Abstract
Key points
Abnormal activation of motoneurons in the spinal cord by sensory pathways is thought to contribute to impaired movement control and spasticity in individuals with cerebral palsy.
Here we use single motor unit recordings to show how individual motoneurons in the spinal cord respond to sensory inputs in a group of participants with cerebral palsy having different degrees of motor dysfunction.
In participants who had problems walking independently and required assistive devices such as wheelchairs, sensory pathways only excited motoneurons in the spinal cord.
In contrast, in participants with cerebral palsy who walked independently for long distances, sensory inputs both inhibited and excited motoneurons in the spinal cord, similar to what we found in uninjured control participants.
These findings demonstrate that in individuals with severe cerebral palsy, inhibitory control of motoneurons from sensory pathways is reduced and may contribute to motor dysfunction and spasticity.
Abstract
Reduced inhibition of spinal motoneurons by sensory pathways may contribute to heightened reflex activity, spasticity and impaired motor function in individuals with cerebral palsy (CP). To measure if the activation of inhibitory post‐synaptic potentials (IPSPs) by sensory inputs is reduced in CP, the tonic discharge rate of single motor units from the soleus muscle was plotted time‐locked to the occurrence of a sensory stimulation to produce peri‐stimulus frequencygrams (PSFs). Stimulation to the medial arch of the foot was used to activate cutaneomuscular afferents in 17 adults with bilateral spastic CP and 15 neurologically intact (NI) peers. Evidence of IPSP activation from the PSF profiles, namely a marked pause or reduction in motor unit firing rates at the onset of the cutaneomuscular reflex, was found in all NI participants but in only half of participants with CP. In the other half of the participants with CP, stimulation of cutaneomuscular afferents produced a PSF profile indicative of a pure excitatory post‐synaptic potential, with firing rates increasing above the mean pre‐stimulus rate for 300 ms or more. The amplitude of motoneuron inhibition during the period of IPSP activation, as measured from the surface EMG, was less in participants with poor motor function as evaluated with the Gross Motor Functional Classification System (r = 0.72, P < 0.001) and the Functional Mobility Scale (r = −0.82, P < 0.001). These findings demonstrate that in individuals with CP, reduced activation of motoneuron IPSPs by sensory inputs is associated with reduced motor function and may contribute to enhanced reflexes and spasticity in CP.
Key points
Abnormal activation of motoneurons in the spinal cord by sensory pathways is thought to contribute to impaired movement control and spasticity in individuals with cerebral palsy.
Here we use single motor unit recordings to show how individual motoneurons in the spinal cord respond to sensory inputs in a group of participants with cerebral palsy having different degrees of motor dysfunction.
In participants who had problems walking independently and required assistive devices such as wheelchairs, sensory pathways only excited motoneurons in the spinal cord.
In contrast, in participants with cerebral palsy who walked independently for long distances, sensory inputs both inhibited and excited motoneurons in the spinal cord, similar to what we found in uninjured control participants.
These findings demonstrate that in individuals with severe cerebral palsy, inhibitory control of motoneurons from sensory pathways is reduced and may contribute to motor dysfunction and spasticity.
Abbreviations
- CM
cerebral malformations
- CMR
cutaneomuscular reflex
- CP
cerebral palsy
- CVA
cerebrovascular accident
- DGMI
deep grey matter injury
- E1
first excitatory response
- EPSP
excitatory post‐synaptic potential
- FLAIR
fluid‐attenuated inversion recovery
- FMS
Functional Mobility Scale
- GMFCS
Gross Motor Function Classification System
- I1
first inhibitory response
- IPSP
inhibitory post‐synaptic potential
- mAsh
modified Ashworth scale
- MRC
Medical Research Council scale
- MRI
magnetic resonance imaging
- MVC
maximal voluntary contraction
- NI
neurologically intact
- Penn
Penn Spasm Frequency scale
- PSF
peri‐stimulus frequencygram
- PSTH
peri‐stimulus time histogram
- PVWMI
periventricular white matter injury
Introduction
Spasticity in cerebral palsy (CP) contributes to deficits in the control of posture and movement and is the primary motor impairment for 90% of those affected with CP (Shevell et al. 2009; Reid et al. 2011). The clinical symptoms of spasticity in CP include hyperreflexia, clonus, spasms, co‐contraction and improper timing of muscle activity (Milner‐Brown & Penn, 1979; Downing et al. 2009; Poon & Hui‐Chan, 2009). The mechanisms producing spasticity in CP are not completely understood. However, we know that damage to the brain and descending motor pathways during early development can result in enhanced activation of spinal motoneurons by peripheral reflex pathways (Evans et al. 1991; Gibbs et al. 1999). In a study of 28 participants with mainly bilateral spastic CP, indirect evidence from reflex recordings measured at rest suggest reduced presynaptic inhibition of Ia afferents and increased activation of excitatory propriospinal pathways but no evidence of reduced excitability of inhibitory pathways to motoneurons (Achache et al. 2010). Here, we examine more directly with single motor unit recordings if there are systematic abnormalities in the activation of inhibitory post‐synaptic potentials (IPSPs) in motoneurons by sensory pathways in a similar population of participants with bilateral, spastic CP as examined in the Achache et al. (2010) study. It is important to understand if inhibition of motoneurons by sensory inputs is abnormal in CP to determine if this may be a contributing factor to the production of spasticity and abnormal motor function.
To estimate the profile of excitatory and inhibitory post‐synaptic potentials (EPSP/IPSPs) evoked in motoneurons, we used the technique of peri‐stimulus frequencygrams (PSFs) whereby the tonic discharge rate of single motor units is plotted time‐locked to the occurrence of a sensory stimulation (Turker & Powers, 2005). Unlike surface EMG profiles, the firing rate profile of a motoneuron is a more accurate representation of its underlying post‐synaptic potential. For example, during an EPSP the firing rate of a motoneuron increases above the mean tonic discharge rate and the overlay of multiple frequency profiles closely follows the trajectory of the underlying membrane potential, especially for profiles lasting longer than 50 ms (Turker & Powers, 1999; Norton et al. 2008). Likewise, during a very weak IPSP, the firing rate of a motoneuron drops below the tonic discharge rate in line with the reduced synaptic potential (Turker & Powers, 1999). A strong IPSP, by contrast, is marked by a pause in the tonic discharge of the motoneuron during the hyperpolarizing phase of the IPSP with a resumption of firing occurring at rates near or slightly below the tonic discharge rate during the repolarization phase of the IPSP (Turker & Powers, 1999; Norton et al. 2008). Thus, the PSF can provide an indication of the amplitude (i.e. profile) of the underlying post‐synaptic potential. In contrast, surface EMG activity is dominated by the occurrence, and not frequency, of motor unit discharge so that its profile during a reflex response can give misleading information about the profile of the post‐synaptic potential (Powers & Turker, 2010 a). This is especially problematic during the later components of a post‐synaptic potential given that a large transient input, such as an EPSP or IPSP, can synchronize the discharge of a tonically firing motoneuron to produce false indications of repeated IPSPs and EPSPs in the surface EMG.
The use of PSFs has revealed that IPSP activation in motoneurons from stimulation of cutaneomuscular afferents is reduced after spinal cord injury, resulting in the activation of a pure, prolonged EPSP. This prolonged EPSP increases the probability of triggering self‐sustained discharge of motoneurons during involuntary muscle spasms, a common feature of spasticity in spinal cord injury (Norton et al. 2008). In this study, we also examined post‐synaptic potentials in motoneurons that were activated by cutaneomuscular afferents given that both excitatory and inhibitory interneurons are activated in this reflex pathway (Pinter et al. 1982). This allowed us to examine if there was a general loss of sensory‐evoked IPSPs in spinal motoneurons to help explain why reflexes are heightened in CP where damage to descending motor pathways occurs during early development. Moreover, we could examine the activation of IPSPs during voluntary contractions given that impairments of inhibitory pathways, like reciprocal and recurrent inhibition, are only revealed in CP and stroke during voluntary contractions (Katz & Pierrot‐Deseilligny, 1999; Crone et al. 2003) compared to when measured at rest (Leonard et al. 2006; Achache et al. 2010). We evaluated sensory activation of motoneurons supplying the soleus muscle, which is particularly spastic in CP (Elder et al. 2003) and involved in gait impairment (Eek et al. 2011). Finally, given that the strength of inhibition evoked in motoneurons can be reduced by inactivity following spinal cord injury (Boulenguez et al. 2010; Murray et al. 2011) and restored by intensive exercise (Cote et al. 2014), we recruited a group of participants with CP with varying levels of motor dysfunction to examine if the strength of motoneuron inhibition was associated with clinical measures of gross motor and walking function.
Methods
Ethical approval and participants
This study was approved by the Health Research Ethics Board at the University of Alberta, in accordance with the Declaration of Helsinki. All participants provided informed consent in writing. Seventeen adults with bilateral spastic CP (10 female, 7 male) and with an average age of 32.8 ± 11.1 years (range 19–56) participated in this study. Fifteen participants who were neurologically intact (NI) and with a similar sex distribution (10 female, 5 male) and average age (30.4 ± 11.6 years, range 18–59) were also recruited. The presence and degree of brain injury in the participants with CP was assessed with magnetic resonance imaging (MRI, described below). Participants with CP having injections of botulinum toxin into the lower leg within 6 months prior to the experiment were excluded although none had such treatment for at least 2 years. Medications taken by the participants with CP which may affect neuronal excitability are listed in Table 1.
Table 1.
Characteristics of participants with CP
| MRC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Sex | Age (years) | Rx | MRI | GMFCS | FMSTOTAL | Plantar | Dorsi | mAsh | Penn |
| CP‐1 | F | 19 | PVWMI+ | I | 17 | 5 | 4 | 0 | 1 | |
| CP‐2 | M | 29 | Baclofen, citalopram | PVWMI++/CM | I | 16 | 4 | 4 | 3 | 3 |
| CP‐3 | M | 25 | PVWMI ++ | I | 18 | 5 | 5 | 1+ | 1 | |
| CP‐4 | F | 31 | Venlafaxine | Contraindication | II | 10 | 5 | 4 | 1+ | 2 |
| CP‐5 | F | 34 | PVWMI ++ | IV | 3 | 1 | 2 | 2 | 1 | |
| CP‐6 | M | 48 | Citalopram, tolterodine | PVWMI ++ | III | 3 | 3 | 1 | 3 | 2 |
| CP‐7* | F | 28 | Normal | II | 14 | 5 | 4 | 1 | 1 | |
| CP‐8* | F | 33 | PVWMI ++/DGMI | III | 6 | 4 | 4 | 3 | 2 | |
| CP‐9 | F | 20 | PVWMI ++ | III | 5 | 1 | 0 | 1 | 1 | |
| CP‐10 | M | 26 | PVWMI +/CM | II | 7 | 2 | 1 | 0 | 1 | |
| CP‐11* | F | 19 | Sertraline, clonazepam | PVWMI ++/CM | IV | 3 | 2 | 1 | 1 | 2 |
| CP‐12 | M | 23 | PVWMI + | IV | 3 | 2 | 4 | 1 | 1 | |
| CP‐13 | M | 42 | PVWMI + | III | 4 | 2 | 4 | 1 | 2 | |
| CP‐14 | F | 38 | Normal | III | 9 | 1 | 2 | 1+ | 1 | |
| CP‐15 | F | 51 | Amitriptyline, citalopram, phenytoin, flunarizine | PVWMI +++/CVA | II | 9 | 1 | 3 | 2 | 2 |
| CP‐16 | F | 56 | PVWMI + | I | 17 | 5 | 5 | 0 | 1 | |
| CP‐17 | M | 30 | PVWMI ++ | II | 14 | 4 | 1 | 1 | 1 | |
Columns represent the participant demographics (Sex & Age), daily medications (Rx), the MRI findings, the Gross Motor Functional Classification System (GMFCS) rating, the total Functional Mobility Scale (FMSTOTAL) score with a maximum score of 18, the strength (MRC) of the plantarflexors (Plantar) and dorsiflexors (Dorsi) with a maximum score of 5, the modified Ashworth score (mAsh) for the plantarflexors with a maximum score of 5 and the Penn Spasm Frequency scale (Penn) where 1 = mild spasms induced by stimulation, 2 = infrequent full spasms occurring < 1 h–1, 3 = spasms occurring > 1 h–1).
*The three CP participants from whom motor unit data were not obtained. PVWMI = periventricular white matter injury, CM = cerebral malformations, DGMI = deep grey matter injury, CVA = cerebrovascular accident.
Motor assessments
Motor function of the participants with CP was evaluated by a physician (E.G.C.) with the results presented in Table 1. The Gross Motor Function Classification System (GMFCS) (Palisano et al. 2008) was used to measure functional abilities in sitting and walking and the need for assistive devices. The Functional Mobility Scale (FMS) was used to categorize the type of mobility a participant uses on a scale of 1 to 6, with 1 = uses wheelchair to 6 = independent on all surfaces, at three distances of 5, 50 and 500 m (Graham et al. 2004). The combined FMS score for all three distances (FMSTOTAL) is presented in Table 1 with a possible maximum score of 18. Although only FMS scores at the individual distances have been validated (Adair et al. 2012), here we summed all the FMS scores because it produced a broader distribution of scores to better compare the functional walking abilities in the participants with CP than any one of the individual distances. Plantarflexor and dorsiflexor strength at the ankle was assessed with the Medical Research Council score (MRC) (Hislop et al. 2014). The modified Ashworth scale (mAsh) (Bohannon & Smith, 1987) and the Penn Spasm Frequency scale (Penn) (Penn et al. 1989) were used to confirm the presence, and not extent, of spasticity given the limited range of these scores.
MRI scans
On a separate day from the reflex experiments, all participants with CP except CP‐4 with a contraindication to MRI (16/17) and 11 of the NI participants were scanned using a 1.5 T Siemens Sonata scanner. Sequences included T1‐weighted, T2‐weighted and fluid‐attenuated inversion recovery (FLAIR). A senior radiology resident and a neuroradiologist (D.T.J. and D.J.E., respectively) reviewed the T1, T2 and FLAIR sequences. Anatomical abnormalities were classified into categories relevant for our CP population, including periventricular white matter injury (PVWMI), cerebral malformations (CM), deep grey matter injury (DGMI) and normal brains (Towsley et al. 2011). The severity of any PVWMI was rated qualitatively as mild, moderate or severe based on cerebral white matter volume loss and T2 hyper‐intensities by consensus between the two radiologists who were blinded to the clinical scores.
Cutaneomuscular reflexes
In the participants with CP, the leg which interfered more with motor function was tested (n = 8 left, n = 9 right). The right leg was tested in the NI group except in three participants with previous injuries to the right ankle. Cutaneomuscular reflexes were elicited by a brief stimulus train (15 ms) delivered to the medial arch of the foot (five pulses, 200 μs pulse width, 300 Hz with a 3 s interstimulus interval) using a DS7A constant‐current stimulator (NL703, Digitimer, Welwyn Garden City, UK). The intensity of stimulation was set just below pain threshold to elicit a clear response in the surface EMG within the average of a few (<10) trials.
Motor unit recordings
Sixteen of the 17 participants with CP agreed to take part in this component of the experiment (CP‐11 declined) and 13 NI controls were recruited to participate. Fine‐wire electrodes made of stainless steel and with 50 μm de‐insulated ends (California Fine Wire, 304, H‐ML) were used to record motor unit activity from the soleus muscle. A 21‐gauge needle was used to guide the fine wires into the soleus muscle. Intronix hardware (Intronix Technology, Bolton, Canada) was used to amplify (5k gain) and filter (200 Hz to 20 kHz) the fine wire EMG signal. A Power1401 A/D and Spike 2 software (version 6, Cambridge Electronic Design, Cambridge, UK) were used to digitize the fine‐wire EMG recordings at a sampling rate of 25 kHz. Surface EMG from the soleus and lateral gastrocnemius was also recorded with Intronix hardware at 1k gain, 20 Hz to 2.5 kHz bandpass filter and at a sampling rate of 5 kHz. Participants were instructed to tonically plantarflex the ankle to produce steady firing of soleus motor units to a target of about 7 Hz. Auditory feedback of motor unit activity as well as verbal cues were provided to maintain sufficient and steady firing rates of the motor units. Most participants lay prone with their foot off the edge of a plinth pushing against a secured board. A few participants in each group were tested sitting or standing but this did not produce systematic differences in reflex responses. We aimed to deliver at least 300 stimuli to the medial arch of the foot (478 ± 190 stimuli were applied on average), typically in blocks of 50, and participants could rest at any time.
Surface EMG recordings
To quantify cutaneomuscular reflexes (CMRs) without the insertion of fine wires into the soleus muscle, which can produce added mechanical sensory activation during muscle contractions, all participants with CP (n = 17) returned for another experiment on a different day where only surface EMG was recorded along with 14 of the 15 NI controls. Surface EMG was recorded using pairs of conductive adhesive hydrogel electrodes (3.81 × 2.24 cm, Covidien, Dublin, Ireland) at 1k gain and 10–1000 Hz bandpass filtering (AMT‐8, Bortec Biomedical, Calgary, Canada), digitized and sampled at 5 kHz using Axon hardware (Digidata 1440A) and software (Axoscope 10.3, Molecular Devices, Sunnyvale, CA, USA). All participants were seated with their upper leg secured to a chair, the knee in 90 deg of flexion and the ankle secured in a custom binding at 0 deg of plantarflexion (±5 deg). With the knee positioned at 90 deg of flexion, all participants with CP were able to comfortably reach and maintain a consistent ankle position for the surface EMG recordings without significant dorsiflexion stretch to reduce the impact of contracture on the surface EMG recordings.
CMRs were recorded while participants voluntarily activated their soleus to a target voluntary contraction of 20% maximum (MVC). Thirty‐five CMRs at 20% MVC were collected and opportunities for rest were provided. A participant's MVC was measured at the start of the experiment. For MVC, the surface EMG was digitally band‐pass filtered (10–500 Hz), rectified and then smoothed with a 1 s moving‐window average applied every 1 ms. At least 60 s of rest was given between each MVC trial. The MVC was calculated from averaging the peak EMG, lasting at least 1 s, from two trials that were within 10% of each other.
Data processing of CMRs
PSFs and post‐stimulus time histograms (PSTHs)
Motor units were fully identified off‐line using Spike 2 wavemarks and manual discrimination. Single motor unit waveforms could be reliably identified in 14 of the 16 participants with CP tested and in all of the NI participants tested (n = 13). PSFs and PSTHs were constructed using custom Matlab software (R2011b, The Mathworks Inc., Natick, MA, USA). For the PSFs, the reciprocal of the interval between spikes was calculated to give instantaneous firing rates which were then plotted at the end of each interspike interval and aligned to the onset of the stimulus train. Multiple stimulation trials were superimposed to generate the PSF. In eight cases, the firing rate profiles of more than one motor unit were used to construct the PSF for a total of 35 motor units that were fully analysed. Only trials with motor unit firing rates that were within 2 standard deviations of the mean pre‐stimulus rate for at least 1 s before the stimulation were included. For visualization purposes, the mean firing rate of the PSF was calculated with a moving‐window average over 10 ms of data and plotted every 1 ms. PSTHs were generated by counting the number of spikes occurring within a 10 ms bin and dividing by the number of stimulation trials to align count values between PSTHs having different trial numbers.
Surface EMG
Both excitatory and inhibitory components of the CMR were measured from the data collected on the second recording day. To mark the different components of the reflex, surface EMGs from the Axoscope files were digitally bandpass filtered (10–500 Hz, 0 phase shift) prior to rectification using a custom Matlab program. The mean level of background EMG over the 100 ms prior to stimulation was calculated (dotted horizontal lines in Fig. 1). Only the first 25 trials having a background EMG that fluctuated < 10% of MVC were averaged together. To compare CMRs between participants, the averaged EMG response was expressed as a percentage of the mean, pre‐stimulus background EMG for each participant (i.e. as 100% of the background EMG). To more readily appreciate how EMG activity during the CMR fluctuated from the pre‐stimulus background, the averaged EMG amplitude was then expressed as an absolute change from the mean background EMG (Fig. 1). For example, the mean background EMG was indicated as 0% change (Δ %BKD EMG) and any EMG activity above and below this was indicated by positive and negative values, respectively.
Figure 1. Components of the cutaneomuscular reflex .
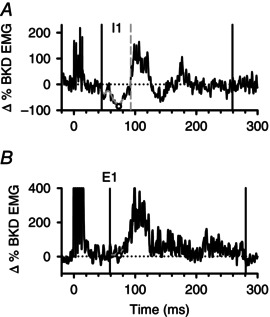
A, example of CMR that started with an EMG suppression (I1). The onset of I1 is marked by the first black vertical line and its termination by the dashed vertical grey line as the EMG falls below and returns to the mean pre‐stimulus EMG as marked by the dashed horizontal line. The pre‐stimulus EMG is expressed as a 0% Change (Δ) BKD EMG value. The maximum suppression of EMG activity between reflex onset and before 80 ms (I1max) is marked by the open circle taken from the moving‐window average (solid grey line). The termination of the CMR is marked by the second black vertical line when the EMG response returns to the pre‐stimulus baseline. B, same as in A but for a CMR with no EMG suppression and only an excitatory response with the onset of the early component (E1) marked by the first black vertical line. Here I1max is marked by the lowest point on the moving‐window average between the onset of the reflex and 80 ms. I1 = first inhibitory response; I1max = maximal amount of EMG suppression within I1; E1 = first excitatory response.
In these experiments, the majority of CMRs started with an initial onset of EMG suppression that was termed I1 (Fig. 1 A). The onset of I1 was determined visually as the time point when the EMG signal fell below 2 standard deviations of the mean, pre‐stimulus EMG for 20 ms or more (I1 onset is marked by the first vertical line in Fig. 1 A). The termination of the reflex was determined when the EMG signal returned to baseline (at the grey dashed vertical line). The magnitude of EMG suppression (I1ave) was measured as the average EMG during the duration of the I1 phase. To measure EMG suppression from mainly spinal mechanisms, the maximum amount of EMG suppression within I1 (I1max) was measured by applying a 10 ms moving‐window average (grey line) that was plotted every 0.5 ms to filter out very fast transients and by measuring the minimum value from response onset to 80 ms after stimulation (marked by circle in Fig. 1 A). An 80 ms cut‐off was used because components of the CMR 80 ms and earlier are probably spinal in origin and not activated by transcortical pathways (Nielsen et al. 1997). In some participants with CP, there was no clear I1 but rather a single excitatory response was evoked (Fig. 1 B). Here, the onset of EMG facilitation above the pre‐stimulus background was determined (E1: first solid vertical line) and the minimum value (I1max) out to 80 ms was measured from the window average (marked by circle). The termination of the CMR was measured as the time point when the EMG returned to the pre‐stimulus background (second solid vertical line, Fig. 1 A and B).
Statistics
Statistical analysis was performed in SPSS (version 21, IBM, Armonk, NY, USA). Differences in motor unit firing rates and features of the CMR (I1ave, I1max, etc.) between the CP and NI groups was determined using a Mann–Whitney U‐test because the data were not normally distributed as tested using the Shapiro–Wilk test. Median and range values are presented for these data. Mean and standard deviation (±SD) were used to describe normally distributed data. In the CP group, Spearman rank correlations were used to measure associations between motor assessments (GMFCS, FMSTOTAL, MVC) and EMG‐based physiological measures (I1ave, I1max). The alpha level for significance was set at 0.05.
Results
Functional motor impairments
The cohort of participants with bilateral, spastic CP had a range of functional motor impairments (Table 1). General gross motor disabilities, as measured by the GMFCS, ranged from visible impairments only when running (e.g. CP‐3 functioning at a GMFCS level I) to the inability to walk, requiring a power wheelchair with trunk support (e.g. CP‐12 functioning at a GMFCS level IV). In general, participants were somewhat evenly distributed between the four GMFCS categories with four in level I, five in levels II and III, and three in level IV. In specific measures of walking function, 4 out of 17 participants with CP required a wheelchair to ambulate 5, 50 or 500 m, as indicated by an FMSTOTAL score of 3, whereas 4/17 could walk independently at all distances using no aids, resulting in an FMSTOTAL score of ≥16. The remaining participants with CP had a mixture of walking abilities in between.
Approximately half of the participants with CP (9/17) could, at a minimum, plantarflex their ankle against gravity throughout their available range of motion, as reflected in an MRC score of 3 or more. The remaining participants with CP (8/17) could only plantarflex their ankle through the full range of movement with gravity eliminated (MRC 2) or only produce a flicker or trace contraction (MRC 1). Plantarflexor MRC scores were often not equal to dorsiflexor scores (Table 1). Despite the range of motor disabilities, all participants with CP had spasticity as their primary motor impairment. In agreement with the diagnosis of spastic CP, most participants (14/17) had discernible muscle tone in response to passive ankle dorsiflexion (mAsh ≥1) and all reported the presence of involuntary muscle spasms as assessed by Penn (Table 1).
Brain imaging
Two of the 16 participants with CP who underwent MRI had scans comparable to the NI participants with no obvious signs of brain injury or structural malformations (CP‐7, CP‐14, Fig. 2). This is in agreement with previous reports demonstrating that ∼13% of participants with CP have normal MRI scans (Towsley et al. 2011; Reid et al. 2014). The remainder had some evidence of PVWMI on both sides of the brain as marked by hyperintensities (white areas) on the FLAIR sequences and loss of white matter. Most participants with CP (13/16) had mild (+) or moderate (++) PVWMI as determined qualitatively by two radiologists blinded to clinical presentation. Five participants with CP had additional findings including three with CM, such as polymicrogyria, and one with a DGMI. Severe PVWMI (+++), particularly on the left but also present on the right, was observed in CP‐15 along with evidence suggestive of periventricular ischaemia.
Figure 2. MRI scans .
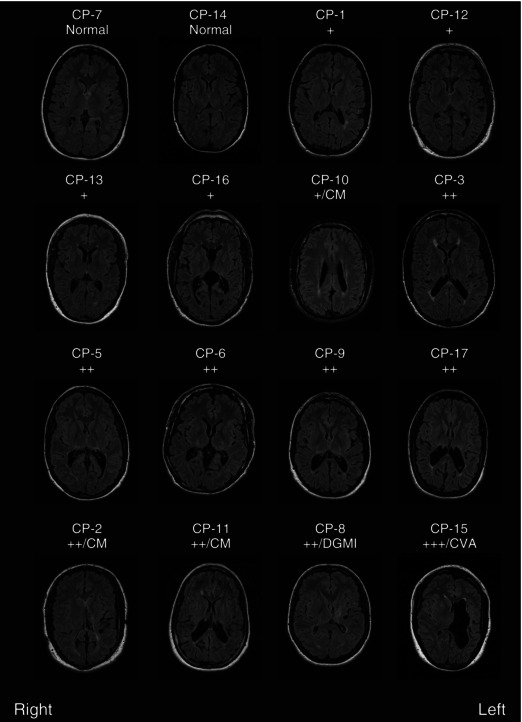
Example axial FLAIR slices from each participant with CP imaged. Slices were selected at similar axial locations across participants except for those with cerebral malformations (CM) or deep grey matter injury (DGMI) where the slice was chosen to best display the abnormality. All participants with abnormal imaging had evidence of mild (+), moderate (++) or severe (+++) periventricular white matter injury (PVWMI) on both sides of the brain. Five participants had additional findings: CP‐2 has partial agenesis of the posterior corpus callosum (a type of cerebral malformation) and evidence of a shunt tract; CP‐10 and CP‐11 have polymicrogyria (a type of cerebral malformation); CP‐8 has injury to the deep grey matter on the left; and CP‐15 has evidence of a perinatal cerebral vascular accident (CVA).
Sensory activation of motoneurons: PSFs
Similar to the clinical and MRI findings, there was a spectrum of PSF profiles in response to cutaneomuscular afferent stimulation in the CP group compared to the consistent responses produced in the NI group. Despite this, conditions for producing the PSF were well matched between the two groups. First, participants in both groups could maintain prolonged periods of steady motor unit discharge so that on average, 140 ± 109 (SD) trials were used to construct PSF profiles in the CP group and 158 ± 72 trials in the NI group (P = 0.17). Second, the median pre‐stimulus firing rate of the motor units in the NI group was 6.7 Hz (5.7–8.2 Hz) and appeared similar to the median rate maintained in the CP group at 6.8 Hz (4.9–10.0 Hz, P = 0.94). Third, the median stimulation intensity applied to the medial arch of the foot to evoke the CMR appeared similar between the two groups [NI: 20.2 mA (15.0–30.0 mA) vs. CP: 22.0 mA (11.0–42.6 mA), P = 0.57].
In all but one of the NI participants (12/13), there was a marked pause in motor unit firing near the onset of the CMR to reflect the activation of a strong IPSP. As shown in Fig. 3 A, in half of the NI participants, the pause in motor unit firing depicted in the PSF (bottom trace) and reflected in the corresponding suppression of EMG activity (top two traces) occurred without a prior excitatory (E1) response. This pause was also reflected in the reduced occurrence of motor unit discharge in the (PSTH, third trace, Fig. 3 A). In the other half of NI participants, a brief increase in firing rate, occurrence of motor unit discharge and EMG activity (E1) preceded the pause (Fig. 3 B). In both cases, the firing rates after the pause fell slightly below the mean pre‐stimulus rate (marked by dotted horizontal line), indicating a resumption of firing on the repolarization phase of the IPSP. Following the pause or decrease in motor unit discharge, firing rates would often increase above the pre‐stimulus rate to indicate a subsequent activation of an EPSP as readily seen in Fig. 3 B.
Figure 3. Representative PSFs in NI participants .
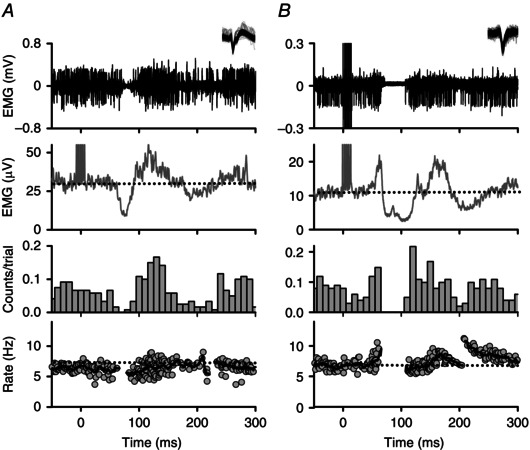
Representative examples of the two main types of PSFs measured in NI participants evoked from cutaneomuscular stimulation (at time = 0 ms). Within the PSF (bottom panel), each dot corresponds to the instantaneous firing rate of a motor unit and the thick black line is a moving‐window average of those rates. Unrectified intramuscular EMG and an inset of the superimposed, isolated motor unit are displayed in the top panel (duration of motor unit waveforms 2–3 ms). A, an initial IPSP is indicated by a pause in firing ∼60 ms following stimulation in the PSF. A pause in firing was noted when there was a break in the PSF and low to no counts in the PSTH (third panel). Dashed horizontal lines indicate mean pre‐stimulus EMG (second panel) and firing rates (bottom panel). B, PSF demonstrating an initial EPSP that preceded the IPSP with increased firing probability and rate near 50 ms following stimulation. Subsequent IPSP is indicated by a pause in firing starting at 60 ms and a resumption of firing near 120 ms with firing rates slightly below baseline. PSF = peri‐stimulus frequencygram; PSTH = peri‐stimulus time histogram.
In 8 of the 14 participants with CP, a pause (Fig. 4 A) or transient decrease in motor unit firing rates below the pre‐stimulus level (Fig. 4 B) also occurred at the start of the CMR, signifying a strong and mild activation, respectively, of an IPSP. Similar to the NI control group, following the resumption of firing after the pause, motor unit firing rates increased above the pre‐stimulus level, as shown in Fig. 4 A, to indicate the presence of a subsequent EPSP. The participant in Fig. 4 A had good motor function with a GMFCS score of I and FMSTOTAL score of 16 and, likewise, the participant in Fig 4 B had a GMFCS score of II and FMSTOTAL score of 14. Interestingly, in the remainder of the participants with CP (6/14), there were no consistent pauses or decreases in motor unit firing rates below the pre‐stimulus level. Rather, the PSF consisted only of a gradual increase and then return of motor unit firing rates to the pre‐stimulus level (Fig. 4 C and D). Such a PSF profile is indicative of a single, long‐duration EPSP and similar to the profiles previously recorded in participants with chronic spinal cord injury (Norton et al. 2008). The participants in Fig. 4 C and D had poor motor function, both having GMFCS scores of III and FMSTOTAL scores of 4 and 3, respectively. In all cases for both CP and NI groups, the mean motor unit firing rates returned to the mean pre‐stimulus rate by 600 ms.
Figure 4. Representative PSFs in participants with CP .
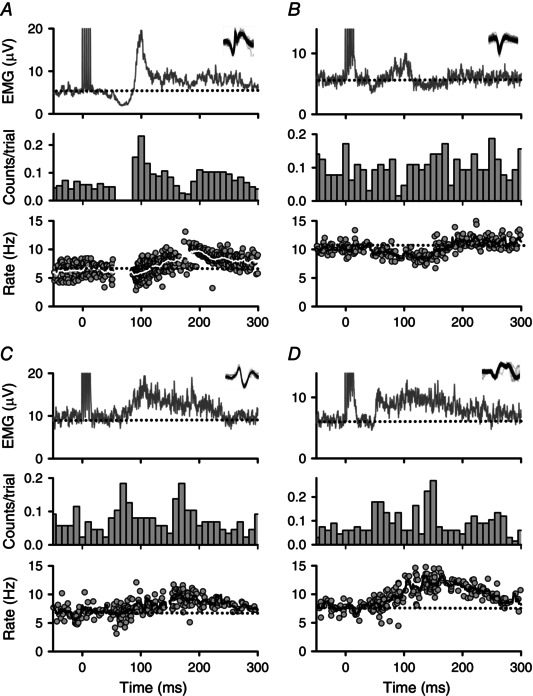
Same as in Fig. 3 but for representative PSFs from four participants with CP. A, PSF indicating a strong initial IPSP with a pause in firing starting near 60 ms and followed by an EPSP (CP‐2). Note the line in the PSF marking the moving‐window average is white for better visualization. B, PSF indicating a weak IPSP as marked by a decrease in the firing rate beginning near 45 ms following stimulation (CP‐17). C and D, pure EPSP indicated by increases in the firing rate starting near 50 ms following stimulation and continuing to 300 ms (CP‐13, CP‐6). PSF = peri‐stimulus frequencygram.
In summary, in 8 of the 14 participants with CP there was evidence for the sensory‐evoked activation of an IPSP followed by an EPSP in soleus motoneurons that was similar to NI controls. In the other 6 participants with CP, only an EPSP was evident from the PSF profiles, similar to that shown previously in participants with chronic spinal cord injury.
Sensory activation of motoneurons: surface EMG
Participants with CP who lacked evidence for the activation of an IPSP, namely a pause or decrease in motor unit firing rates below the pre‐stimulus level, also had more severe deficits in voluntary muscle activation and walking function. Thus, to examine if the strength of IPSP activation was associated with motor dysfunction, we quantified the amount of early‐onset EMG suppression (I1) within the CMR. Based on the PSF profiles we were confident that the magnitude of early‐onset EMG suppression was probably related to the magnitude of IPSP activation, even for reflexes where EMG suppression occurred with a prior excitation (E1). To avoid extraneous sensory inputs that could affect the magnitude of EMG suppression, CMRs were measured on a separate recording day without the insertion of fine wires into the muscle. Moreover, all participants were seated to better control positioning of the ankle and knee.
In all NI participants, removing the sensory stimulation from the intramuscular wire abolished the occurrence of the early‐onset excitation (E1) that could sometimes precede I1, as shown for three representative participants in Fig. 5 A. Thus, the amount of EMG suppression was probably produced by the activation of an IPSP and not influenced by motoneuron refractoriness or discharge synchronization as would occur when there is a prior motoneuron excitation (e.g. E1). To measure the amount of EMG suppression that was mediated at a spinal latency, the maximum amount of EMG suppression from response onset to 80 ms following stimulation was determined (I1max), as shown by the dots in Fig. 5 A (see Surface EMG in Methods for rationale). The presence of a marked EMG suppression was very consistent across all NI controls and the median I1max was 69.5% lower than the pre‐stimulus background EMG (i.e. −69.5% Δ BKD EMG, see Table 2 for median and range values).
Figure 5. Cutaneomuscular reflexes: surface EMG .
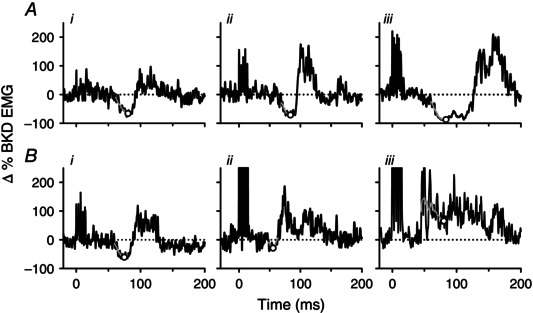
A, black lines represent the averaged surface EMG response (25 trials) to cutaneomuscular stimulation at time = 0 ms for three representative NI participants (i to iii). The grey line represents the moving‐window average from response onset to 80 ms following stimulation with the lowest point on the moving‐window average (I1max) indicated by the open circle. EMG values are normalized to the mean pre‐stimulation EMG (marked by the dotted horizontal line) and expressed as an absolute change (Δ) from that value (mean pre‐stimulation EMG = 0% Δ). B, same as in A but for three representative participants with CP showing progressively lower amounts of EMG suppression (i to ii) and EMG facilitation (iii). I1max = maximal amount of EMG suppression within the first inhibitory response.
Table 2.
Characteristics of the cutaneomuscular reflex
| Reflex components | CP | NI | P‐value |
|---|---|---|---|
| I1max (Δ % BKD EMG) | −36.1 (−66.7 to 68.0) | −69.5 (−87.7 to −30.3) | <0.001 |
| I1ave (Δ % BKD EMG) | −26.4 (−46.3 to 0.0) | −48.6 (−63.6 to −21.7) | 0.001 |
| I1 onset (ms) | 58.4 (40.7 to 60) | 57.4 (43.3 to 70.9) | 0.98 |
| Cutaneomuscular reflex termination (ms) | 286 (89 to 825) | 273 (152 to 449) | 0.30 |
Median and range values for various components of the CMR in both the CP and the NI groups and the associated P‐values from the Mann–Whitney U‐test comparisons between the two groups. I1max = maximal amount of EMG suppression within the first inhibitory response. I1ave = average EMG during the first inhibitory response.
In 10 of the 17 participants with CP, the CMR started with a sustained period of EMG suppression (I1) similar to that measured in NI participants. The amount of early‐onset EMG suppression was strongest in the four participants with CP who could walk at least 500 m with no gait aids (e.g. Fig. 5 Bi). In the other six participants with CP who had an initial I1 (6/10), the amount of EMG suppression was smaller, as shown for a representative example in Fig. 5 Bii. Very little to no sustained suppression of EMG activity was present in the remaining participants with CP (7/17). Instead, the CMR consisted solely of an excitatory response (e.g. Fig. 5 Biii) and this typically occurred in participants requiring wheeled mobility.
Overall, the maximum amount of EMG suppression (I1max, see dots in Fig. 5) was less in the CP group at −36.1% compared to the NI control group at −69.5% (P < 0.001). A positive I1max occurred when the EMG activity from response onset to 80 ms after stimulation did not fall below the pre‐stimulus background (as in Fig. 5 Biii). Likewise, the average EMG over the entire period of EMG suppression (I1ave) was also smaller in the CP group with a median I1ave of −26.4% compared to the NI control group at −48.6% (P = 0.001, Table 2). Note that when there was no visible I1 in the participants with CP, an I1ave value of 0 was given. When present, the median onset of I1 was 58.4 ms in the CP group and appeared similar to the onset of I1 in the NI control group at 57.4 ms (P = 0.56, Table 2). Likewise, the total duration of the CMR appeared similar between the two groups with the reflex terminating at 279.2 ms following stimulation in the CP group and at 285.7 ms in the NI group (P = 0.78, Table 2).
Associations of spinal inhibition to motor function in CP
To determine if our estimate of spinally mediated IPSPs (i.e. I1max) was related to the functional motor abilities of the participants with CP, both the FMSTOTAL and the GMFCS for each participant with CP was plotted against I1max. There was a significant negative association between I1max and the FMSTOTAL scores (r = −0.82, P < 0.001, Spearman rank) indicating that as the walking ability of the participants with CP was more affected (low FMS scores), there was less EMG suppression (more positive I1max values) and probably fewer IPSPs activated in the motoneurons (left graph, Fig. 6). Significant associations were also present for each individual FMS score at 5, 50 and 500 m (P all ≤ 0.001). In participants with CP who relied on wheeled mobility for even short distances (having FMSTOTAL = 3), I1max was more positive than any value recorded in the NI group. These participants also had evidence of pure EPSPs in their PSF profiles. In the participants with CP who could walk for at least 500 m with no gait aids (FMSTOTAL ≥ 16), I1max values were all within the 25th and 75th percentile limits of the NI control group (i.e. within the grey bar of Fig. 6). These participants also had evidence of strong IPSP activation with marked pauses in motor unit firing in the PSF. There was also a positive association between GMFCS and I1max (r = 0.72, P < 0.001, Spearman rank, right graph, Fig. 6), signifying that the greater impairment in functional sitting and walking abilities (as indicated by a higher GMFCS score), the lower the EMG suppression (more positive I1max) from reduced IPSP activation. Associations remained significant when the five participants with CP who had additional MRI findings (e.g. CM, DGMI and periventricular ischaemia) were removed from the group (all P < 0.008). Similar to the FMSTOTAL and GMFCS, there was a significant association between I1max and MVC that was generated during plantarflexion (not shown, r = −0.56, P = 0.02). Significant associations were also produced when comparing these clinical measures with the average EMG measured across the entire EMG suppression period, i.e. I1ave (all P < 0.01).
Figure 6. Spinal inhibition and motor function .
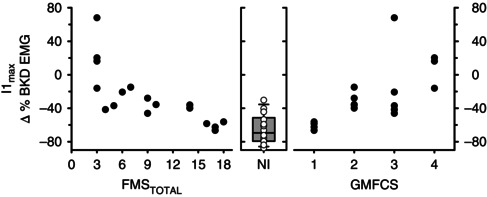
The magnitude of I1max is plotted against FMSTOTAL (left panel) and GMFCS (right panel) in the CP group (n = 17). The box plot in the middle panel illustrates the median (thick black line), 25th and 75th percentiles (box bounds), and 95th and 5th percentiles (whiskers) of I1max in the NI group. The scatter of all data points for the NI group (n = 14) is also displayed over the box plot. FMSTOTAL = total Functional Mobility Scale, GFMCS = Gross Motor Functional Classification System, I1max = maximal amount of EMG suppression within the first inhibitory response.
Discussion
General summary
Similar to studies examining the activation of IPSPs in soleus motoneurons from tendon afferent stimulation (Binboga et al. 2011; Rogasch et al. 2012), there was consistent evidence for the activation of IPSPs in the PSF profiles of NI controls in response to cutaneomuscular afferent stimulation. In contrast, evidence for the activation of an IPSP was observed in only half of the participants with CP and, in the other half, only a pure EPSP was indicated in the PSF profiles, consistent with findings from surface EMG recordings in children and adolescents with CP (Gibbs et al. 1999). As discussed below, the presence and magnitude of sensory‐evoked IPSPs in the participants with CP was associated with their gross motor and walking function, suggesting that post‐synaptic inhibition of motoneurons may be dependent upon motor activity.
PSFs and what they tell us about the profile of post‐synaptic potentials
Although there are some limits with the PSF technique in providing an accurate measure of IPSP or EPSP amplitude (Powers & Turker, 2010 b), data from intracellular experiments confirm that the presence of a long‐lasting (>50 ms) EPSP or IPSP can be accurately represented by the modulation in firing rate of a tonically discharging motor unit/motoneuron (Turker & Powers, 2005; Norton et al. 2008; Powers & Turker, 2010 a). For example, during both the rising and the falling phase of a single EPSP, the time it takes for a tonically discharging motoneuron to reach firing threshold is shortened, producing elevated firing rates above the pre‐stimulus background on both the rising and the falling phase of the EPSP (Turker & Powers, 1999; Norton et al. 2008). A pure, single EPSP evoked from cutaneomuscular stimulation was demonstrated in the PSF profiles for approximately half of the participants with CP (e.g. Fig. 4 C and D). Even though the baseline firing rates were low at ∼7 Hz (Powers & Turker, 2010 a), a clear EPSP‐like profile emerged in the PSF. This may have been possible because at 7 Hz the motor unit would fire every 140 ms so that two action potentials could occur over the course of a single 300 ms EPSP. Coupled with a large number of PSF trials (∼150), a sufficient number of sampling points were probably obtained to measure the underlying membrane potential of the EPSP. Moreover, the PSF indicated a pure, single EPSP unlike the multiple peaks produced in the PSTH as a result of synchronization in motoneuron firing.
The profile of more complicated, multi‐component post‐synaptic potentials can also be reflected in the PSF profile. For example, when using current injection to mimic a strong IPSP followed by an EPSP, a PSF is produced whereby a sustained pause in motor unit firing occurs during the falling phase of the IPSP, especially when the rate of change in membrane potential is 100 mV s−1 or faster as this flattens and prolongs the upward trajectory of the afterhyperpolarization (Turker & Powers, 2003, 2005; Norton et al. 2008). A cluster of resumed firing is then often produced near or slightly below the mean pre‐stimulus rate as the rising phase of the IPSP accelerates the afterhyperpolarization potential to firing threshold (Norton et al. 2008). The firing rate then increases above the pre‐stimulus level during both the rising and the falling phase of the subsequent EPSP. A post‐synaptic potential having an IPSP followed by an EPSP was reflected in the PSFs of both the NI control group (e.g. Fig. 3) and in some of the participants with CP (Fig. 4 A).
In summary, there is robust intracellular data to support our claim for the presence or absence of an IPSP from the PSF profiles measured in the NI and CP groups in this study. However, it is more difficult to measure the amplitude of IPSPs from the PSF profile because the motor unit often stops firing with the rate going to 0 Hz. Instead, as discussed below, we quantified the amount of IPSP activation from the surface EMG to determine if the strength of motoneuron inhibition was related to motor function in the participants with CP.
Estimating early‐onset IPSP amplitude from surface EMG
Because surface EMG activity is dominated by the occurrence, and not frequency, of motor unit discharge, its profile during a reflex response can give misleading information concerning the profile of the post‐synaptic potential in the motoneuron (Powers & Turker, 2010 a). This is especially problematic during the later components of a post‐synaptic potential given that a large transient input, such as an EPSP or IPSP, can synchronize the discharge of a tonically firing motoneuron to produce false indications of repeated IPSPs and EPSPs. However, this is not a problem for the first component of a post‐synaptic potential whereby the surface EMG (and PSTH) reflect what is happening to the post‐synaptic potential because it is not influenced by a prior transient event. We propose that our estimation of IPSP amplitude from measuring the amplitude of the surface EMG during the first component of the CMR (I1max) is reasonable because a large IPSP will strongly suppress the occurrence of motor unit discharge (probably in multiple motoneurons), a small IPSP will reduce it to a lesser extent and an EPSP will increase the occurrence of motor unit discharge to modify, correspondingly, the surface EMG signal (Turker & Powers, 1999, 2003).
In the surface EMG profiles where we measured I1max (for both NI and CP participants), a prior excitatory response (E1) was not present when the first component of the CMR contained a sustained, marked suppression of EMG activity (e.g. Fig. 6). Thus, the EMG suppression in these cases was not affected by motoneuron refractoriness or firing synchronization from a prior excitatory response and probably resulted from the activation of an initial IPSP as reflected in the PSF profiles for these participants. In the remainder of the trials, which occurred in the CP group only, a sustained increase in surface EMG activity was measured out to 80 ms following stimulation (positive or near positive I1max values) and probably reflected the activation of an EPSP, as also indicated in the PSF profiles of these same participants. We took the measurement of I1max out to 80 ms not only to reflect changes in motoneuron activation from spinal mechanisms (Nielsen et al. 1997) but also to avoid effects from motoneuron synchronization which, at a mean discharge rate of 7 Hz (140 ms interspike interval) and with a reflex onset near 60 ms, would have occurred near 200 ms following stimulation. Thus, based on the supporting PSF profiles, we are fairly confident that the magnitude of surface EMG suppression within the first 80 ms from stimulation onset is a reasonable estimate of the magnitude of IPSP (or EPSP) activation from within the entire soleus motoneuron pool.
IPSPs and motor function
The presence and magnitude of IPSP activation was strongest in participants with CP who had good motor function (e.g. daily walkers) and absent in those with poor motor function (e.g. wheelchair dependent). We cannot determine if decreases in IPSP activation contributed to the reduced motor function of the participants with CP or if motor activity itself is necessary for the development and maintenance of post‐synaptic inhibition in motoneurons. Work in rat models provides evidence for the importance of motor activity in maintaining the activation of IPSPs in motoneurons. For example, following a complete spinal cord injury where motor activity is greatly reduced, insertion of potassium‐chloride (KCC2) transporters into the motoneuron membrane is reduced (Boulenguez et al. 2010). This lowers the intracellular chloride concentration so that when glycine or GABA receptors are activated, chloride flows out of the motoneuron to reduce or abolish the activation of IPSPs. Increased activation of the spinal cord from passive cycling increases the insertion of KCC2 transporters into the motoneuron membrane and associated GABA‐mediated inhibition (Cote et al. 2014). Likewise, in patients with incomplete spinal cord injury, the excitability of spinal inhibitory pathways can be facilitated by daily locomotor training (Manella & Field‐Fote, 2013; Knikou & Mummidisetty, 2014; Zewdie et al. 2015). Thus, it would be interesting to determine if intensive motor training in the CP participants with poor motor function could increase the amount of post‐synaptic inhibition of motoneurons and if this would decrease symptoms of spasticity in addition to improving motor control.
Regardless of the cause and effect, reduced activation of inhibitory interneurons and/or enhanced activation of excitatory interneurons within the CMR pathway may impair voluntary movements in individuals with CP. During the generation of voluntary movements, descending and other peripheral afferent pathways may also utilize spinal interneurons that are contained within the CMR pathway studied here (Geertsen et al. 2011). For example, spinal interneurons that are activated by corticospinal pathways to the soleus muscle are implicated in setting the excitability levels of agonist/antagonist motoneuron pairs to aid in fast transitions of dorsi‐ and plantarflexion movements (Geertsen et al. 2010). An imbalance of excitation and inhibition in CMR pathways may directly impair the timing of agonist/antagonist muscle activation to explain, in part, why participants with CP have difficulty in making rapid, alternating movements (Milner‐Brown & Penn, 1979). Likewise, reduced inhibition in cutaneous reflex pathways from the foot may also impede the descending and afferent control of both posture (Aniss et al. 1992) and walking (Bouyer & Rossignol, 2003) where cutaneous reflexes are thought to stabilize the ankle and mediate fine control of foot placement, respectively.
Effects of reduced IPSPs on spasticity
A reduced level of IPSP activation and predominance of EPSP activation by sensory inputs may contribute to the activation of involuntary muscle spasms in people with spastic CP. Sustained involuntary activity during a muscle spasm is produced by the activation of persistent inward currents in the motoneuron that are triggered by a prolonged (>500 ms) EPSP evoked by cutaneous afferents in a rat model of spinal cord injury (Li et al. 2004). Similarly, stimulation of cutaneomuscular afferents in participants with incomplete spinal cord injury failed to evoke IPSPs (based on PSF profiles) but instead produced an ∼1000 ms EPSP which probably facilitated the triggering of persistent inward currents and involuntary muscle spasms (Norton et al. 2008). The reduced sensory‐evoked IPSP and facilitated EPSP activation observed in the participants with CP could also facilitate the triggering of involuntary muscle spasms reported in this group. Although not as prolonged compared to spinal cord injury, the 300 to 600 ms pure EPSPs evoked from cutaneomuscular stimulation in some of the participants with CP, if temporally summed, may trigger the activation of persistent inward currents in motoneurons to produce self‐sustained activity and prolonged, involuntary muscle spasms. Such pure EPSPs would more readily active persistent inward currents compared to a mixed IPSP–EPSP as measured in controls.
Two of the participants with CP were on anti‐spastic medications that theoretically could have enhanced inhibition in the CMR (Table 1). Although intermittently taking clonazepam to enhance GABAa receptor activity, participant CP‐11 still had no evidence of IPSP activation. In contrast, CP‐2 who was on a low dose of baclofen (10 mg, two times a day), a GABAb receptor agonist to enhance pre‐synaptic inhibition, had evidence of strong IPSP activation (−59%), similar to other participants with CP who had good motor function and who were not on anti‐spastics. Thus, it is difficult to determine with certainty if the strong IPSP activation in this participant was due to baclofen or preserved motor function.
Conclusion
We have found that in some adult participants with CP, post‐synaptic inhibition of motoneurons is reduced or lost, most notably in those with more severe motor deficits. This is in contrast to previous studies showing that post‐synaptic inhibitory pathways measured at rest, such as reciprocal inhibition, are normal in CP (Achache et al. 2010) and illustrates the importance of measuring the presence or strength of inhibition during voluntary contractions. Although spasticity in people with CP is multifactorial, ranging in causes from muscle and joint tissue changes to abnormal activation of brain and spinal cord pathways, data from this study suggest that in adults with CP, many years of reduced motor activity may affect how spinal motoneurons transduce sensory information, which may contribute to motor dysfunction in this population.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
The neurophysiology experiments were performed in the laboratory of M.A.G. and the MRI at the Peter S. Allen MR Research Centre at the University of Alberta. M.A.G. and E.G.C. contributed to conceiving, designing and conducting the electrophysiological experiments as well as analysis of the electrophysiological data. D.J.E. and D.T.J. designed and analysed the MRI data. All authors contributed to the writing and editing of the manuscript and approved the final version submitted for publication.
Funding
Funding for this work was provided by CIHR MOP106549. E.G.C. was supported through the Clinical Investigator Program and Alberta Innovates: Health Solutions 201100202.
Authors’ Translational Perspective
Abnormal activation of motoneurons in the spinal cord by sensory pathways is thought to contribute to impaired motor control and spasticity in persons with cerebral palsy (CP). We examined how spinal motoneurons respond to sensory inputs from the foot in a group of participants with CP who had different degrees of motor dysfunction. In the strongest participants who walked regularly, there was no evidence of abnormal sensory activation of their motoneurons. This might have resulted from less injury to descending systems that regulate spinal inhibition or because these participants generated sufficient motor activity to develop and maintain motoneuron inhibition. The latter possibility introduces the idea that intensive motor training may improve the control over motoneuron inhibition and spinal cord excitability as a means to reduce and treat spasticity in CP. Moreover, the assessment of sensory‐evoked motoneuron inhibition may also be used as a screening tool. For example, selective dorsal rhizotomies are used to reduce aberrant sensory activation of the spinal cord to reduce spasticity in CP. The ability to walk is a commonly utilized criterion for such surgeries (Grunt et al. 2014). However, we demonstrate that individuals who walk regularly have normal sensory activation of their motoneurons. Thus, selecting only individuals who exhibit abnormal sensory‐evoked activation of motoneurons may further optimize the selection criterion for dorsal rhizotomies. In summary, our findings shed light on spinal mechanisms involved in the production of spasticity and suggest a potential screening and assessment tool for interventions aimed at reducing spasticity in people with CP.
Acknowledgements
We thank the participants for their involvement in this work and the administrative and technical contributions of Ms Jennifer Duchcherer. We thank Drs Kelvin Jones, Francois Roy and David Collins for their advice and helpful comments on the manuscript.
References
- Achache V, Roche N, Lamy JC, Boakye M, Lackmy A, Gastal A, Quentin V & Katz R (2010). Transmission within several spinal pathways in adults with cerebral palsy. Brain 133, 1470–1483. [DOI] [PubMed] [Google Scholar]
- Adair B, Said CM, Rodda J & Morris ME (2012). Psychometric properties of functional mobility tools in hereditary spastic paraplegia and other childhood neurological conditions. Dev Med Child Neurol 54, 596–605. [DOI] [PubMed] [Google Scholar]
- Aniss AM, Gandevia SC & Burke D (1992). Reflex responses in active muscles elicited by stimulation of low‐threshold afferents from the human foot. J Neurophysiol 67, 1375–1384. [DOI] [PubMed] [Google Scholar]
- Binboga E, Prasartwuth O, Pehlivan M & Turker KS (2011). Responses of human soleus motor units to low‐threshold stimulation of the tibial nerve. Exp Brain Res 213, 73–86. [DOI] [PubMed] [Google Scholar]
- Bohannon RW & Smith MB (1987). Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67, 206–207. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean‐Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M & Vinay L (2010). Down‐regulation of the potassium‐chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med 16, 302–307. [DOI] [PubMed] [Google Scholar]
- Bouyer LJ & Rossignol S (2003). Contribution of cutaneous inputs from the hindpaw to the control of locomotion. I. Intact cats. J Neurophysiol 90, 3625–3639. [DOI] [PubMed] [Google Scholar]
- Cote MP, Gandhi S, Zambrotta M & Houle JD (2014). Exercise modulates chloride homeostasis after spinal cord injury. J Neurosci 34, 8976–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Johnsen LL, Biering‐Sorensen F & Nielsen JB (2003). Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 126, 495–507. [DOI] [PubMed] [Google Scholar]
- Downing AL, Ganley KJ, Fay DR & Abbas JJ (2009). Temporal characteristics of lower extremity moment generation in children with cerebral palsy. Muscle Nerve 39, 800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eek MN, Tranberg R & Beckung E (2011). Muscle strength and kinetic gait pattern in children with bilateral spastic CP. Gait Posture 33, 333–337. [DOI] [PubMed] [Google Scholar]
- Elder GC, Kirk J, Stewart G, Cook K, Weir D, Marshall A & Leahey L (2003). Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol 45, 542–550. [DOI] [PubMed] [Google Scholar]
- Evans AL, Harrison LM & Stephens JA (1991). Cutaneomuscular reflexes recorded from the first dorsal interosseous muscle of children with cerebral palsy. Dev Med Child Neurol 33, 541–551. [DOI] [PubMed] [Google Scholar]
- Geertsen SS, van de Ruit M, Grey MJ & Nielsen JB (2011). Spinal inhibition of descending command to soleus motoneurons is removed prior to dorsiflexion. J Physiol 589, 5819–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geertsen SS, Zuur AT & Nielsen JB (2010). Voluntary activation of ankle muscles is accompanied by subcortical facilitation of their antagonists. J Physiol 588, 2391–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J, Harrison LM, Stephens JA & Evans AL (1999). Cutaneomuscular reflex responses recorded from the lower limb in children and adolescents with cerebral palsy. Dev Med Child Neurol 41, 456–464. [PubMed] [Google Scholar]
- Graham HK, Harvey A, Rodda J, Nattrass GR & Pirpiris M (2004). The Functional Mobility Scale (FMS). J Pediatr Orthop 24, 514–520. [DOI] [PubMed] [Google Scholar]
- Grunt S, Fieggen AG, Vermeulen RJ, Becher JG & Langerak NG (2014). Selection criteria for selective dorsal rhizotomy in children with spastic cerebral palsy: a systematic review of the literature. Dev Med Child Neurol 56, 302–312. [DOI] [PubMed] [Google Scholar]
- Hislop HJ, Avers D, Brown M & Daniels L (2014). Daniels and Worthingham's Muscle Testing: Techniques of Manual Examination and Performance Testing. Elsevier/Saunders, St. Louis, MO. [Google Scholar]
- Katz R & Pierrot‐Deseilligny E (1999). Recurrent inhibition in humans. Prog Neurobiol 57, 325–355. [DOI] [PubMed] [Google Scholar]
- Knikou M & Mummidisetty CK (2014). Locomotor training improves premotoneuronal control after chronic spinal cord injury. J Neurophysiol 111, 2264–2275. [DOI] [PubMed] [Google Scholar]
- Leonard CT, Sandholdt DY, McMillan JA & Queen S (2006). Short‐ and long‐latency contributions to reciprocal inhibition during various levels of muscle contraction of individuals with cerebral palsy. J Child Neurol 21, 240–246. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA & Bennett DJ (2004). Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol 91, 767–783. [DOI] [PubMed] [Google Scholar]
- Manella KJ & Field‐Fote EC (2013). Modulatory effects of locomotor training on extensor spasticity in individuals with motor‐incomplete spinal cord injury. Restor Neurol Neurosci 31, 633–646. [DOI] [PubMed] [Google Scholar]
- Milner‐Brown HS & Penn RD (1979). Pathophysiological mechanisms in cerebral palsy. J Neurol Neurosurg Psychiatry 42, 606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D'Amico J, Gorassini MA & Bennett DJ (2011). Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5‐HT1B and 5‐HT1F receptors. J Neurophysiol 106, 925–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Petersen N & Fedirchuk B (1997). Evidence suggesting a transcortical pathway from cutaneous foot afferents to tibialis anterior motoneurones in man. J Physiol 501, 473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton JA, Bennett DJ, Knash ME, Murray KC & Gorassini MA (2008). Changes in sensory‐evoked synaptic activation of motoneurons after spinal cord injury in man. Brain 131, 1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palisano RJ, Rosenbaum P, Bartlett D & Livingston MH (2008). Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol 50, 744–750. [DOI] [PubMed] [Google Scholar]
- Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B & Kroin JS (1989). Intrathecal baclofen for severe spinal spasticity. N Engl J Med 320, 1517–1521. [DOI] [PubMed] [Google Scholar]
- Pinter MJ, Burke RE, O'Donovan MJ & Dum RP (1982). Supraspinal facilitation of cutaneous polysynaptic EPSPs in cat medical gastrocnemius motoneurons. Exp Brain Res 45, 133–143. [DOI] [PubMed] [Google Scholar]
- Poon DM & Hui‐Chan CW (2009). Hyperactive stretch reflexes, co‐contraction, and muscle weakness in children with cerebral palsy. Dev Med Child Neurol 51, 128–135. [DOI] [PubMed] [Google Scholar]
- Powers RK & Turker KS (2010. a). Deciphering the contribution of intrinsic and synaptic currents to the effects of transient synaptic inputs on human motor unit discharge. Clin Neurophysiol 121, 1643–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK & Turker KS (2010. b). Estimates of EPSP amplitude based on changes in motoneuron discharge rate and probability. Exp Brain Res 206, 427–440. [DOI] [PubMed] [Google Scholar]
- Reid SM, Carlin JB & Reddihough DS (2011). Distribution of motor types in cerebral palsy: how do registry data compare? Dev Med Child Neurol 53, 233–238. [DOI] [PubMed] [Google Scholar]
- Reid SM, Dagia CD, Ditchfield MR, Carlin JB & Reddihough DS (2014). Population‐based studies of brain imaging patterns in cerebral palsy. Dev Med Child Neurol 56, 222–232. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Burne JA & Turker KS (2012). Comparison of the inhibitory response to tendon and cutaneous afferent stimulation in the human lower limb. J Neurophysiol 107, 564–572. [DOI] [PubMed] [Google Scholar]
- Shevell MI, Dagenais L & Hall N (2009). The relationship of cerebral palsy subtype and functional motor impairment: a population‐based study. Dev Med Child Neurol 51, 872–877. [DOI] [PubMed] [Google Scholar]
- Towsley K, Shevell MI & Dagenais L (2011). Population‐based study of neuroimaging findings in children with cerebral palsy. Eur J Paediatr Neurol 15, 29–35. [DOI] [PubMed] [Google Scholar]
- Turker KS & Powers RK (1999). Effects of large excitatory and inhibitory inputs on motoneuron discharge rate and probability. J Neurophysiol 82, 829–840. [DOI] [PubMed] [Google Scholar]
- Turker KS & Powers RK (2003). Estimation of postsynaptic potentials in rat hypoglossal motoneurones: insights for human work. J Physiol 551, 419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turker KS & Powers RK (2005). Black box revisited: a technique for estimating postsynaptic potentials in neurons. Trends Neurosci 28, 379–386. [DOI] [PubMed] [Google Scholar]
- Zewdie ET, Roy FD, Yang JF & Gorassini MA (2015). Facilitation of descending excitatory and spinal inhibitory networks from training of endurance and precision walking in participants with incomplete spinal cord injury. Prog Brain Res 218, 127–155. [DOI] [PubMed] [Google Scholar]


