Electrical synapses are most commonly known by their role in the adult nervous system, where they generally promote the coordinated activity of groups of neurons (Connors & Long, 2004). However, electrical synapses play a critical role in the formation of neural circuits during development. A wealth of data obtained for both invertebrate and vertebrate nervous systems indicates that electrical synapses lead to the formation of neural circuits via interactions with chemical synapses (for review see Pereda, 2014). Consistent with this role, a new study by Zolnik & Connors (2016), reported in this issue of The Journal of Physiology, now shows that disruption of electrical transmission alters the inhibitory circuitry in the mammalian thalamus.
Electrical synaptic transmission is mediated by groups of intercellular channels known as gap junctions. In vertebrates, these channels are formed by proteins called connexins, encoded by a family of over 20 genes of which only a few are expressed in neurons. Because of its almost exclusive neuronal expression and widespread distribution, connexin 36 (Cx36) is considered the main neuronal connexin in the mammalian brain. GABAergic neurons in the reticular nucleus of the thalamus (TRN) are interconnected by Cx36‐containing electrical synapses and powerfully inhibit ventrobasal (VB) thalamic relay neurons. The thalamic relay nuclei contain groups of neurons that process peripheral sensory information and relay it to specific regions of the cortex (Fig. 1 A). Amongst them, the ventrobasal complex is dedicated to nociception, and inhibition by TRN neurons is thought to shape the receptive field of VB neurons.
Figure 1. Connexin 36‐containing electrical synapses influence the development of inhibitory circuits in the thalamus .
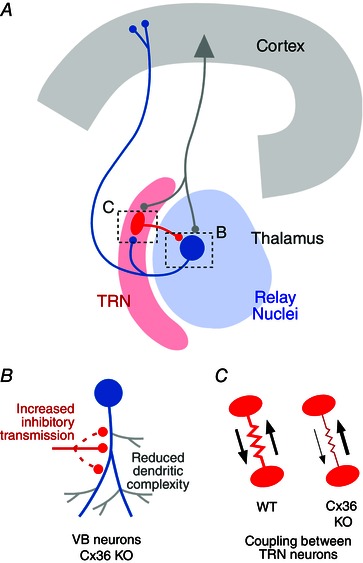
A, schematic representation of the involved thalamic networks (TRN: thalamic reticular nucleus (red); relay nuclei (blue)) and their functional relationship with the cortex. Dashed boxes represent the areas at which the changes illustrated in B and C were found. B, ventrobasal (VB) neurons (blue) at relay nuclei in Cx36 KO mice exhibit reduced dendritic complexity and increased inhibitory transmission from TRN cells (depicted for illustration purposes as an increase in the number of connections). TRN projections onto VB neurons are also less divergent (not shown). C, in the absence of Cx36, electrical transmission between TRN neurons is weaker and asymmetric (right) when compared with WT animals (left).
By combining functional and anatomical analysis in mice lacking Cx36 (Cx36 knockout (KO) mice) the authors showed that, in addition to the expected reduction in electrical coupling, the synaptic connectivity between TRN and VB neurons was substantially altered. Functional analysis of GABAergic responses was consistent with TRN neurons having either new synaptic contacts or more release sites at the already existing contacts as well as less divergent inputs onto VB neurons. Furthermore, anatomical reconstructions revealed that VB neurons in Cx36 KO mice had simpler dendritic trees (Fig. 1 B). While the presence of dendro‐dendritic gap junctions is required for proper dendritic formation in the leech (Baker et al. 2013), the much lower incidence of electrical synapses between VB neurons suggests that changes in dendritic morphology, together with those observed in GABAergic transmission, are likely to be secondary to the impaired electrical transmission between TRN neurons, as the formation of these connections precedes the development of their inhibitory connections on VB neurons. The nature of the signal exchanged between TRN neurons via their electrical synapses under normal conditions remains unknown. Coordinated electrical activity was shown, in some cases, to be required for synapse elimination suggesting a role for electrical coupling, whereas diffusion of regulatory molecules was suggested to be required in others (reviewed in Pereda, 2014). Non‐conductive functions of gap junctions (adhesive properties) could also contribute to the function of electrical synapses during development. It is possible that the changes triggered by the absence of Cx36 are not restricted to the examined synapses but involve downstream connections, such as projections of VB neurons on the cortex.
Another exciting aspect of the study is the properties of electrical transmission between TRN neurons in the Cx36 KO mice (Fig. 1 C). Consistent with the presence of Cx36 at TRN–TRN electrical synapses, the incidence of electrical coupling in Cx36 KO mice was markedly reduced. However, although weaker, electrical coupling was still present in a significant number of the recorded pairs suggesting the presence of gap junctions formed by other connexins. Moreover, electrical transmission in Cx36 KO mice had different biophysical properties. In contrast to Cx36‐containing synapses, which are virtually voltage insensitive over the explored range of transjunctional voltage, junctional currents in the Cx36 KO mice exhibited faster voltage‐dependent decay kinetics. In addition, conductance at these junctions was most often asymmetric, indicating that electrical synapses in Cx36 KO mice are rectifying. The presence of electrical rectification has been associated with heterotypic gap junctions at which intercellular channels are formed by hemichannels of dissimilar molecular composition, raising the possibility that more than one connexin is present at these electrical contacts. The identity of the involved connexins remains undetermined. Fast voltage‐dependent kinetics is typical of gap junctions containing Cx45, a neuronal connexin reported to be present in the developing thalamus; Cx30.2 was also reported in thalamus and is capable of forming heterotypic channels with Cx45, making these two connexins the prime candidates.
The presence of electrical coupling in Cx36 KO mice offers interesting insights into the properties of electrical transmission. What does this coupling represent? On one hand, coupling might represent an attempt of functional compensation by new gap junctions formed by different connexin isoforms. On the other hand, the coupling could represent electrical synapses that are normally obscured by the presence of the more numerous Cx36‐containing junctions. Interestingly, Cx36 and Cx45 were proposed to coexist in the same GJ plaques, forming bi‐homotypic channels (Li et al. 2008), as Cx36 and Cx45 are incompatible for forming heterotypic channels. In this context, coupling in the TRN of Cx36 KO mice could represent a compensation by the remnant connexin(s). This possibility is supported by observations on gap junctions in the cerebellum, where abundant Cx36 at gap junctions is co‐localized with a low level of Cx45, and where expression of Cx45 is dramatically increased at gap junctions in Cx36 KO mice (J. I. Nagy, personal communication). A provocative possibility would be that such compensation actually represents the expression of a dynamic regulatory process, at which the amount of channels formed by each connexin changes under different functional conditions. As with receptor subunits at glutamatergic synapses, this process could shape the functional properties of an electrical synapse by combining channels with different functional characteristics. The existence of such hypothetical mechanism would greatly expand our views of the plastic properties of electrical synapses.
In summary, as also reported in the olfactory bulb (Maher et al. 2009), the presence of Cx36‐containing electrical synapses seems to be required for the formation of normal neural circuits. Analysis of Cx36 KO mice should therefore be taken with caution, as in addition to a profound reduction in electrical transmission, these animals might have altered neural connectivity. From a more general perspective, the findings suggest that altered electrical transmission during development could play important pathological roles by leading to subtle modifications of neural circuits relevant to various neurological conditions.
Additional information
Competing interests
None declared.
References
- Baker MW, Yazdani N & Macagno ER (2013). Gap junction‐dependent homolog avoidance in the developing CNS. J Neurosci 33, 16673–16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW & Long MA (2004). Electrical synapses in the mammalian brain. Annu Rev Neurosci 27, 393–418. [DOI] [PubMed] [Google Scholar]
- Li X, Kamasawa N, Ciolofan C, Olson CO, Lu S, Davidson KG, Yasumura T, Shigemoto R, Rash JE & Nagy JI (2008). Connexin45‐containing neuronal gap junctions in rodent retina also contain connexin36 in both apposing hemiplaques, forming bihomotypic gap junctions, with scaffolding contributed by zonula occludens‐1. J Neurosci 28, 9769–9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher BJ, McGinley MJ & Westbrook GL (2009). Experience‐dependent maturation of the glomerular microcircuit. Proc Natl Acad Sci USA 106, 16865–16870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE (2014). Electrical synapses and their functional interactions with chemical synapses. Nat Rev Neurosci 15, 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolnik TA & Connors BW (2016). Electrical synapses and the development of inhibitory circuits in the thalamus. J Physiol 594, 2579–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]


