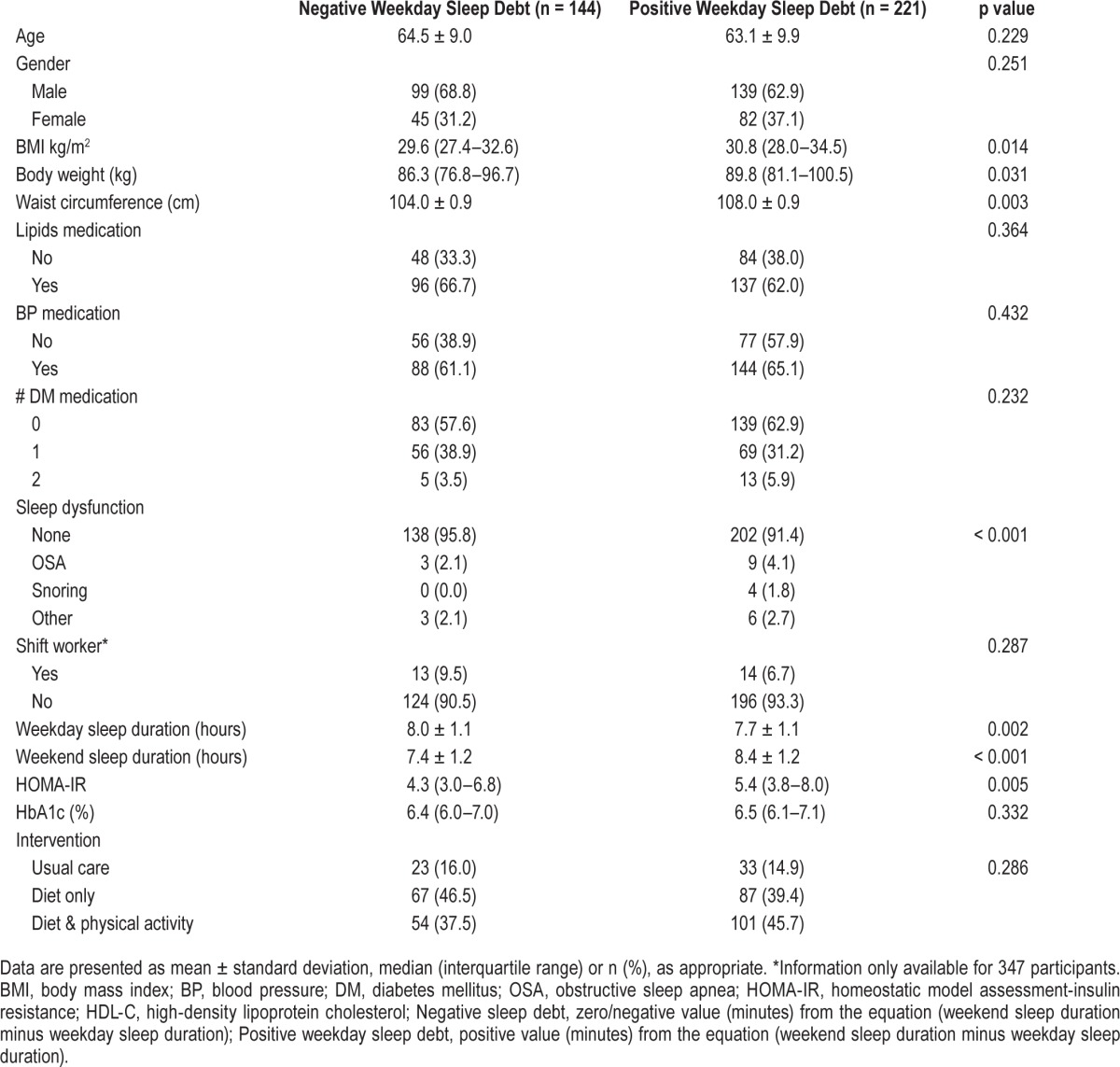
Figure 1. Proportion of individuals with and without sleep debt according to obesity, central adiposity, and insulin resistance at each of the three study time points (baseline, 6 months, and 12 months post-intervention).
(A) percentage of participants with obesity at each assessed time point according to presence/absence of weekday sleep debt at baseline; (B) percentage of participants with excess central adiposity at each assessed time point according to presence/absence of weekday sleep debt at baseline; (C) percentage of participants with insulin resistance at each assessed time.
There was a cross-sectional relationship between positive weekday sleep debt and obesity, after adjustment OR = 1.72 (95% CI: 1.02–1.92). Longitudinal multivariate analyses demonstrated a significant, increased risk of obesity at 6 months and a further increase at 12 months post-intervention with positive weekday sleep debt at baseline (see Table 2). The strongest effect of weekday sleep debt at baseline was observed for obesity at 12 months post-intervention OR = 2.10 (95% CI: 1.14–3.87), after adjustment.
Table 2.
The associations between positive weekday sleep debt and adiposity, insulin resistance and HbA1c at baseline, 6 months, and 12 months post-intervention in 365 newly diagnosed type 2 diabetes mellitus patients.

We observed a significant cross-sectional association between weekday sleep debt and waist circumference OR = 1.87 (95% CI: 1.10–3.17), adjusted for age, although this relationship diminished with further adjustment (see Table 2). After full adjustment, a significant association was present between weekday sleep debt and central adiposity at 6 months only, OR = 1.99 (95% CI: 1.14–3.48).
No cross-sectional relationship was observed between positive weekday sleep debt at baseline and insulin resistance, before or after adjustment (see Table 2). There was, however, a significant increased risk of insulin resistance, after adjustment, at 6 months and 12 months post-intervention in those with positive weekday sleep debt. At 6 months post-intervention, individuals with a positive weekday sleep debt at baseline were more than 2 times more likely to be insulin resistant. At 12 months post-intervention, compared to those without week-day sleep debt at baseline, those with sleep debt were more than 3 times more likely to be insulin resistant.
Those with weekday sleep debt had a significantly increased risk of HbA1c ≥ 6.5% at baseline (OR = 1.85 [95% CI: 1.08–3.14]) and 6 months post-intervention (OR = 1.95 [95% CI: 1.12–3.40]). No association was found at 12 months post-intervention between baseline weekday sleep debt and HbA1c ≥ 6.5%.
For every 30 minutes of additional weekday sleep debt at baseline, the risk of obesity and insulin resistance cross-sectionally showed no significant associations. A positive risk for obesity and insulin resistance, however, were observed longitudinally at 6 months post-intervention. Each additional 30 minutes of weekday sleep debt at baseline was associated with an 18% increased risk of excess central adiposity at baseline. Six months post-intervention, 30 minutes of weekday sleep debt was significantly associated with an increased risk of all 3 outcomes: 15% for obesity, 24% for insulin resistance, and 23% for central adiposity. A further significant increase was present at 12 months post-intervention for both obesity (18%) and insulin resistance (41%) (Table 3). Baseline weekday sleep debt of 30 minutes was associated with HbA1c ≥ 6.5% at 6 months post-intervention only (OR = 1.18 [95% CI: 1.03–1.36]).
Table 3.
The associations between every 30 minutes of positive weekday sleep debt and obesity, central adiposity, and insulin resistance at baseline, 6 months, and 12 months post-intervention in 365 newly diagnosed type 2 diabetes mellitus patients.
No differences were observed for average weekday or weekend sleep duration at any of the 3 study time points according to the randomized condition (p > 0.05). The only exception was weekday sleep duration for those randomized to usual care (8.2 ± 1.0 h) compared to the diet intervention (7.7 ± 1.1 h) at 12 months post-intervention, where the post-hoc Bonferroni test was p = 0.020.
DISCUSSION
Our study investigated the potential cross-sectional and prospective impact of weekday sleep debt upon obesity and insulin resistance in a large cohort of newly diagnosed patients with type 2 diabetes mellitus. Our findings demonstrate an increasing risk of obesity over the three time points assessed in those with positive weekday sleep debt at baseline. The effect of weekday sleep debt at baseline was also significantly and prospectively associated with central adiposity, increased insulin resistance, as well as glycemic control. Intervention type did not alter these associations (p > 0.05 for both intervention arms). We observed a small amount of weekday sleep debt, as little as 30 minutes, to be important for prospectively increasing the risk of three common features in early type 2 diabetes mellitus patients.
Previous studies have examined the impact of shorter sleep duration on body composition and metabolic parameters.6,19,20 It has been hypothesized that sleep loss predisposes to excess adiposity and metabolic derangements such as insulin resistance.6,10,20 Sleep loss is usually accumulated on weekdays, being associated with work and social commitments, with an opportunity to repay the accumulated sleep debt on weekend days. Few previous studies have examined the impact of sleep debt on body composition and insulin resistance. Specifically, there are no studies examining the impact of sleep debt in patients with early diabetes.
Previous studies have shown that sleep loss is accumulated, creating a greater sleep debt, with potential downstream effects on sleepiness and cognition.21,22 In one study, the greater sleep debt accumulated through chronic partial sleep deprivation in healthy volunteers (14 nights of 4 h or 6 h of sleep opportunity versus 8 h), the more impeded the participant's cognitive performance.21 Thus, if sleep loss accumulates, resulting in a sleep debt that is not repaid, then neurobehavioral consequences ensue. Our findings now show that sleep debt also has a signifi-cant impact on body composition and metabolic health.
There has been an important focus on the link between short sleep duration and metabolic disease in recent decades with systematic reviews and meta-analyses demonstrating strong associations with obesity (odds ratio = 1.55),23 waist circumference (r = −0.10),24 and type 2 diabetes mellitus (relative risk = 1.28).25 Previous studies, however, have concentrated on the impact of extremes of sleep duration (e.g., < 5 h of sleep/ night), but few studies have examined the cumulative effect of smaller degrees of sleep loss. We have shown that as little as 30 minutes of weekday sleep debt may contribute to the development and/or progression of obesity, central adiposity, and insulin resistance over time in patients with early diabetes. Furthermore, given our prospective analysis, sleep debt over time is likely to have a longer-term dose-dependent effect on body composition and insulin resistance. Sleep debt at baseline, for example, was associated with increased insulin resistance at 12 months by almost three-fold.
The mechanisms for the impact of sleep debt on body composition and metabolism remain to be determined. However, previous population and human sleep laboratory studies of healthy volunteers have highlighted several potential pathways. These include alterations in hormones regulating appetite, energy expenditure, and glucose homeostasis such as ghrelin, leptin, cortisol, and growth hormone.6,22 An early report by Spiegel and colleagues, subjected 11 healthy, young male participants to 3 nights of 8 hours of sleep opportunity (baseline) and then 6 nights of 4 hours' time in bed (sleep debt condition), and finally 7 nights of 12 hours in bed (sleep recovery). This study highlighted the impact of sleep deprivation on glucose homeostasis (reduced glucose tolerance) and cortisol secretion (elevated evening cortisol levels). Similar findings were observed for subjective sleepiness and sympatho-vagal balance (measured objectively by examining beat-to-beat heart-rate intervals). The link between sleep (generally believed to be a brain phenomenon) and hormone secretion appears to be explained by increased sympathetic activation with sleep loss.22 The metabolic alterations with sleep deprivation were reversed during sleep recovery suggesting that repayment of sleep debt may normalize some sleep-related hormone alterations.22 Encouraging sleep debt repayment may be a novel and relatively straightforward approach to promoting optimum health and wellbeing. Other potential pathways include increased wakefulness and opportunity to eat.26 For example, a number of recent experimental studies enforcing sleep reduction that results in additional wakefulness, have demonstrated increased food intake/calorie consumption, particularly during evening/early hours.27–29 One study found the order of sleep manipulation to be important with beneficial effects on body weight with decreases in the consumption of energy (fats and carbohydrates) in those experiencing adequate/recovery sleep subsequent to sleep loss.27 If this concept is applied to our own study findings then energy intake may improve during weekends in those with positive weekday sleep debt, although weight gain may still occur given the higher proportion of weekdays to weekends. This, however, requires further investigation in future studies to confirm or refute this notion.
In a six-year prospective study, short sleepers (≤ 6 h), as compared to those with healthy sleep duration (7–8 h), increased waist circumference and gained more weight over the study period. Statistically significant increases in these outcomes were found in individuals who scored high, compared to low for disinhibited eating (tendency to overeat and/or eat opportunistically). Over the six-year period, the risk of incident over-weight/obesity in short sleepers with high disinhibited eating was OR = 4.49 (95% CI: 3.06–6.06).4 The downstream physiological impact of persistent short sleep are likely to have consequential effects on feeding behaviors that may lead to weight gain and diabetes mellitus onset. Recent advances that have explored the relationship between experimental sleep loss, appetitive food desire and neuronal activity have revealed potential mechanistic pathways. For example, Greer and colleagues demonstrated reduced activity in multiple brain regions, known to be instrumental in appetitive desire.30 Participants (n = 23) completed a food-desire task (desire for 80 presented food items) and underwent fMRI after two sleep conditions (24 hours total sleep deprivation and normal rested sleep of ∼8 h). Compared to when participants were rested, after total sleep deprivation a significant reduction in activity across all three brain regions were observed. Moreover, an increase in the desire for unhealthy food types (high in calories) after sleep deprivation (T = 2.21, p = 0.04) was observed.30 These recent findings are pivotal in elucidating our understanding of how sleep loss is linked to energy homeostasis and ultimately lifestyle-driven disease onset/progression.
Our study benefitted from a large sample of newly diagnosed type 2 diabetes mellitus patients with detailed information concerning medication use and objective measures of metabolic markers. The study also benefitted from consistent dietary and physical advice delivered to the intervention groups. A limitation of our study is the use of sleep diaries as opposed to more objective measures such as actigraphy. We, and others, have, however, previously demonstrated that sleep diary data has a good level of agreement with wrist actigraphy and can be an accurate estimate of sleep duration.13 Rogers et al. also demonstrated an acceptable agreement level (κ = 0.87) with high sensitivity and specificity (92.3% and 95.6%, respectively) between sleep duration reported in sleep diaries compared to 24-hour portable polysomnographic monitoring in a sleep-disordered patient population as well as healthy controls.31 However, we also acknowledge that sleep diary data is less accurate compared to actigraphy in some patient populations.32 Also, although our study did not objectively screen for sleep-disordered breathing; instead, we obtained information using self-reported physician diagnosed data. Nevertheless, it is possible that the observed associations could been confounded by obstructive sleep apnea, given that a substantial proportion of sleep apnea cases have been documented in healthy, pre-diabetes and diabetes populations.33–37 It is possible that the effects of weekday sleep debt and/or other aspects of sleep may be more powerful prior to the onset of diabetes mellitus, or even contribute to its development, but we were only able to assess the prospective effects of weekday sleep debt subsequent to diabetes diagnoses.
In conclusion, we report prospective findings in a large sample of newly diagnosed type 2 diabetes mellitus patients, demonstrating the impact of sleep debt in this population. Increasing evidence shows the importance of tackling type 2 diabetes mellitus at the earliest stage possible. Reducing excess adiposity and insulin resistance is important for achieving good diabetes outcomes. Our data suggest that weekday sleep debt is a potential factor that affects body composition and glucose homeostasis. Thus, addressing this factor may be important in metabolic improvement, and could be incorporated into future interventions aimed at tackling diabetes.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding was provided by Diabetes UK and the UK Department of Health. The funding bodies did not contribute to the design/conduct of the study; collection, management, analysis or interpretation of the data; preparation, review or approval of the manuscript or the decision to submit the manuscript for publication. Dr. Andrews has participated in speaking engagements for Novonordisk, Lilly, MSD, and Astra Zenica. Dr. Chen has participated in speaking engagements for Novonordisk and Astra Zenica. ST and TA are funded by the Biomedical Research Program (BMRP) at Weill Cornell Medicine in Qatar, supported by Qatar Foundation. ARC is supported by the National Institute for Health Research (NIHR) Bristol Nutrition Biomedical Research Unit based at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the members of the steering committee, Jonathan Levy, Susan Jebb, Edwin Gale, and Chris Riddoch. We also thank Lisa Treeby, Koba Koplatadze, Mimi Chen, Isy Douek, Nicola McLintock, and Sarah Sainsbury for their help with the study.
ABBREVIATIONS
- ACTID
ACTivity In Diabetes
- ANOVA
analysis of variance
- BMI
body mass index
- CI
confidence interval
- DM
diabetes mellitus
- HbA1c
glycated hemoglobin
- HOMA-IR
homeostatic model assessment-insulin resistance
- IR
insulin resistance
- LDL
low-density lipoprotein
- OSA
obstructive sleep apnea
- OR
odds ratio
REFERENCES
- 1.International Diabetes Federation. Diabetes Atlas 6th Edition. 2014. [Accessed February 5, 2015]. Availalbe at: http://www.idf.org/diabetesatlas/update-2014.
- 2.Arora T, Chen MZ, Omar OM, Cooper AR, Andrews RC, Taheri S. An investigation of the associations among sleep duration and quality, body mass index and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. Ther Adv Endocrinol Metab. 2016;7:3–11. doi: 10.1177/2042018815616549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaput JP, Despres JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: analyses of the Quebec Family Study. Sleep Med. 2009;10:919–24. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between short sleep duration and weight gain is dependent on disinhibited eating behavior in adults. Sleep. 2011;34:1291–7. doi: 10.5665/SLEEP.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28:1289–96. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 6.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luyster FS, Strollo PJ, Jr., Zee PC, et al. Sleep: a health imperative. Sleep. 2012;35:727–34. doi: 10.5665/sleep.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mota MC, De-Souza DA, Rossato LT, et al. Dietary patterns, metabolic markers and subjective sleep measures in resident physicians. Chronobiol Int. 2013;30:1032–41. doi: 10.3109/07420528.2013.796966. [DOI] [PubMed] [Google Scholar]
- 9.Araghi MH, Thomas GN, Taheri S. The potential impact of sleep duration on lipid biomarkers of cardiovascular disease. Future Medicine. 2012;7:443–53. [Google Scholar]
- 10.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–33. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews RC, Cooper AR, Montgomery AA, et al. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011;378:129–39. doi: 10.1016/S0140-6736(11)60442-X. [DOI] [PubMed] [Google Scholar]
- 12.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 13.Arora T, Broglia E, Pushpakumar D, Lodhi T, Taheri S. An investigation into the strength of the association and agreement levels between subjective and objective sleep duration in adolescents. PloS One. 2013;8:e72406. doi: 10.1371/journal.pone.0072406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Cooper AR, Sebire S, Montgomery AA, et al. Sedentary time, breaks in sedentary time and metabolic variables in people with newly diagnosed type 2 diabetes. Diabetologia. 2012;55:589–99. doi: 10.1007/s00125-011-2408-x. [DOI] [PubMed] [Google Scholar]
- 17.Dickerson EH, Cho LW, Maguiness SD, Killick SL, Robinson J, Atkin SL. Insulin resistance and free androgen index correlate with the outcome of controlled ovarian hyperstimulation in non-PCOS women undergoing IVF. Human Reprod. 2010;25:504–9. doi: 10.1093/humrep/dep393. [DOI] [PubMed] [Google Scholar]
- 18.Salgado AL, Carvalho L, Oliveira AC, Santos VN, Vieira JG, Parise ER. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Ggastroenterol. 2010;47:165–9. doi: 10.1590/s0004-28032010000200009. [DOI] [PubMed] [Google Scholar]
- 19.St-Onge MP, Perumean-Chaney S, Desmond R, et al. Gender Differences in the Association between Sleep Duration and Body Composition: The Cardia Study. Int J Endocrinol. 2010;2010:726071. doi: 10.1155/2010/726071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nedeltcheva AV, Kessler L, Imperial J, Penev PD. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab. 2009;94:3242–50. doi: 10.1210/jc.2009-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–9. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 23.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–26. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperry SD, Scully ID, Gramzow RH, Jorgensen RS. Sleep duration and waist circumference in adults: a meta-analysis. Sleep. 2015;38:1269–76. doi: 10.5665/sleep.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taheri S. The link between short sleep duration and obesity: we should recommend more sleep to prevent obesity. Arch Dis Child. 2006;91:881–4. doi: 10.1136/adc.2005.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36:981–90. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greer SM, Goldstein AN, Walker MP. The impact of sleep deprivation on food desire in the human brain. Nat Comm. 2013;4:2259. doi: 10.1038/ncomms3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers AE, Caruso CC, Aldrich MS. Reliability of sleep diaries for assessment of sleep/wake patterns. Nurs Res. 1993;42:368–72. [PubMed] [Google Scholar]
- 32.McCall C, McCall WV. Comparison of actigraphy with polysomnography and sleep logs in depressed insomniacs. J Sleep Res. 2012;21:122–7. doi: 10.1111/j.1365-2869.2011.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storgaard H, Mortensen B, Almdal T, Laub M, Tarnow L. At least one in three people with Type 2 diabetes mellitus referred to a diabetes centre has symptomatic obstructive sleep apnoea. Diabetic Med. 2014;31:1460–7. doi: 10.1111/dme.12477. [DOI] [PubMed] [Google Scholar]
- 35.Fredheim JM, Rollheim J, Omland T, et al. Type 2 diabetes and pre-diabetes are associated with obstructive sleep apnea in extremely obese subjects: a cross-sectional study. Cardiovasc Diabetol. 2011;10:84. doi: 10.1186/1475-2840-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–88. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pamidi S, Aronsohn RS, Tasali E. Obstructive sleep apnea: role in the risk and severity of diabetes. Best Pract Res Clin Endocrinol Metab. 2010;24:703–15. doi: 10.1016/j.beem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.