Abstract
Study Objectives:
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia characterized by REM sleep without atonia and elaborate motor activity in association with dream mentation. The melatonin receptor agonist ramelteon has been documented as being effective in two patients with secondary RBD. However, there are no reports on ramelteon treatment for idiopathic RBD.
Methods:
In an open-labeled trial, we treated 12 consecutive patients with idiopathic RBD for at least 4 w with 8 mg ramelteon given within 30 min before bedtime.
Results:
Ramelteon treatment did not have a clear effect on REM sleep without atonia or an RBD severity scale measured by video-supported polysomnography. However, clinical assessment using a visual analog scale showed a trend toward significance and there were also definitely positive changes in some individual cases. Ramelteon was well tolerated in most patients, with minor side effects.
Conclusions:
Considering that ramelteon is associated with few side effects, further study may ascertain whether patients with RBD could be effectively treated by ramelteon, especially when clonazepam may not be suitable due to its side effects.
Commentary:
A commentary on this article appears in this issue on page 643.
Citation:
Esaki Y, Kitajima T, Koike S, Fujishiro H, Iwata Y, Tsuchiya A, Hirose M, Iwata N. An open-labeled trial of ramelteon in idiopathic rapid eye movement sleep behavior disorder. J Clin Sleep Med 2016;12(5):689–693.
Keywords: REM sleep behavior disorder, ramelteon, parasomnia, RBD severity scale, REM sleep without atonia
INTRODUCTION
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia that emerges during REM sleep and may cause injury or sleep disruption. RBD is also associated with electro-myographic (EMG) abnormalities during REM sleep, including an excess of muscle tone or phasic EMG twitch activity.1,2 RBD occurs in approximately 0.5% in the general population1 and can be idiopathic or due to secondary cause, usually neurological diseases (e.g., synucleinopathies) or neuropsychiatric medications (e.g., tricyclic antidepressants, selective serotonin reuptake inhibitors, and serotonin/norepinephrine reuptake inhibitors).3,4 The treatment of RBD is symptomatic; clonazepam is the first-line treatment, but it should be used with caution in patients with dementia, daytime sleepiness, or concomitant obstructive sleep apnea (OSA).5 RBD usually emerges after the age of 50 y; therefore, the risk of falls also often prevents clonazepam from being used in elderly patients.
As an alternative treatment option, the beneficial effects of melatonin in RBD were first described in 1997 by Kunz and Bes.6 Subsequently, further literature and guidelines have suggested clinical benefits of melatonin in patients who require pharmacological treatment for RBD.3,5,7 However, in Japan, melatonin is rarely used for treatment in clinical practice because it is not available as a formally approved drug. Recently, the melatonin receptor agonist ramelteon was approved for the treatment of insomnia in Japan.8 Ramelteon effectively improved sleep measures in adults with primary chronic insomnia, with low adverse events.9,10 Some studies reported no next-day residual effect of ramelteon on behavioral or cognitive tasks.11 Furthermore, ramelteon has been documented to be effective in two patients with RBD secondary to multiple system atrophy and Parkinson disease.12 However, to the best of our knowledge, there are no reports on ramelteon treatment for idiopathic RBD. In this open-labeled study, we examined the effects of ramelteon on 12 consecutive patients with idiopathic RBD.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Clonazepam is a first-line treatment for rapid eye movement sleep behavior disorder (RBD). However, clonazepam is not always effective for treating RBD symptoms, and produces numerous side effects. Recently the melatonin receptor agonist ramelteon has been documented as being effective in two patients with secondary RBD. This study investigated the effect of ramelteon on patients with idiopathic RBD.
Study Impact: Ramelteon treatment did not have a clear effect on symptoms of idiopathic RBD. However, ramelteon had high tolerability for elderly individuals with RBD, with minor side effects, and there were some individual cases in which RBD symptoms were improved after ramelteon treatment. Ramelteon might be useful for patients with RBD who are unable to take clonazepam or are resistant to clonazepam treatment.
METHODS
Subjects and Clinical Assessment
The study protocol was approved by the ethics committee of Toyohashi Mates Sleep Disorders Center. All participants provided written informed consent. All patients were seeking treatment for neuropsychiatric sleep related disturbances. Video-supported polysomnography (v-PSG) was performed in cases meeting the American Academy of Sleep Medicine (AASM) indications for diagnostic polysomnography,1 and patients were included in the study if the International Classification for Sleep Disorders, Second Edition, criteria for RBD were met; including even very mild cases. If patients had OSA, they were treated with nasal continuous positive airway pressure therapy or an intraoral device. The study, therefore, included these treated cases. Exclusion criteria were (1) comorbid psychiatric disorders (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,) or neurological disorders, including Parkinson disease and dementia with Lewy bodies, and (2) the intake of any medication that might interfere with melatonin production/secretion or REM sleep (e.g., benzodiazepines, antidepressants, beta-blockers, and anti-inflammatory drugs).6,13 After the diagnosis was made by v-PSG, patients were treated with 8 mg ramelteon for at least 4 w. Patients were instructed to take ramelteon within 30 min before bedtime. We used a visual analog scale (VAS)14 for assessment of the daily symptoms with regard to RBD frequency and severity at baseline and following treatment; the VAS rating was provided by a bed partner or family member who had observed the patient's RBD symptoms (such as dream enactment behaviors, falls, and injuries) during sleep over the previous month. As a more objective measurement, we checked the severity of RBD using the RBD severity scale (RBDSS) by v-PSG.15 RBDSS classified the severity as to both of motor events and vocalizations. Motor events in REM sleep were rated on a digital scale from 0 to 3 according to the localization and severity of movements. No visible movement but registration of REM sleep without atonia scored as 0; slight movements restricted to the distal extremities, jerks, or facial movements scored as 1; movements involving the proximal extremities, with complex and/or violent behaviors scored as 2; and any axial involvement with a possibility of falling out of bed or observed bed falls scored as 3. Vocalizations were rated as absent (a score of “0”) or present (a score of “1”) for any sound other than respiratory noises generated during REM sleep. Motor and vocalization scores were separated by a full stop. The final RBD severity score was defined by the highest scoring given during second-by-second video review of all REM epochs during the v-PSG night. The RBDSS rating assessments, thus, included the REM epochs that contained apneas.
Sleep Analysis
v-PSG involved six electroencephalography leads (F4-A1, F3-A2, C4-A1, C3-A2, O2-A1, O1-A2), electrooculogram, EMG, submentalis and bilateral anterior tibialis, snore sensor, finger pulse oximeter, nasal/oral airflow monitors, and thoracoabdominal respiratory effort sensors. Sleep, periodic leg movements, and apneic events were scored in 30-sec epochs according to AASM criteria.16 REM sleep without atonia (RWA) and phasic REM sleep muscle activity were also assessed using the AASM criteria,16 including the REM epochs that contained apneas. The v-PSG and quantitative RWA scorings were, as a result, made by impartial raters blinded to the dates of acquisition.
Statistical Analysis
Our primary outcome measures with respect to efficacy were as follows: (1) percentage of RWA per whole REM sleep epochs by v-PSG, (2) family ratings of frequency and severity of dream enactment behavior with VAS, and (3) RBDSS by v-PSG. Data were analyzed for statistical significance using the Wilcoxon signed-rank test (IBM SPSS statistics version 21, IBM Japan, Tokyo, Japan). The significance level was set at p < 0.05.
RESULTS
We enrolled 12 patients with idiopathic RBD (eight males and four females, mean age 70.9 ± 8.7 y, range 52–81 y). The mean duration of the treatment before the second v-PSG was 8.3 ± 6.8 w. Two patients dropped out of the study because of drug rash or dizziness occurring during ramelteon treatment. Our results are summarized in Table 1 and Table S1, supplemental material.
Table 1.
Demographics and results of 12 patients with rapid eye movement sleep behavior disorder undergoing ramelteon treatment.
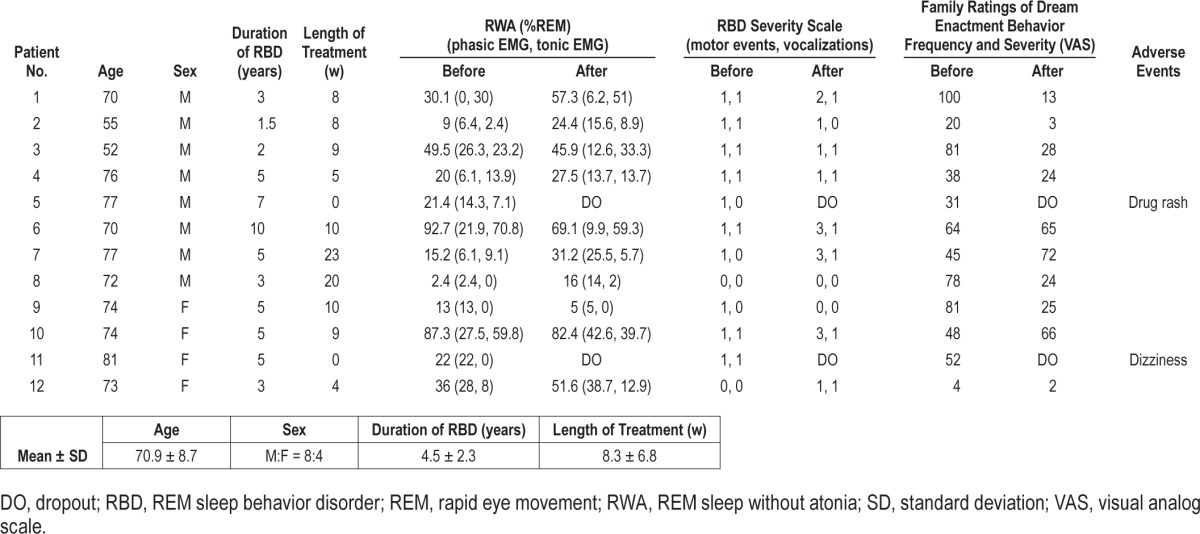
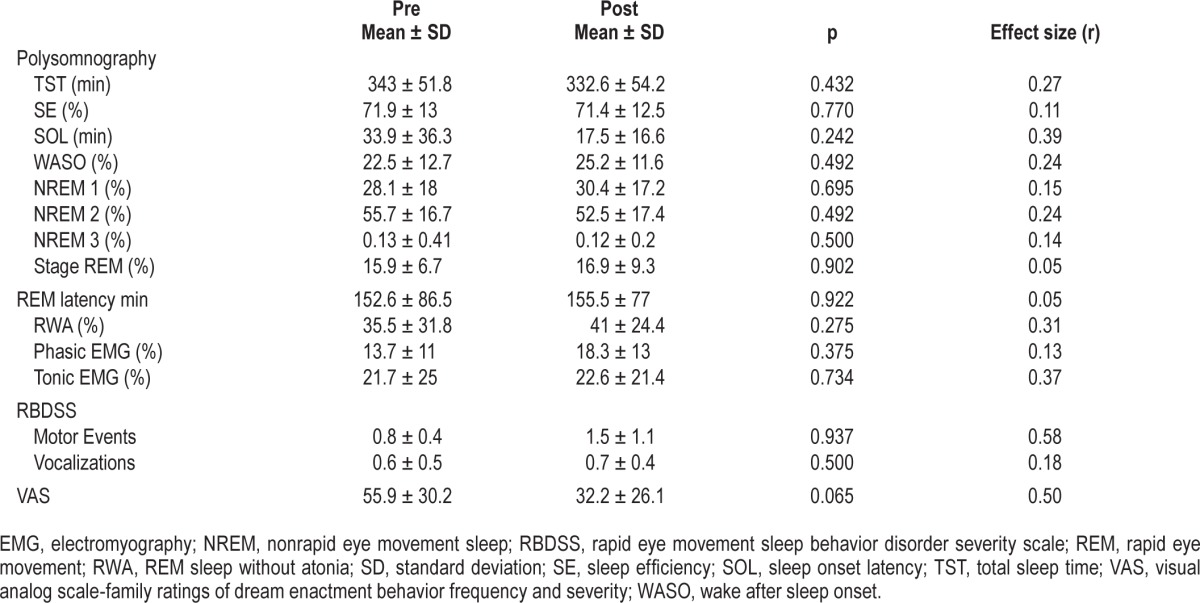
Regarding v-PSG measures, no significant difference in the total sleep time, sleep efficiency, sleep onset latency, wake after sleep onset, or sleep stage variables, including the amount of RWA, was found between those assessed before and after treatment with ramelteon (Table 2). We did not observe significant changes in RBDSS, although the VAS score showed a trend toward significance (Table 2). There were also notable changes in individual cases, where 2 of the 10 patients showed a reduction in the RWA percentage of more than 5%, and 6 showed an increase of more than 5%. In 2 of 10 patients, RBDSS score (motor events or vocalizations) decreased; in 3, it remained unchanged; and in 5, it increased. Seven of 10 patients exhibited a decrease in VAS, whereas this score increased in 3 patients.
Table 2.
Statistical analyses of polysomnographic rapid eye movement sleep behavior disorder severity scale and visual analog scale data of patients with rapid eye movement sleep behavior disorder before and after ramelteon treatment.
DISCUSSION
As a melatonin receptor agonist, ramelteon was approved in the United States and Japan as a treatment for insomnia. Melatonin (MT) 1 and MT2 receptors have been detected in the suprachiasmatic nucleus of the hypothalamus, and are believed to be involved in the regulation of the circadian rhythm of the sleep–wake cycle.17 Ramelteon has a high selectivity for the MT1/MT2 receptors, rather than acting via the gamma-aminobutyric acid A–benzodiazepine receptor complexes located throughout the brain.18 Ramelteon was found to be effective in two patients with RBD secondary to multiple system atrophy and Parkinson disease.12 In the report, patients took 8 mg ramelteon prior to bedtime and demonstrated clinical RBD improvement and a decrease in RWA. In addition, another case series of three patients found that agomelatine, an MT1 and MT2 receptor agonist with 5-hydroxytrypamine 2C receptor antagonist activity, reduced the frequency and severity of RBD episodes over the 6-mo follow-up period.19 Moreover, treatment with exogenous melatonin for RBD was found to yield a statistically significant decrease in RWA in one randomized, placebo-controlled trial and two open-label trials.6,20,21 From the aforementioned evidence, we thought that the melatonin receptor agonist might be effective for RBD.
Our results over the whole case series failed to show that ramelteon resulted in a statistically significant improvement in RBD symptoms, although the improvement in the daily symptoms with regard to RBD frequency and severity, as assessed by VAS, showed a trend toward significance. A larger sample size may have, therefore, revealed a significant difference. Unfortunately, we could not calculate a priori for the required sample size, as we were unable to obtain sufficient appropriate data for this calculation from previous reports concerning VAS measurements for RBD treatment responses.
Interestingly, six patients showed a marked increase in RWA, but four of these nevertheless showed an improvement on VAS. Hence, ramelteon may improve RBD symptoms, as well as increase RWA. The mechanism for the effect of ramelteon on RBD is, however, unclear. Possible explanations have been proposed for the effect of melatonin on RBD, such as direct inhibition of RWA, modulation of REM sleep, circa-dian resetting, and sleep stabilization.22 Ramelteon could be expected to have similar effects; but previous reports of melatonin treatment on RBD showed RWA to be reduced, so these hypotheses cannot be directly applied to our results. We did not analyze the change in other variables of REM sleep (such as stage shift from REM sleep, movement time in REM sleep, or REM density); these may have, instead, brought further insights. Another hypothesis, that ramelteon might have an effect on emotion processing and thereby affect the enactment of dream mentation without direct effect on RWA, could explain the dissociation between the changes of RBD symptoms and RWA. It is well accepted that emotional stress can worsen RBD symptoms. The MT1 receptor has also been reported to be involved in the mesolimbic dopamine system,23 suggesting a melatonergic influence on emotion. Unfortunately, we could not make use of data regarding patients' dream contents before and after ramelteon treatment, so we cannot discuss this further. Taking into account the hypothesis, the effect of ramelteon on RBD may be mild and unable to reduce severe RBD symptoms; this could also explain why the two patients who showed higher RWA (92.7% and 87.3%) did not show a reduction in RBD symptoms. Other possible explanations for the increased RWA after treatment in the six cases could be that it was due to the natural progress of RBD over months,24 or to simple variation. Further studies are needed to clarify these mechanisms.
This study has some limitations. First, this study was not a randomized controlled investigation. Second, the participating subjects were enrolled from one institute, and the number of study subjects was small. Third, the evaluation of RBDSS differed in 60% of the patients between nights 1 and 2 in the original report.15 In the study, 40% of patients showed violent behavior, but only on one night15; therefore, RBDSS may be considered as only a weak measurement method for treatment response. Fourth, the previous case report described the improvement of RBD with ramelteon in two patients with secondary RBD12; however, our subjects were all idiopathic cases. Thus, it remains possible that ramelteon treatment might only improve secondary RBD. Fifth, the duration of treatment before measuring its outcomes varied widely between the subjects, ranging from 4 to 23 w. This is because we gave priority to the convenience of the patients undergoing PSG; this variation may have resulted in a greater variability of the outcomes.
In conclusion, this small, open trial showed a trend toward significant reduction in bedpartner/family rated dream enactment frequency-severity in RBD, and there were also definitely positive changes in some individual cases. Ramelteon had high tolerability for elderly individuals with RBD, with minor side effects. Based on our results, a prospective, blinded, randomized controlled trial could be undertaken to determine whether ramelteon is effective and safe for the treatment of RBD. A preliminary sample size calculation suggests that approximately 14 subjects may be sufficient to show a significant effect of ramelteon treatment. Considering that ramelteon has few side effects, more studies may also provide valuable information as to whether some specific subpopulation of patients with RBD could be more effectively treated by ramelteon, especially when clonazepam may not be suitable due to its side effects.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Iwata has received research support from Eli Lilly and Daiichi-Sankyo and has consulted for Janssen and Mebix. Dr. Kitajima has received research support from Takeda and MSD and has participated in speaking engagements for Mitsubishi Tanabe Pharma Corp., MSD, Yoshitomiyakuhin Corp., Fukuda Life Tech Chubu KK, Takeda, Eizai, and Dainippon Suimitomo Pharma. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to the patients who participated in our study, and thank
MARUZEN Editing Light service (http://kw.maruzen.co.jp/kousei-honyaku/) for the English language review.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- EMG
electromyography
- MT
melatonin
- OSA
obstructive sleep apnea
- RBD
REM sleep behavior disorder
- RBDSS
RBD severity scale
- REM
rapid eye movement
- RWA
REM sleep without atonia
- v-PSG
video-supported polysomnography
- VAS
visual analog scale
REFERENCES
- 1.American Academy of Sleep Medicine. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. International classification of sleep disorders: diagnostic and coding manual. [Google Scholar]
- 2.Schenck CH, Mahowald MW. REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP. Sleep. 2002;25:120–38. doi: 10.1093/sleep/25.2.120. [DOI] [PubMed] [Google Scholar]
- 3.Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann N Y Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trotti LM. REM sleep behaviour disorder in older individuals: epidemiology, pathophysiology and management. Drugs Aging. 2010;27:457–70. doi: 10.2165/11536260-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aurora RN, Zak RS, Maganti RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD) J Clin Sleep Med. 2010;6:85–95. [PMC free article] [PubMed] [Google Scholar]
- 6.Kunz D, Bes F. Melatonin as a therapy in REM sleep behavior disorder patients: an open-labeled pilot study on the possible influence of melatonin on REM-sleep regulation. Mov Disord. 1999;14:507–11. doi: 10.1002/1531-8257(199905)14:3<507::aid-mds1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Boeve BF, Silber MH, Ferman TJ. Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med. 2003;4:281–4. doi: 10.1016/s1389-9457(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 8.Uchiyama M, Hamamura M, Kuwano T, Nishiyama H, Nagata H, Uchimura N. Evaluation of subjective efficacy and safety of ramelteon in Japanese subjects with chronic insomnia. Sleep Med. 2011;12:119–26. doi: 10.1016/j.sleep.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of Ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Richardson GS, Zammit G, Wang-Weigand S, Zhang J. Safety and subjective sleep effects of ramelteon administration in adults and older adults with chronic primary insomnia: a 1-year, open-label study. J Clin Psychiatry. 2009;70:467–76. doi: 10.4088/jcp.07m03834. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MW, Suess PE, Griffiths RR. Ramelteon: a novel hypnotic lacking abuse liability and sedative adverse effects. Arch Gen Psychiatry. 2006;63:1149–57. doi: 10.1001/archpsyc.63.10.1149. [DOI] [PubMed] [Google Scholar]
- 12.Nomura T, Kawase S, Watanabe Y, Nakashima K. Use of ramelteon for the treatment of secondary REM sleep behavior disorder. Intern Med. 2013;52:2123–6. doi: 10.2169/internalmedicine.52.9179. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon JF, Postuma RB, Montplaisir J. Update on the pharmacology of REM sleep behavior disorder. Neurology. 2006;67:742–7. doi: 10.1212/01.wnl.0000233926.47469.73. [DOI] [PubMed] [Google Scholar]
- 14.McCarter SJ, Boswell CL, St Louis EK, et al. Treatment outcomes in REM sleep behavior disorder. Sleep Med. 2013;14:237–42. doi: 10.1016/j.sleep.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sixel-Doring F, Schweitzer M, Mollenhauer B, Trenkwalder C. Intraindividual variability of REM sleep behavior disorder in Parkinson's disease: a comparative assessment using a new REM sleep behavior disorder severity scale (RBDSS) for clinical routine. J Clin Sleep Med. 2011;7:75–80. [PMC free article] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson AL, et al. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. [Google Scholar]
- 17.Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI. Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J. 1998;12:1211–20. doi: 10.1096/fasebj.12.12.1211. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology. 2005;48:301–10. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Bonakis A, Economou NT, Papageorgiou SG, Vagiakis E, Nanas S, Paparrigopoulos T. Agomelatine may improve REM sleep behavior disorder symptoms. J Clin Psychopharmacol. 2012;32:732–4. doi: 10.1097/JCP.0b013e31826866f8. [DOI] [PubMed] [Google Scholar]
- 20.Kunz D, Mahlberg R. A two-part, double-blind, placebo-controlled trial of exogenous melatonin in REM sleep behaviour disorder. J Sleep Res. 2010;19:591–6. doi: 10.1111/j.1365-2869.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi N, Uchimura N, Hashizume Y, et al. Melatonin therapy for REM sleep behavior disorder. Psychiatry Clin Neurosci. 2001;55:267–9. doi: 10.1046/j.1440-1819.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 22.McGrane IR, Leung JG, St Louis EK, Boeve BF. Melatonin therapy for REM sleep behavior disorder: a critical review of evidence. Sleep Med. 2015;16:19–26. doi: 10.1016/j.sleep.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog Neurobiol. 2008;85:335–53. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Stefani A, Gabelia D, Hogl B, et al. Long-term follow-up investigation of isolated rapid eye movement sleep without atonia without rapid eye movement sleep behavior disorder: a pilot study. J Clin Sleep Med. 2015;11:1273–9. doi: 10.5664/jcsm.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


