Abstract
Key points
Cerebrovascular reactivity (CVR) reflects the vasodilatory reserve of cerebral resistance vessels.
Normal development in children is associated with significant changes in blood pressure, cerebral blood flow (CBF) and cerebral oxygen metabolism. Therefore, it stands to reason that CVR will also undergo changes during this period.
The study acquired magnetic resonance imaging measures of CVR and CBF in healthy children and young adults to trace their changes with age.
We found that CVR changes in two phases, increasing with age until the mid‐teens, followed by a decrease. Baseline CBF declined steadily with age.
We conclude that CVR varies with age during childhood, which prompts future CVR studies involving children to take into account the effect of development.
Abstract
Cerebrovascular reactivity (CVR) reflects the vasculature's ability to accommodate changes in blood flow demand thereby serving as a critical imaging tool for mapping vascular reserve. Normal development is associated with extensive physiological changes in blood pressure, cerebral blood flow and cerebral metabolic rate of oxygen, all of which can affect CVR. Moreover, the evolution of these physiological parameters is most prominent during childhood. Therefore, the aim of this study was to use non‐invasive magnetic resonance imaging (MRI) to characterize the developmental trajectories of CVR in healthy children and young adults, and relate them to changes in cerebral blood flow (CBF). Thirty‐four healthy subjects (17 males, 17 females; age 9–30 years) underwent CVR assessment using blood oxygen level‐dependent MRI in combination with a computer controlled CO2 stimulus. In addition, baseline CBF was measured with a pulsed arterial spin labelling sequence. CVR exhibited a gradual increase with age in both grey and white matter up to 14.7 years. After this break point, a negative correlation with age was detected. Baseline CBF maintained a consistent negative linear correlation across the entire age range. The significant age‐dependent changes in CVR and CBF demonstrate the evolution of cerebral haemodynamics in children and should be taken into consideration. The shift in developmental trajectory of CVR from increasing to decreasing suggests that physiological factors beyond baseline CBF also influence CVR.
Key points
Cerebrovascular reactivity (CVR) reflects the vasodilatory reserve of cerebral resistance vessels.
Normal development in children is associated with significant changes in blood pressure, cerebral blood flow (CBF) and cerebral oxygen metabolism. Therefore, it stands to reason that CVR will also undergo changes during this period.
The study acquired magnetic resonance imaging measures of CVR and CBF in healthy children and young adults to trace their changes with age.
We found that CVR changes in two phases, increasing with age until the mid‐teens, followed by a decrease. Baseline CBF declined steadily with age.
We conclude that CVR varies with age during childhood, which prompts future CVR studies involving children to take into account the effect of development.
Abbreviations
- ASL
arterial spin labelling
- BOLD
blood oxygen level‐dependent
- CBF
cerebral blood flow
- CBV
cerebral blood volume
cerebral metabolic rate of oxygen
- CVR
cerebrovascular reactivity
- fMRI
functional magnetic resonance imaging
- GM
grey matter
- MRI
magnetic resonance imaging
end‐tidal partial pressures of carbon dioxide
end‐tidal partial pressures of oxygen
- SNR
signal‐to‐noise ratio
- WM
white matter
Introduction
The developing brain undergoes significant physiological and organizational changes during childhood and adolescence as a gradual increase in volume is simultaneously accompanied by systematic pruning of excess neuronal connections to form more efficient networks (Huttenlocher, 1979; Luo & O'Leary, 2005; Biagi et al. 2007). This period is also associated with increased myelination and prominent alterations of the vasculature leading to an evolution in cerebral haemodynamics (Tortori‐Donati & Rossi, 2005; Biagi et al. 2007; Labarthe et al. 2009; Hales et al. 2014). The cerebral blood flow (CBF) of children, in particular, has been a subject of much interest as it has been shown to mirror the development of specific cognitive functions (Wintermark et al. 2004). Previous imaging studies have demonstrated that while normal baseline CBF varies across the entire lifespan, the most significant changes occur over the first two decades of life (Chiron et al. 1992; Takahashi et al. 1999; Biagi et al. 2007; Hales et al. 2014).
CBF, however, does not sufficiently describe the dynamics of cerebral circulation as CBF itself is actively regulated by changes in vascular resistance. This ability to mediate blood flow in the brain is characterized as cerebrovascular reserve, and is a vital function of the vasculature that helps maintain adequate blood supply in response to fluctuations in metabolic demand and blood pressure. Assessment of cerebrovascular reserve can provide information about the vascular health of the brain that is not available from baseline CBF alone (Yonas & Pindzola, 1994). Currently, it is unknown whether the longitudinal changes in baseline CBF observed during childhood development have any impact on the vasculature's ability to regulate blood flow in the brain.
Changes in vascular resistance are primarily driven by the constriction and dilatation of cerebral blood vessels. This can be measured in terms of cerebrovascular reactivity (CVR), a surrogate for cerebrovascular reserve. CVR is acquired by quantifying the relative change in CBF following the introduction of a vasoconstrictive or vasodilatory stimulus (Fierstra et al. 2013). Carbon dioxide (CO2) gas is commonly used, as it is non‐invasive, is easily administered and can induce vessel dilatation within the order of seconds (Ainslie & Duffin, 2009). Healthy vessels that are highly distensible and have sufficient capacity for vasodilatation are associated with high reactivity, whereas impaired cerebral vessels that are unable to properly regulate blood flow in the brain will exhibit low reactivity. When acquired using high resolution imaging techniques such as magnetic resonance imaging (MRI), CVR data can be displayed as tissue‐level parametric maps, which have been used clinically to help identify target regions for surgical planning, detect risk of future ischaemic injury, and monitor recovery after surgical and drug interventions (Rao & Pillai, 2006; Conklin et al., 2010, 2011; Han et al. 2011; Mandell et al. 2011; Pillai & Zacá, 2011; Zaca et al. 2011; Glodzik et al. 2013).
Despite the potential of CVR as a clinical tool, previous research has primarily focused on adult data, which may not necessarily translate to the childhood population. Most of the literature on CVR alterations with age do not include subjects younger than 18 years (Lu et al. 2011; Gauthier et al. 2013), so little is known about how CVR may differ between adults and children, and how it may change during normal brain development. Obtaining CVR data from healthy paediatric controls across a broad age range would provide valuable insight into the developing brain's ability to regulate CBF. Furthermore, the data would represent a set of normal reference values necessary for the clinical interpretation of cerebral haemodynamic abnormalities in children, especially in systemic or bilateral cerebrovascular disease where isolated regional abnormalities are not evident.
Of the few publications that involve CVR in paediatric volunteers, results relating CVR changes with age are almost non‐existent. In one early study, Brouwers et al. (1990) used Doppler ultrasound to measure hyperventilation‐induced changes in mean arterial velocity and found no significant differences in CVR between children of various age groups. However, ultrasound‐based CVR measures can be unreliable as they are influenced by a number of physiological and technical factors, such as angle of insonation and operator variability (Leung et al. 2013). Furthermore, hyperventilation‐induced CBF changes are difficult to control, and decreases in arterial partial pressures of CO2 are accompanied by a concomitant rise in partial pressures of O2. In animal literature, Winter et al. (2011) utilized MRI to demonstrate a log‐linear relation between CVR and body weight (as a surrogate for age) in intubated juvenile piglets. However, the CVR changes in humans during childhood and adolescence have yet to be fully characterized.
MRI is the most suitable image modality for paediatric CVR mapping as it is sensitive to microvascular changes in blood flow and does not expose subjects to invasive procedures or ionizing radiation. Blood oxygen level‐dependent (BOLD) MRI detects relative changes in CBF via the susceptibility difference between oxygenated and deoxygenated blood. It is commonly used due to its widespread availability as well as high temporal resolution and signal‐to‐noise ratio (SNR). Unlike arterial spin labelling (ASL), it does not require pharmacokinetic modelling assumptions that may be confounded by variations in blood flow transit time and is less sensitive to physiological noise (Buxton et al. 1998). BOLD MRI is well suited for CVR measurements as the signal response to hypercapnia has been shown to predominantly reflect relative changes in CBF (Mandell et al. 2008). Moreover, the advancement of high field MRI systems and novel robust methods of administering the stimulus have enabled CVR maps to be created with ever improving spatial resolution and reproducibility (Winter et al. 2009; Kassner et al. 2010; Thomas et al. 2013).
The purpose of our study was to quantify the developmental trajectory of CVR from childhood through early adulthood in healthy volunteers using BOLD MRI. In addition, we investigated the association between changes in CBF and CVR during this period. Since increased blood flow is primarily mediated by vasodilatation, the capacity for vessels to further dilate may be diminished in younger children as they have higher baseline CBF compared with adults (Biagi et al. 2007). We therefore hypothesized that CVR is lower in young children and increases with age, in response to the gradual decline in baseline CBF.
Methods
Ethical approval
This study conformed to the standards of the Declaration of Helsinki for medical research involving human subjects. All procedures were approved by the Research Ethics Board at The Hospital for Sick Children, and informed, written consent was obtained from each subject or their parent or guardian.
Data collection
Imaging data of healthy volunteers collected from a larger study were reviewed for secondary analysis. In total, 34 data sets (17 males, 17 females) were available. The subjects were aged between 9 and 30 years and had no history of respiratory, cardiovascular or cerebrovascular disease. Participants were asked to refrain from consuming vasoactive substances such as caffeine or alcohol on the day of imaging. An outline of subject demographics is provided in Table 1.
Table 1.
Demographic characteristics of all subjects
| Subject | Age (years) | Sex | Height (cm) | Weight (kg) |
|---|---|---|---|---|
| 1 | 9.1 | F | 143 | 38 |
| 2 | 10.2 | F | 152 | 43 |
| 3 | 10.5 | F | 152 | 61 |
| 4 | 11.0 | F | 147 | 35 |
| 5 | 12.3 | F | 165 | 72 |
| 6 | 13.1 | F | 171 | 121 |
| 7 | 14.3 | F | 161 | 50 |
| 8 | 14.7 | F | 165 | 63 |
| 9 | 15.4 | F | 159 | 46 |
| 10 | 15.6 | F | 159 | 56 |
| 11 | 15.7 | F | 159 | 43 |
| 12 | 17.0 | F | 167 | 54 |
| 13 | 17.5 | F | 170 | 51 |
| 14 | 17.5 | F | 163 | 46 |
| 15 | 17.8 | F | 164 | 91 |
| 16 | 17.9 | F | 163 | 57 |
| 17 | 23.5 | F | 162 | 51 |
| 18 | 9.4 | M | 130 | 23 |
| 19 | 11.8 | M | 147 | 61 |
| 20 | 11.9 | M | 161 | 68 |
| 21 | 12.0 | M | 147 | 39 |
| 22 | 13.6 | M | 149 | 49 |
| 23 | 14.7 | M | 175 | 70 |
| 24 | 16.3 | M | 166 | 61 |
| 25 | 16.6 | M | 171 | 58 |
| 26 | 16.6 | M | 175 | 68 |
| 27 | 17.1 | M | 169 | 75 |
| 28 | 17.3 | M | 175 | 90 |
| 29 | 17.5 | M | 176 | 68 |
| 30 | 17.5 | M | 180 | 62 |
| 31 | 20.2 | M | 169 | 53 |
| 32 | 21.2 | M | 185 | 70 |
| 33 | 27.9 | M | 180 | 65 |
| 34 | 30.2 | M | 172 | 71 |
CO2 stimulus
A CO2 breathing challenge was administered using a model‐driven prospective end‐tidal system (RespirActTM; Thornhill Research Inc., Toronto, Canada) to induce periods of hypercapnia while the subjects were in the MRI. This computer‐controlled system regulates the flow and composition of gases (CO2, O2 and N2) based on each subject's physiological parameters (age, height, weight, etc.) and delivers the gas mixture via a rebreathing mask and circuit. The dynamic delivery of specific gas concentrations enables fast and accurate simultaneous targeting of end‐tidal () and (), which have been shown to closely correlate to arterial blood gas levels. A more detailed description of the gas delivery system used in our study is provided by Slessarev et al. (2007).
The breathing challenge was a block design consisting of alternating 60 s periods of normocapnia ( = 40 mmHg) and 45 s periods of hypercapnia ( = 45 mmHg). Concurrently, normoxia was maintained ( = 100 mmHg) throughout the gas sequence. The total runtime was 8 min. Sampling lines in the breathing mask fed into the RespirAct to continuously monitor partial pressures of each subject's expired gas. End‐tidal values were recorded at the end of each expired breath to define and plot the measured and temporal waveforms. This type of breathing challenge has previously demonstrated high subject compliance and good reproducibility in both adults (Kassner et al. 2010; Mark et al. 2010) and children (Leung et al. 2016).
Magnetic resonance imaging
All imaging data were acquired on a clinical 3.0 T MRI system (MAGNETOM Tim Trio; Siemens Medical Solutions; Erlangen, Germany) with a 32‐channel head coil. The CVR protocol consisted of an 8 min BOLD acquisition utilizing a single‐shot T2*‐weighted echo‐planar imaging sequence (TE/TR = 30/2000 ms, FA = 70°, FOV = 220 mm, matrix = 64 × 64, slices = 25, thickness = 4.5 mm, volumes = 240), which was run in synchrony with the previously described CO2 breathing challenge. High resolution T1‐weighted anatomical images (MPRAGE) with isotropic 1.0 mm voxel size were then collected under normocapnia for coregistration and segmentation purposes. In addition, baseline CBF data were acquired using a pulsed ASL sequence (PICORE Q2TIPS; TE/TR = 13/2500 ms, TI1 = 700 ms, TI2 = 1800 ms, FA = 90 deg, FOV = 220 mm, matrix = 64×64, slices = 13, thickness = 4.5 mm, volumes = 91). The ASL imaging slab was aligned to the anterior posterior commissures and centred at the superior portion of the corpus callosum to maximize coverage.
Data processing and analysis
End‐tidal waveforms and MRI data were transferred to an independent workstation for secondary analysis. Using in‐house scripts written in MATLAB (The Mathworks Inc., Natick, MA, USA), we temporally aligned each waveform to its corresponding BOLD dataset based on a cross‐correlation with the mean whole‐brain BOLD signal, then resampled the data to match the BOLD sequence temporal resolution. This removed any lag between the stimulus and MRI measurements. Whole‐brain CVR maps were then generated using FSL (FMRIB Software Library; the University of Oxford, UK). The BOLD dynamics were first corrected for motion, spatially smoothed with a 5 mm Gaussian kernel to reduce noise, and temporally filtered to remove low frequency artifacts. Each voxel value on the CVR map was computed from a linear regression (FSL‐FEAT) between the temporal BOLD signal and the resampled waveform. These values were then normalized to the temporal mean BOLD signal of each respective voxel to represent the CVR map in terms of percentage of ΔMR signal per millimetre of mercury (co 2). Finally, each map was coregistered (FSL‐FLIRT) to its corresponding high resolution T1‐weighted anatomical image.
Baseline CBF maps were computed from ASL data using a single‐compartment kinetic model described by Buxton et al. (1998). All volumes were corrected for motion and the fully relaxed reference signal (M 0) was estimated from the first volume. Tag‐control difference images were calculated from the remaining 45 data pairs and averaged to generate a CBF map. CBF was quantified in units of millilitres per 100 grams per minute by factoring in the blood–tissue partition coefficient (λ = 0.9 ml g−1), the inversion efficiency (α = 0.95) and the T1 of blood at 3.0 T (T1a = 1650 ms) (Alsop et al. 2015). As with CVR, the CBF maps were coregistered to their corresponding T1‐weighted image space.
Grey matter (GM) and white matter (WM) masks were generated from the T1‐weighted images by first using a brain extraction algorithm (FSL‐BET) to remove non‐brain regions followed by automated tissue segmentation (FSL‐FAST). These masks were applied to the coregistered CVR and CBF maps to calculate the global mean reactivity and mean CBF in the GM and WM for each subject.
Statistical tests
Linear regression analysis was performed between CVR and age, CBF and age, and CVR and CBF. Additionally, a segmented regression analysis was performed, as Biagi et al. (2007) have shown that CBF changes with age appear to exhibit a sigmoidal pattern. The identification of break points in the regression slope would indicate that the data are more appropriately modelled with multiple linear segments. Pearson's correlation coefficients were calculated for each identified segment, and statistical significance was defined as P < 0.05. All statistical tests were executed using R (v3.2.1).
Results
Subject was accurately and consistently targeted with an average coefficient of variation of 1.92% during normocapnia and 1.79% during hypercapnia. None of the BOLD‐CVR data sets acquired were compromised by subject motion, but three ASL data sets had to be discarded due to motion artifacts. Representative slices from the CVR maps of three subjects are provided in Fig. 1, showing how CVR is distributed across GM and WM regions at different ages (10, 16 and 23 years old).
Figure 1.
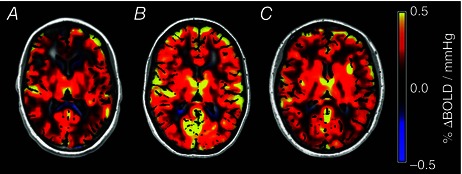
Representative slices of CVR maps for a 10‐year‐old healthy female (A), a 16‐year‐old healthy female (B) and a 23‐year‐old female (C) showing the change in CVR at different ages
The segmented regression test between CVR and age revealed statistical evidence of two distinct slopes with the break point at age 14.7 for both GM and WM (Fig. 2 A). Results from Pearson correlation analyses are provided in Table 2. A statistically significant linear relation was observed on both the upward slopes before the break point for GM (P = 0.00459) and WM (P = 0.0379) and the downward slopes after the break point for GM (P = 0.0369) and WM (P = 0.0390). The corresponding relative CBF values in the GM and WM were plotted against subject age in Fig. 2 B. Unlike CVR, the CBF measurements exhibited a monotonic reduction over the entire age range for our study, which is in agreement with previous ASL literature (Biagi et al. 2007; Hales et al. 2014). Segmented regression could not identify a significant break point in the CBF data, likely to be due to insufficient data points in adulthood to properly characterize the expected plateau of the CBF decline (Biagi et al. 2007). Instead, a linear model was applied. The correlations from the linear regression of CBF with age were statistically significant in both GM and WM (P < 0.001). However, correlational analysis between CVR and CBF data did not reveal any significant correlation in GM (P = 0.834 before break point, P = 0.125 after break point) or WM (P = 0.569 before break point, P = 0.376 after break point).
Figure 2. Plots of CVR (A) and CBF (B) with age .
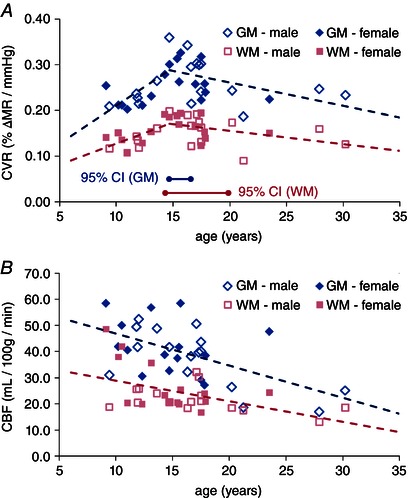
Each data point represents values averaged over GM (diamonds) and WM (squares) for each subject, where an open symbol indicates male and filled symbol indicates female. The dashed line shows the best linear fit. For the CVR, a regression break point was computed at 14.7 years with the 95% confidence intervals indicated by horizontal lines along the bottom of the graph. No break point was detected for CBF.
Table 2.
Pearson regression results between CVR, CBF and age for data prior to and after the break point
| Grey matter | White matter | |||
|---|---|---|---|---|
| r 2 | P | r 2 | P | |
| Correlation before break point | ||||
| CVR vs. age | 0.502 | 0.0046 | 0.364 | 0.0379 |
| CBF vs. age | 0.306 | 0.0012 | 0.246 | 0.0045 |
| CVR vs. CBF | 0.0039 | 0.8347 | 0.0334 | 0.5694 |
| Correlation after break point | ||||
| CVR vs. age | 0.220 | 0.0369 | 0.196 | 0.0390 |
| CBF vs. age | n/a* | |||
| CVR vs. CBF | 0.150 | 0.1249 | 0.046 | 0.3763 |
*CBF did not exhibit any break point. CBF, cerebral blood flow; CVR, cerebrovascular reactivity.
Discussion
Our study demonstrates, for the first time, the developmental trajectories of both CVR and CBF from childhood into adulthood. As plotted in Fig. 2 A, CVR undergoes significant changes with age during childhood as calculated data points take on a biphasic pattern. Segmented regression analysis identified a break point in the CVR data at 14.7 years, indicating the transition from a positively correlated to a negatively correlated trend.
In contrast to CVR, CBF exhibited a steady decline throughout the entire age range in the study (r = −0.55, P = 0.001 in GM, r = −0.50, P = 0.005 in WM). The mean CBF computed from our data declined by approximately 50% in both the GM and WM over the span of our subject age range, which is in line with findings published by Biagi et al. (2007). Hales et al. (2014) noted similar results but the rate of change was slower, possibly as a result of using a more robust acquisition sequence to account for differences in transit time with age. As of yet, ASL quantification is not fully standardized so variations in sequence parameters and data processing can contribute to differing results.
The elevated CBF observed in younger subjects is most likely a means to support the higher energy demands in the brain during this critical stage of development (Biagi et al. 2007). Previous PET studies in children have demonstrated that cerebral oxygen metabolism () throughout the brain peaks at age 7 and remains elevated into early adolescence (Chugani et al. 1987; Takahashi et al. 1999). There are also other physiological parameters such as blood pressure that differ during childhood and can affect cerebral haemodynamics (Regan et al. 2014). While blood pressure measurements were not collected as part of the original study, population studies have shown that blood pressure in children is lower compared with adults (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, 2004). CBF is maintained constant across a range of mean arterial pressures, and therefore the lower blood pressure in children would be met with cerebral vasodilatation to preserve flow. Taken together, the simultaneous high CBF and low blood pressure in childhood requires extensive vasodilatation. Cerebrovascular reserve is, therefore, likely to be reduced in children as their cerebral blood vessels will be less compliant to further increases in blood flow. This supports our initial hypothesis that CVR is inversely correlated to CBF over time. After the mid‐teens, however, the trajectory of CVR alters while CBF continues to steadily decrease with age. This sudden deviation from the trend may be associated with the tail end of physical maturation during puberty. Most notably, systolic blood pressure stabilizes around the late teens and transitions from a steep incline to a much shallower slope (Labarthe et al. 2009). The relationship between CVR and CBF may, therefore, be influenced by factors such as blood pressure, which are strongly associated with normal growth patterns.
In addition, the influence of and cerebral blood volume (CBV) on BOLD‐based measurements must be acknowledged (Leontiev & Buxton, 2007; Blockley et al. 2012; Zong et al. 2012). These parameters are intrinsically associated with the principles of the BOLD effect as specified in the Davis model (Buxton & Frank, 1997; Davis et al. 1998) and may contribute to the observed changes in CVR. Measurements of and CBF during childhood development may reveal physiological mechanisms specific to the BOLD response in younger subjects, as well as elucidate differences between physiological CVR and CVR measured by BOLD. However, as our study is based on retrospective data and there are currently no published data on and CBV changes throughout adolescence, we were unable to examine any potential correlations. Such relations will need to be investigated in future studies.
CVR and CBF changes with age were shown to follow linear or piecewise linear trajectories, but we were unable to find a statistically significant correlation between CVR and CBF. The lack of correlation may be a consequence of measurement variability such as in pulsed ASL, which suffers from lower SNR compared with continuous and pseudo‐continuous ASL (Alsop et al. 2015). Furthermore, the ASL sequence used for quantifying CBF has approximately half the spatial coverage of the BOLD sequence used for CVR imaging. This may skew the mean CBF plots if blood flow changes with age do not occur uniformly across the entire brain. The CBF measured in our study also appears to be lower compared with values reported in previous ASL literature (Wang et al. 2003; Biagi et al. 2007; Hales et al. 2014). However, the mean GM and WM CBF values presented in these studies were averaged from manually selected regions of interest. In contrast, the results in the current study was derived from automated segmentation of the entire GM and WM. This approach is more systematic but can potentially cause underestimation due to partial voluming effects. The lower CBF may also be due to limitations of the physiological assumptions used in the quantification model, such as transit time. In particular, the change in CBF between children and adults suggests that the ASL imaging protocol may need to be adapted for differences in flow velocity to acquire a more accurate measurement. One potential solution for future studies would be to implement multi‐phase ASL to characterize the bolus transit through each voxel.
Although we were able to demonstrate significant developmental changes in CVR and CBF with age, a larger sample size would allow a more comprehensive statistical comparison between groups within our cohort, such as sex differences. In a previous adult study, CVR was shown to be higher in men compared with women (Kassner et al. 2010), while females tend to have higher resting CBF values (Rodriguez et al. 1988). However, our current subject numbers do not have the statistical power to facilitate a comparison of developmental trajectories between males and females in our study. No significant differences could be determined between sex, and therefore the two groups were presented as a single data set. Additional subject data may also provide sufficient statistical power to fit a more robust model of the CVR and CBF trajectories. The piecewise linear fit for CVR presented in this study is an approximation for the purpose of demonstrating the presence of a break point. As such, the precise age at which the break point occurs during adolescence may vary depending on the model chosen. Application of higher order polynomials similar to the models of cortical development presented by Shaw et al. (2008), or even exponential decay models, may further improve the CVR correlation with age as well as shift the break point. Alternatively, the collection of more detailed biometric information may also aid in the analysis of the results. This current study proposes chronological age as developmental metric and regressor for CVR and CBF, but this can vary between individuals, especially in younger subjects. Physical maturity (Mirwald et al. 2002), for example, may be better associated with cerebrovascular development, but these measures were not collected as part of the original study.
While CBF is a well established and clinically relevant physiological parameter, the applications of CVR have only recently expanded with potential utility in assessing vasculopathies, psychological disorders and tumour vasculature (Rao & Pillai, 2006; Conklin et al., 2010, 2011; Han et al. 2011; Mandell et al. 2011; Pillai & Zacá, 2011; Zaca et al. 2011; Glodzik et al. 2013). To further improve the diagnostic interpretation of CVR imaging, an atlas of reference CVR values has also been proposed (Sobczyk et al. 2014). However, these advances have primarily focused on the adult population and very little is known about how CVR differs between children and adults. Our findings provide an impetus for future CVR studies in paediatric subjects to control for age‐related changes when assessing CVR across age groups.
Another useful application of this work is in the subsequent analysis of other BOLD‐related studies such as functional magnetic resonance imaging (fMRI). Gaillard et al. (2001) commented on potential confounders of fMRI studies in children, which included cognitive ability, physiological development and specific technical challenges. One study by Thomason et al. (2005) examined the BOLD haemodynamic response during breath‐hold and found that the extent of activation was significantly reduced in pre‐teens compared with adults. Conversely, other studies utilizing specific task‐based stimuli have suggested that local BOLD activation amplitudes are equivalent or greater in children compared with adults (Kang et al. 2003; Church et al. 2008; Moses et al. 2014). This discrepancy may be a result of the nature of the stimulus, where functional tasks can induce significantly greater changes in oxygen metabolism compared with CO2. More importantly, fMRI primarily focuses on the quantification of activated voxels and neglects regions with weaker signal responses. This approach may bias results as functionally associated regions that happen to have lower reactivity may be excluded from analysis. As noted by Thomason et al. (2005), BOLD data from younger subjects were significantly noisier than those from adults, which is inevitably reflected in the resulting activation maps. Since CVR is assessed across all regions of the brain irrespective of signal or noise level, it can serve as a means to contextualize these age‐related differences in BOLD‐fMRI activation.
In conclusion, our data represent the first characterization of CVR evolution from childhood to early adulthood (age 9–30 years). These findings broaden our understanding of cerebrovascular development in the maturing brain by demonstrating significant CVR changes during children. Furthermore, these changes are not solely dependent on the age‐related decrease in CBF observed during the first two decades of life. The differences in CVR between age groups imply that the cerebral vasculature's capacity to accommodate increases in blood flow can vary across the lifespan. The physiological influence of age may have important implications for the way BOLD imaging results are interpreted, from the assessment of CVR in cerebrovascular diseases to the identification of fMRI activation signals.
Additional information
Competing interests
The authors have no competing interests with regard to the subject matter of this manuscript to disclose.
Author contributions
The experiments were carried out at The Hospital for Sick Children, Toronto, Ontario, Canada. J.L. and A.K. were responsible for the conception and design of the research; J.L. and P.D.K. performed the data collection; J.L., P.D.K., P.L.C. and A.K. performed data analysis and interpretation of results; J.L. and P.D.K. drafted the manuscript; All authors contributed to the editing and revision of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was been funded by a Canadian Institutes of Health Research operating grant (FRN# 111113) and a Canadian Research Chairs award (FRN# 72029767).
Translational perspective
Cerebrovascular reactivity (CVR) describes the capacity of cerebral blood vessels to mediate changes in blood flow in response to various stimuli such as changes in perfusion pressure, blood‐gas concentrations, and metabolic demand. CVR is an important physiological parameter as it can be used as a marker of cerebrovascular health. In addition, measures of CVR provide valuable insight into functional magnetic resonance imaging (fMRI) data as CVR assesses the vascular reserve utilized during functional tasks. This study presents the first characterization of CVR in healthy subjects from childhood to early adulthood using blood oxygen level‐dependent MRI in combination with a controlled hypercapnic stimulus. We demonstrated that CVR exhibits a bi‐phasic profile between the ages of 9 and 30 years, consisting of an initial positive linear correlation with age followed by a negative slope after a break point occurring in the mid‐teenage years. Furthermore, the observed changes in CVR throughout childhood elucidate physiological differences between children and adults and emphasize the need for appropriate reference values for different ages. Specifically, our findings introduce new considerations for the interpretation of paediatric fMRI and CVR studies as the effect of age can be a confounding factor.
Acknowledgements
We wish to acknowledge Thornhill Research Inc. for providing technical support on the RespirAct. We also thank Garry Detzler, Tammy Rayner, Annette Weekes, and Ruth Weiss for their MRI support throughout our study, as well as George Tomlinson for his guidance on statistics analysis.
References
- Ainslie PN & Duffin J (2009). Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296, R1473–R1495. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA, Golay X, Günther M, Hendrikse J, Hernandez‐Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC & Zaharchuk G (2015). Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi L, Abbruzzese A, Bianchi MC, Alsop DC, Del Guerra A & Tosetti M (2007). Age dependence of cerebral perfusion assessed by magnetic resonance continuous arterial spin labeling. J Magn Reson Imaging 25, 696–702. [DOI] [PubMed] [Google Scholar]
- Blockley NP, Griffeth VEM & Buxton RB (2012). A general analysis of calibrated BOLD methodology for measuring CMRO2 responses: Comparison of a new approach with existing methods. Neuroimage 60, 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers PJ, Vriens EM, Musbach M, Wieneke GH & van Huffelen AC (1990). Transcranial pulsed Doppler measurements of blood flow velocity in the middle cerebral artery: reference values at rest and during hyperventilation in healthy children and adolescents in relation to age and sex. Ultrasound Med Biol 16, 1–8. [DOI] [PubMed] [Google Scholar]
- Buxton RB & Frank LR (1997). A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab 17, 64–72. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Frank LR, Wong EC, Siewert B, Warach S & Edelman RR (1998). A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 40, 383–396. [DOI] [PubMed] [Google Scholar]
- Chiron C, Raynaud C, Mazière B, Zilbovicius M, Laflamme L, Masure MC, Dulac O, Bourguignon M & Syrota A (1992). Changes in regional cerebral blood flow during brain maturation in children and adolescents. J Nucl Med 33, 696–703. [PubMed] [Google Scholar]
- Chugani HT, Phelps ME & Mazziotta JC (1987). Positron emission tomography study of human brain functional development. Ann Neurol 22, 487–497. [DOI] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE & Schlaggar BL (2008). A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb Cortex 18, 2054–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin J, Fierstra J, Crawley AP, Han JS, Poublanc J, Silver FL, Tymianski M, Fisher JA, Mandell DM & Mikulis DJ (2011). Mapping white matter diffusion and cerebrovascular reactivity in carotid occlusive disease. Neurology 77, 431–438. [DOI] [PubMed] [Google Scholar]
- Conklin J, Fierstra J, Crawley AP, Han JS, Poublanc J, Mandell DM, Silver FL, Tymianski M, Fisher JA & Mikulis DJ (2010). Impaired cerebrovascular reactivity with steal phenomenon is associated with increased diffusion in white matter of patients with moyamoya disease. Stroke 41, 1610–1616. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM & Rosen BR (1998). Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci USA 95, 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierstra J, Sobczyk O, Battisti‐Charbonney A, Mandell DM, Poublanc J, Crawley AP, Mikulis DJ, Duffin J & Fisher JA (2013). Measuring cerebrovascular reactivity: what stimulus to use? J Physiol 591, 5809–5821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Grandin CB & Xu B (2001). Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. Neuroimage 13, 239–249. [DOI] [PubMed] [Google Scholar]
- Gauthier CJ, Madjar C, Desjardins‐Crépeau L, Bellec P, Bherer L & Hoge RD (2013). Age dependence of hemodynamic response characteristics in human functional magnetic resonance imaging. Neurobiol Aging 34, 1469–1485. [DOI] [PubMed] [Google Scholar]
- Glodzik L, Randall C, Rusinek H & de Leon MJ (2013). Cerebrovascular reactivity to carbon dioxide in Alzheimer's disease. J Alzheimer's Dis 35, 427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales PW, Kawadler JM, Aylett SE, Kirkham FJ & Clark CA (2014). Arterial spin labeling characterization of cerebral perfusion during normal maturation from late childhood into adulthood: normal “reference range” values and their use in clinical studies. J Cereb Blood Flow Metab 34, 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Mikulis DJ, Mardimae A, Kassner A, Poublanc J, Crawley AP, deVeber GA, Fisher JA & Logan WJ (2011). Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level‐dependent MRI. Stroke 42, 1261–1269. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR (1979). Synaptic density in human frontal cortex—Developmental changes and effects of aging. Brain Res 163, 195–205. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE & Schlaggar BL (2003). Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage 19, 16–28. [DOI] [PubMed] [Google Scholar]
- Kassner A, Winter JD, Poublanc J, Mikulis DJ & Crawley AP (2010). Blood‐oxygen level dependent MRI measures of cerebrovascular reactivity using a controlled respiratory challenge: reproducibility and gender differences. J Magn Reson Imaging 31, 298–304. [DOI] [PubMed] [Google Scholar]
- Labarthe DR, Dai S, Fulton JE, Harrist RB, Shah SM & Eissa MA (2009). Systolic and fourth‐ and fifth‐phase diastolic blood pressure from ages 8 to 18 years. Project HeartBeat! Am J Prev Med 37, S86–S96. [DOI] [PubMed] [Google Scholar]
- Leontiev O & Buxton RB (2007). Reproducibility of BOLD, perfusion, and CMRO2 measurements with calibrated‐BOLD fMRI. Neuroimage 35, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung J, Behpour A, Sokol N, Mohanta A & Kassner A (2013). Assessment of intracranial blood flow velocities using a computer controlled vasoactive stimulus: A comparison between phase contrast magnetic resonance angiography and transcranial doppler ultrasonography. J Magn Reson Imaging 38, 733–738. [DOI] [PubMed] [Google Scholar]
- Leung J, Kim JA & Kassner A (2016). Reproducibility of cerebrovascular reactivity measures in children using BOLD MRI. J Magn Reson Imaging 43, 1191–1195. [DOI] [PubMed] [Google Scholar]
- Lu H, Xu F, Rodrigue KM, Kennedy KM, Cheng Y, Flicker B, Hebrank AC, Uh J & Park DC (2011). Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex 21, 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L & O'Leary DDM (2005). Axon retraction and degeneration in development and disease. Annu Rev Neurosci 28, 127–156. [DOI] [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Fierstra J, Tymianski M, Fisher JA & Mikulis DJ (2011). Quantitative measurement of cerebrovascular reactivity by blood oxygen level‐dependent MR imaging in patients with intracranial stenosis: Preoperative cerebrovascular reactivity predicts the effect of extracranial‐intracranial bypass surgery. Am J Neuroradiol 32, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DM, Han JS, Poublanc J, Crawley AP, Stainsby JA, Fisher JA & Mikulis DJ (2008). Mapping cerebrovascular reactivity using blood oxygen level‐dependent MRI in patients with arterial steno‐occlusive disease: comparison with arterial spin labeling MRI. Stroke 39, 2021–2028. [DOI] [PubMed] [Google Scholar]
- Mark CI, Slessarev M, Ito S, Han J, Fisher JA & Pike GB (2010). Precise control of end‐tidal carbon dioxide and oxygen improves BOLD and ASL cerebrovascular reactivity measures. Magn Reson Med 64, 749–756. [DOI] [PubMed] [Google Scholar]
- Mirwald RL, Baxter‐Jones AD, Bailey DA & Beunen GP (2002). An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc 34, 689–694. [DOI] [PubMed] [Google Scholar]
- Moses P, DiNino M, Hernandez L & Liu TT (2014). Developmental changes in resting and functional cerebral blood flow and their relationship to the BOLD response. Hum Brain Mapp 35, 3188–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004). The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114, 555–576. [PubMed] [Google Scholar]
- Pillai JJ & Zacá D (2011). Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World J Clin Oncol 2, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GSU & Pillai SV (2006). Cerebrovascular reactivity to carbon dioxide in the normal and abnormal cerebral hemispheres under anesthesia in patients with frontotemporal gliomas. J Neurosurg Anesthesiol 18, 185–188. [DOI] [PubMed] [Google Scholar]
- Regan RE, Fisher JA & Duffin J (2014). Factors affecting the determination of cerebrovascular reactivity. Brain Behav 4, 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez G, Warkentin S, Risberg J & Rosadini G (1988). Sex differences in regional cerebral blood flow. J Cereb Blood Flow Metab 8, 783–789. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN & Wise SP (2008). Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci 28, 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slessarev M, Han J, Mardimae A, Prisman E, Preiss D, Volgyesi G, Ansel C, Duffin J & Fisher JA (2007). Prospective targeting and control of end‐tidal CO2 and O2 concentrations. J Physiol 581, 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk O, Battisti‐Charbonney A, Poublanc J, Crawley AP, Sam K, Fierstra J, Mandell DM, Mikulis DJ, Duffin J & Fisher JA (2014). Assessing cerebrovascular reactivity abnormality by comparison to a reference atlas. J Cereb Blood Flow Metab 35, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Shirane R, Sato S & Yoshimoto T (1999). Developmental changes of cerebral blood flow and oxygen metabolism in children. AJNR Am J Neuroradiol 20, 917–922. [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Logan W, Donner EJ & Shroff M (2013). Assessment of cerebrovascular reactivity using real‐time BOLD fMRI in children with moyamoya disease: a pilot study. Childs Nerv Syst 29, 457–63. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Burrows BE, Gabrieli JD & Glover GH (2005). Breath holding reveals differences in fMRI BOLD signal in children and adults. Neuroimage 25, 824–837. [DOI] [PubMed] [Google Scholar]
- Tortori‐Donati P & Rossi A (2005). Pediatric Neuroradiology. Springer, Berlin, Heidelberg. [Google Scholar]
- Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, Zimmerman RA & Detre JA (2003). Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging 18, 404–413. [DOI] [PubMed] [Google Scholar]
- Winter JD, Dorner S, Lukovic J, Fisher JA, St Lawrence KS & Kassner A (2011). Noninvasive MRI measures of microstructural and cerebrovascular changes during normal swine brain development. Pediatr Res 69, 418–424. [DOI] [PubMed] [Google Scholar]
- Winter JD, Poublanc J, Crawley AP & Kassner A (2009). Comparison of spiral imaging and SENSE‐EPI at 1.5 and 3.0 T using a controlled cerebrovascular challenge. J Magn Reson Imaging 29, 1206–1210. [DOI] [PubMed] [Google Scholar]
- Wintermark M, Lepori D, Cotting J, Roulet E, van Melle G, Meuli R, Maeder P, Regli L, Verdun FR, Deonna T, Schnyder P & Gudinchet F (2004). Brain perfusion in children: evolution with age assessed by quantitative perfusion computed tomography. Pediatrics 113, 1642–1652. [DOI] [PubMed] [Google Scholar]
- Yonas H & Pindzola RR (1994). Physiological determination of cerebrovascular reserves and its use in clinical management. Cerebrovasc Brain Metab Rev 6, 325–340. [PubMed] [Google Scholar]
- Zaca D, Hua J & Pillai JJ (2011). Cerebrovascular reactivity mapping for brain tumor presurgical planning. World J Clin Oncol 2, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Kim T & Kim SG (2012). Contributions of dynamic venous blood volume versus oxygenation level changes to BOLD fMRI. Neuroimage 60, 2238–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]


