Abstract
An update of the 2012 systematic review and meta-analyses were performed and a modified-GRADE approach was used to update the recommendation for the use of adaptive servo-ventilation (ASV) for the treatment of central sleep apnea syndrome (CSAS) related to congestive heart failure (CHF). Meta-analyses demonstrated an improvement in LVEF and a normalization of AHI in all patients. Analyses also demonstrated an increased risk of cardiac mortality in patients with an LVEF of ≤ 45% and moderate or severe CSA predominant sleep-disordered breathing. These data support a Standard level recommendation against the use of ASV to treat CHF-associated CSAS in patients with an LVEF of ≤ 45% and moderate or severe CSAS, and an Option level recommendation for the use of ASV in the treatment CHF-associated CSAS in patients with an LVEF > 45% or mild CHF-related CSAS. The application of these recommendations is limited to the target patient populations; the ultimate judgment regarding propriety of any specific care must be made by the clinician.
Citation:
Aurora RN, Bista SR, Casey KR, Chowdhuri S, Kristo DA, Mallea JM, Ramar K, Rowley JA, Zak RS, Heald JL. Updated adaptive servo-ventilation recommendations for the 2012 AASM guideline: “The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence-Based Literature Review and Meta-Analyses”. J Clin Sleep Med 2016;12(5):757–761.
Keywords: central sleep apnea, adaptive servo-ventilation, clinical practice guideline
INTRODUCTION
The most recent practice parameter paper by the American Academy of Sleep Medicine (AASM) on the treatment of central sleep apnea syndrome (CSAS) in adults was published in 2012.1 Since the publication of the current practice parameters, the scientific literature on adaptive servo-ventilation (ASV) for the treatment of CSAS has grown considerably. In particular, recent evidence from the SERVE-HF trial demonstrated an increase in cardiovascular mortality in heart failure patients with a reduced ejection fraction using ASV compared to a control group.2 These findings resulted in the device manufacturer (ResMed) issuing a Field Safety Notice in 2015 stating that ASV therapy is contraindicated in this specific patient population.3
Due to this new evidence, the AASM recommissioned the same physician Task Force members who formulated the 2012 practice parameter paper to update the specific recommendations pertaining to the use of ASV to treat CSAS associated with congestive heart failure, i.e. 4.2.3a Adaptive Servo-Ventilation (ASV) targeted to normalize the apnea-hypopnea index (AHI) is indicated for the treatment of CSAS related to CHF (STANDARD).
BACKGROUND
Adaptive servo-ventilation (ASV) is a form of bilevel positive airway pressure (BPAP) therapy that is increasingly used to treat sleep-related breathing disorders, particularly central sleep apnea (CSA). Similar to BPAP and continuous positive airway pressure (CPAP), ASV provides expiratory positive airway pressure (EPAP) that can be adjusted to control obstructive events. However, ASV therapy differs from CPAP or BPAP by providing dynamic (i.e. breath-by-breath) adjustment of inspiratory pressure support (IPS) and utilizing an auto-backup rate to normalize breathing rate relative to a predetermined target. Two manufacturers currently offer ASV devices in North America: ResMed and Phillips Respironics.
The ResMed ASV (AirCurve 10 ASV, S9 VPAP Adapt, VPAP SV, VPAP Adapt, or VPAP Adapt Enhanced) uses a three minute moving average to monitor and determine an appropriate target minute ventilation, set to 90% of their most recent minute ventilation. This target threshold prevents under and over ventilation by dynamically increasing (for hypopneas) or decreasing (for hyperpneas) inspiratory pressure support (IPS) as needed. Together with a back-up respiratory rate (set dynamically at 15 breaths/min), when a patient's minute ventilation falls below the set target, ResMed's ASV automatically adjusts the inspiratory pressure support to provide the ventilation needed. As breathing stabilizes, the pressure delivered is rapidly reduced back towards the minimum required. ResMed's newest ASV device, AirCurve 10 ASV, has a maximum pressure of 25 cmH2O and can be set to an IPS of 0 to 20 cmH2O.
The Philips Respironics ASV (BiPAP autoSV Advanced System One) utilizes inspiratory flow as the primary variable to identify and respond to sleep-related breathing disorders (SRBD). In the absence of SRBD, EPAP is automatically determined based on the REMstar Auto algorithm. The algorithm identifies and responds to obstructive sleep disordered breathing events as they occur. During periods of airway stability, the algorithm will proactively assess the airway to minimize pressure while optimizing airway patency. The maximum inspiratory positive airway pressure is 30 cmH2O with a minimum EPAP of 4 cmH2O. The Philips Respironics ASV devices are also capable of withdrawing IPS entirely during periods of normal breathing. All Respironics ASV devices have two methods of setting a backup rate: a fixed rate determined by the operator, or an auto mode that synchronize with the patient's intrinsic rate.
METHODOLOGY
Expert Task Force
In order to develop this recommendation update, the AASM re-commissioned the authors of the 2012 Practice Parameters paper and the AASM Science and Research Department staff members. Prior to appointment, the authors were required to disclose all potential conflicts of interest (COI) according to the AASM's policy. All relevant conflicts of interest are listed in the Disclosures section.
PICO Question
An amended PICO question 2 was used in order to update this recommendation:
“ Does adaptive servo-ventilation improve clinical (transplant-free survival) or surrogate (left ventricular ejection fraction or apnea-hypopnea index) outcomes in patients with CSAS and congestive heart failure”
Literature Searches, Evidence Review and Data Extraction
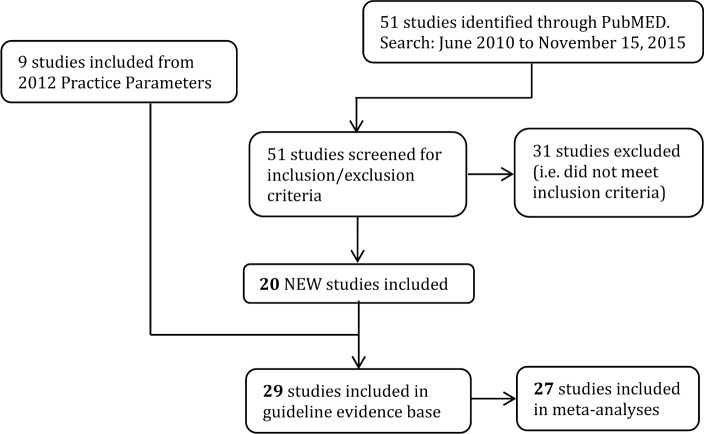
A literature search was performed on November 15, 2015 by the AASM research staff using the PubMed database (see Figure 1), using the following criteria:
(adaptive servo ventilation OR ASV) AND
sleep apnea syndromes AND
articles published from June 2010–present AND
limits of adults 19+, English, and humans
This resulted in 51 publications. Full keywords and MeSH terms for the literature search can be found in the appendix (see supplemental material, “Literature Search String”). Abstracts from all retrieved articles, including “pearled” publications, were individually assessed by two task force (TF) members to deter mine whether the publication should be included for further consideration in the project. Inclusion criteria were:
-
A minimum of 5 patients plus clinical outcomes measures of mortality/transplant-free survival, left ventricular ejection fraction (LVEF), or apneahypopnea index (AHI).
AND
-
Sleep-disordered breathing is clearly differentiated between CSA and OSA.
AND
The central sleep apnea index is greater than the obstructive sleep apnea index OR the percentage of central events is greater than 50% of respiratory events.
Twenty new studies were approved for inclusion. Of the studies used for the 2012 practice parameters, 9 were included, for a total of 29 studies included in the new evidence base. Twenty-seven were used for the meta-analysis. Full texts of accepted articles were inspected closely; data pertaining to the outcomes of interest were extracted into spreadsheets by AASM staff. If outcome data were not presented in the format necessary for statistical analysis (i.e. mean, standard deviation, and sample size), the paper was discussed but not used in the meta-analyses.
Figure 1. Evidence base flow diagram.
Statistical and Meta-Analysis
For the outcomes of interest, data from baseline and last treatment time points were used for all statistical and meta-analyses. For adverse events, all data presented in the included papers were used for statistical and meta-analysis. All calculations and meta-analyses were performed using Review Manager 5.3 software. Whenever possible, meta-analyses were performed by pooling data across studies for each outcome and adverse event. The evidence was grouped for analysis based on the clinical outcome of interest and LVEF inclusion criteria (≤ 45% and > 45%).
Meta-analyses for continuous outcomes were performed as pre-post analyses using the random effects model, while relative risk was used for dichotomous outcomes. For most interventions, absolute effects of treatments are represented by the mean difference (MD) ± standard deviation (SD) of post-treatment vs post-placebo. The result of each meta-analysis is displayed as a forest plot. Pooled results for continuous outcomes are expressed as the total number of patients, mean difference (MD) and 95% confidence interval (CI) between the experimental treatment and placebo. Relative risk is presented as baseline risk of the control group (events per thousand) and comparative risk of the intervention (events per thousand).
Strength of Recommendations
The assessment of evidence quality was performed by AASM staff and the task force. The task force followed the GRADE process that was used in the 2012 Practice Parameters, with slight modifications to the initial quality rating based on recent publications from the GRADE working group.4 The results are reported in Table S1 in the supplemental material.
Briefly, risk of bias includes aspects of study design (randomized control trials [RCTs] versus non-randomized controlled trials or before-after trials) and conduct such as blinding, allocation concealment, large loss to follow up, or selective outcome reporting. Imprecision refers to wide confidence intervals around the estimate of effect when there are relatively few patients and few events. Indirectness occurs when the question being addressed is different than the available evidence regarding population, intervention, comparator, or outcome. There is inconsistency when there is unexplained heterogeneity of the results. Reporting bias can occur if there is selective reporting of studies or outcomes, which may occur if the published evidence is limited to a small number of trials funded by a for-profit organization.
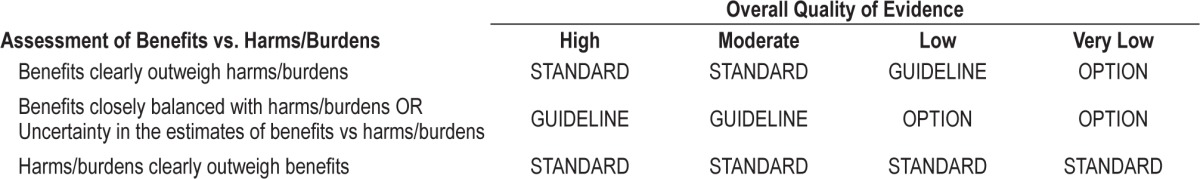
To determine the strength of the recommendation, the TF assessed the quality of evidence, balance of beneficial and harmful effects to determine the strength of a recommendation, according to Table 1.
Table 1.
AASM strengths of recommendations.
Approval and Interpretation of Recommendations
The final guideline was submitted to the AASM Board of Directors who approved these recommendations.
The recommendations in this guideline define principles of practice that should complement the 2012 Practice Parameters to meet the needs of most patients in most situations. This guideline should not, however, be considered inclusive of all proper methods of care or exclusive of other methods of care reasonably used to obtain the same results. The ultimate judgment regarding propriety of any specific care must be made by the clinician, in light of the individual circumstances presented by the patient, available diagnostic tools, accessible treatment options, and resources.
The AASM expects this guideline to have an impact on professional behavior, patient outcomes, and, possibly, health care costs. This clinical practice guideline reflects the state of knowledge at the time of publication and will be reviewed and updated as new information becomes available.
RECOMMENDATIONS
Adaptive Servo-Ventilation for the Treatment of Central Sleep Apnea Syndrome Related to Congestive Heart Failure
Recommendation 1: Adaptive servo-ventilation (ASV) targeted to normalize the apnea-hypopnea index (AHI) should not be used for the treatment of CSAS related to CHF in adults with an ejection fraction ≤ 45% and moderate or severe CSA predominant, sleep-disordered breathing. (STANDARD AGAINST)
Remarks: The recommendation against using ASV is based on evidence for increased risk of death in CHF patients with LVEF ≤ 45%.
Recommendation 2: Adaptive servo-ventilation (ASV) targeted to normalize the apnea-hypopnea index (AHI) can be used for the treatment of CSAS related to CHF in adults with an ejection fraction > 45% or mild CHF-related CSAS. (OPTION)
Summary
The overall quality of evidence for ASV is low due to mixed study designs (i.e. a combination of randomized controlled trials and observational studies). The quality of evidence for LVEF and AHI are low due to a combination of study methodologies, which differs from the previous assessment of quality. The quality of evidence for mortality (cardiac death) is high. Meta-analyses indicate that ASV improves LVEF by 5.49% (95% CI: 4.16% to 6.82%) and decreases the AHI by 30 events/h (95% CI: −27 to −34 events/h) over baseline. Data from Cowie et. al.2 demonstrate a relative risk of cardiac death of 1.25 (95% CI: 1.02 to 1.53) in CHF patients with an LVEF ≤ 45% receiving ASV compared to standard care. (see Table S1)
Discussion
One study5 compared therapeutic to sub-therapeutic ASV, 10 studies2,6–14 compared ASV treatment to standard care, 7 studies15–21 compared ASV treatment to baseline, 4 studies22–25 compared ASV to CPAP, 3 studies26–28 compared it to BPAP-ST, 1 study29 compared ASV to either CPAP or BPAP (these 2 treatment results were combined), and 3 study compared ASV to oxygen.30–32
LEFT VENTRICULAR EJECTION FRACTION: Nineteen studies5,7–15,18,19,22–24,27,29,32 reported on the effects of ASV on LVEF. The meta-analysis of the change in LVEF with treatment versus baseline is shown in Figure S1 in the supplemental material. The data show that ASV improves LVEF by 5.49% (95% CI: 4.16 to 6.82%). Five longer-term (3–6 months) studies7,11,12,18,19 showed a statistically significant increase in LVEF with ASV. A fourth study by Randerath et al. showed no significant change in LVEF with ASV.23 Finally, a study by Bitter et al. could not be included in the meta-analysis because no standard deviations were provided, but demonstrated a small increase in LVEF with ASV.6 (see Figure S1)
APNEA-HYPOPNEA INDEX: Twenty-six studies5,7–27,29–32 reported data on the effects of ASV on AHI and were consistent in showing that ASV improves AHI over baseline. Meta-analyses indicate that ASV decreases AHI by 30 events/h (95% CI: −27 to −34 events/h) over baseline. Furthermore, 12 of the studies7,8,16,18,22,24–26,28–31 showed a normalization of AHI to 5 or less. Finally, studies by Bitter et al., Cowie et al. and Morgenthaler et al. could not be included in the meta-analysis because they did not provide standard deviation, but demonstrated a normalization of AHI with ASV.2,6,28 (see supplemental material, Figure S2)
MORTALITY (CARDIAC DEATH): Four studies2,12–14 reported data on mortality in CHF patients using ASV compared to standard care. Cowie et al.2 examined all-cause mortality as a primary end point and cardiovascular death as a secondary end point, following 1,325 patients with a LVEF of ≤ 45% (mean 32% ± 8%) an average of 31 months. The remaining three studies12–14 captured cardiac death during follow-up of 176 patients with an average LVEF ranging from 34% to 56% for an average of 12–18 months.
Cowie et al.2 reported a significantly higher incidence of cardiac death in ASV patients compared to patients receiving standard care (25% vs 20%, respectively; p = 0.03). The remaining three studies reported significantly lower incidence of cardiac death in ASV patients compared to patients receiving standard care (0–4% vs 11–25%, respectively; p = 0.01 to < 0.01).12–14 However due to the heterogeneity of the study results, the small study populations and shorter follow-up periods of these three studies, the task force relied exclusively on the data from Cowie et al. for the recommendations. (see supplemental material, Figures S3A and S3B)
VALUES AND TRADEOFFS: Although there is only one study demonstrating a small but statistically significant increase in mortality with ASV use in CHF patients with an EF ≤ 45% and moderate or severe CSA, the strength of the evidence is high given the study design, duration of follow-up, and sample size. Thus, at this time, ASV therapy should not be prescribed to heart failure patients with moderate or severe CSA predominant, sleep-disordered breathing (SDB), and an ejection fraction ≤ 45%. However, the results from this singular study cannot be generalized to other types of heart failure, i.e. those with preserved ejection fraction (EF > 45%), mild sleep-disordered breathing, or those with obstructive sleep apnea (OSA)-predominant SDB. It is also recommended that until further data are available, other ASV devices not be prescribed for the subgroup of heart failure patients with an ejection fraction ≤ 45% and moderate or severe central sleep apnea.
DISCUSSION AND FUTURE DIRECTIONS
Undoubtedly, there have been significant strides made over recent years to enhance our understanding of CHF-associated CSAS and its treatment. However, critical gaps remain in the literature. The current update highlights that, with the exception of the recent SERVE-HF, there continues to be a paucity of high quality research, and especially clinical trials. Thus, there are several potential investigative avenues to pursue.
For example, given that the SERVE-HF trial focused on CHF patients with a reduced ejection fraction of < 45%, the results cannot be extrapolated to patients with CHF and preserved ejection fraction. Thus, studies that examine the sizable patient population with CHF, CSAS, and an ejection fraction ≥ 45% should be considered. There is likely to be considerable debate regarding the details of study design and the implications of the SERVE-HF trial, including the possibility of post-hoc data analysis (since the study used an intent-to-treat design) which could influence these recommendations in the future.33 Furthermore, manufacturer-based differences in algorithms between ASV devices limit generalizability. Different ASV devices need to be tested in clinical trials to ensure that inferences regarding the use of ASV devices in CHF-associated CSAS from current, available data are in fact appropriate. Additionally, outcomes with ASV use in those with CHF and mild CSAS need to be explored. Overall, the data on the use of ASV (and other positive airway pressure devices) in CHF-associated CSAS continues to improve. However, investigative efforts need to be expanded both in terms of the quality and quantity of studies.
DISCLOSURE STATEMENT
This was not an industry supported study. Drs. Kristo and Ramar serve on the American Academy of Sleep Medicine's Board of Directors. Mr. Heald is employed by the American Academy of Sleep Medicine. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The task force would like to thank and acknowledge the contributions of Carin I. Lamm, MD, who served as an author on the 2012 Practice Parameters.
REFERENCES
- 1.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35:17–40. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373:1095–105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ResMed. ResMed Provides Update on Phase IV SERVE-HF Study of Adaptive Servo-Ventilation (ASV) Therapy In Central Sleep Apnea and Chronic Heart Failure. 2015. [Accessed March 2016]. Available from: http://www.resmed.com/us/en/consumer/newsandinformation/news-releases/2015/resmed-presents-data-on-phase-iv-serve-hf-study.html.html.
- 4.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Pepperell JC, Maskell NA, Jones DR, et al. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med. 2003;168:1109–14. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 6.Bitter T, Gutleben KJ, Nolker G, et al. Treatment of Cheyne-Stokes respiration reduces arrhythmic events in chronic heart failure. J Cardiovasc Electrophysiol. 2013;24:1132–40. doi: 10.1111/jce.12197. [DOI] [PubMed] [Google Scholar]
- 7.Joho S, Oda Y, Ushijima R, Hirai T, Inoue H. Effect of adaptive servoventilation on muscle sympathetic nerve activity in patients with chronic heart failure and central sleep apnea. J Card Fail. 2012;18:769–75. doi: 10.1016/j.cardfail.2012.08.360. [DOI] [PubMed] [Google Scholar]
- 8.Koyama T, Watanabe H, Kobukai Y, et al. Beneficial effects of adaptive servo ventilation in patients with chronic heart failure. Circ J. 2010;74:2118–24. doi: 10.1253/circj.cj-10-0082. [DOI] [PubMed] [Google Scholar]
- 9.Koyama T, Watanabe H, Tamura Y, Oguma Y, Kosaka T, Ito H. Adaptive servo-ventilation therapy improves cardiac sympathetic nerve activity in patients with heart failure. Eur J Heart Fail. 2013;15:902–9. doi: 10.1093/eurjhf/hft049. [DOI] [PubMed] [Google Scholar]
- 10.Miyata M, Yoshihisa A, Suzuki S, et al. Adaptive servo ventilation improves Cheyne-Stokes respiration, cardiac function, and prognosis in chronic heart failure patients with cardiac resynchronization therapy. J Cardiol. 2012;60:222–7. doi: 10.1016/j.jjcc.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Oldenburg O, Bitter T, Lehmann R, et al. Adaptive servoventilation improves cardiac function and respiratory stability. Clin Res Cardiol. 2011;100:107–15. doi: 10.1007/s00392-010-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owada T, Yoshihisa A, Yamauchi H, et al. Adaptive servoventilation improves cardiorenal function and prognosis in heart failure patients with chronic kidney disease and sleep-disordered breathing. J Card Fail. 2013;19:225–32. doi: 10.1016/j.cardfail.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Yoshihisa A, Shimizu T, Owada T, et al. Adaptive servo ventilation improves cardiac dysfunction and prognosis in chronic heart failure patients with Cheyne-Stokes respiration. Int Heart J. 2011;52:218–23. doi: 10.1536/ihj.52.218. [DOI] [PubMed] [Google Scholar]
- 14.Yoshihisa A, Suzuki S, Yamaki T, et al. Impact of adaptive servo-ventilation on cardiovascular function and prognosis in heart failure patients with preserved left ventricular ejection fraction and sleep-disordered breathing. Eur J Heart Fail. 2013;15:543–50. doi: 10.1093/eurjhf/hfs197. [DOI] [PubMed] [Google Scholar]
- 15.Carnevale C, Georges M, Rabec C, Tamisier R, Levy P, Pepin JL. Effectiveness of Adaptive Servo Ventilation in the treatment of hypocapnic central sleep apnea of various etiologies. Sleep Med. 2011;12:952–8. doi: 10.1016/j.sleep.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Kourouklis SP, Vagiakis E, Paraskevaidis IA, et al. Effective sleep apnoea treatment improves cardiac function in patients with chronic heart failure. Int J Cardiol. 2013;168:157–62. doi: 10.1016/j.ijcard.2012.09.101. [DOI] [PubMed] [Google Scholar]
- 17.Oldenburg O, Bitter T, Wellmann B, et al. Trilevel adaptive servoventilation for the treatment of central and mixed sleep apnea in chronic heart failure patients. Sleep Med. 2013;14:422–7. doi: 10.1016/j.sleep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg O, Schmidt A, Lamp B, et al. Adaptive servoventilation improves cardiac function in patients with chronic heart failure and Cheyne-Stokes respiration. Eur J Heart Fail. 2008;10:581–6. doi: 10.1016/j.ejheart.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Yoshihisa A, Miyata M, et al. Adaptive servo-ventilation therapy improves long-term prognosis in heart failure patients with anemia and sleep-disordered breathing. Int Heart J. 2014;55:342–9. doi: 10.1536/ihj.13-354. [DOI] [PubMed] [Google Scholar]
- 20.Szollosi I, O'Driscoll DM, Dayer MJ, Coats AJ, Morrell MJ, Simonds AK. Adaptive servo-ventilation and deadspace: effects on central sleep apnoea. J Sleep Res. 2006;15:199–205. doi: 10.1111/j.1365-2869.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoshihisa A, Suzuki S, Owada T, et al. Short-term use of adaptive servo ventilation improves renal function in heart failure patients with sleep-disordered breathing. Heart Vessels. 2013;28:728–34. doi: 10.1007/s00380-012-0303-0. [DOI] [PubMed] [Google Scholar]
- 22.Kasai T, Kasagi S, Maeno K, et al. Adaptive servo-ventilation in cardiac function and neurohormonal status in patients with heart failure and central sleep apnea nonresponsive to continuous positive airway pressure. JACC Heart Fail. 2013;1:58–63. doi: 10.1016/j.jchf.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Randerath WJ, Nothofer G, Priegnitz C, et al. Long-term auto-servoventilation or constant positive pressure in heart failure and coexisting central with obstructive sleep apnea. Chest. 2012;142:440–7. doi: 10.1378/chest.11-2089. [DOI] [PubMed] [Google Scholar]
- 24.Philippe C, Stoica-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92:337–42. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasai T, Usui Y, Yoshioka T, et al. Effect of flow-triggered adaptive servo-ventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail. 2010;3:140–8. doi: 10.1161/CIRCHEARTFAILURE.109.868786. [DOI] [PubMed] [Google Scholar]
- 26.Arzt M, Schroll S, Series F, et al. Auto-servoventilation in heart failure with sleep apnoea: a randomised controlled trial. Eur Respir J. 2013;42:1244–54. doi: 10.1183/09031936.00083312. [DOI] [PubMed] [Google Scholar]
- 27.Fietze I, Blau A, Glos M, Theres H, Baumann G, Penzel T. Bi-level positive pressure ventilation and adaptive servo ventilation in patients with heart failure and Cheyne-Stokes respiration. Sleep Med. 2008;9:652–9. doi: 10.1016/j.sleep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30:468–75. doi: 10.1093/sleep/30.4.468. [DOI] [PubMed] [Google Scholar]
- 29.Arzt M, Wensel R, Montalvan S, et al. Effects of dynamic bilevel positive airway pressure support on central sleep apnea in men with heart failure. Chest. 2008;134:61–6. doi: 10.1378/chest.07-1620. [DOI] [PubMed] [Google Scholar]
- 30.Campbell AJ, Ferrier K, Neill AM. Effect of oxygen versus adaptive pressure support servo-ventilation in patients with central sleep apnoea-Cheyne Stokes respiration and congestive heart failure. Intern Med J. 2012;42:1130–6. doi: 10.1111/j.1445-5994.2011.02623.x. [DOI] [PubMed] [Google Scholar]
- 31.Yoshihisa A, Suzuki S, Miyata M, et al. ‘A single night’ beneficial effects of adaptive servo-ventilation on cardiac overload, sympathetic nervous activity, and myocardial damage in patients with chronic heart failure and sleep-disordered breathing. Circ J. 2012;76:2153–8. doi: 10.1253/circj.cj-12-0453. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XL, Yin KS, Li XL, Jia EZ, Su M. Efficacy of adaptive servoventilation in patients with congestive heart failure and Cheyne-Stokes respiration. Chin Med J (Engl) 2006;119:622–7. [PubMed] [Google Scholar]
- 33.Bradley TD, Floras JS. Adaptive servo-ventilation and the treatment of central sleep apnea in heart failure. Let's not throw the baby out with the bathwater. Am J Respir Crit Care Med. 2016;193:357–9. doi: 10.1164/rccm.201511-2198ED. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.