Abstract
Key points
The CA3 hippocampal region generates sharp waves (SPW), a population activity associated with neuronal representations. The synaptic mechanisms responsible for the generation of these events still require clarification.
Using slices maintained in an interface chamber, we found that the firing of single CA3 pyramidal cells triggers SPW like events at short latencies, similar to those for the induction of firing in interneurons.
Multi‐electrode records from the CA3 stratum pyramidale showed that pyramidal cells triggered events consisting of putative interneuron spikes followed by field IPSPs. SPW fields consisted of a repetition of these events at intervals of 4–8 ms. Although many properties of induced and spontaneous SPWs were similar, the triggered events tended to be initiated close to the stimulated cell.
These data show that the initiation of SPWs in vitro is mediated via pyramidal cell synapses that excite interneurons. They do not indicate why interneuron firing is repeated during a SPW.
Abstract
Sharp waves (SPWs) are a hippocampal population activity that has been linked to neuronal representations. We show that SPWs in the CA3 region of rat hippocampal slices can be triggered by the firing of single pyramidal cells. Single action potentials in almost one‐third of pyramidal cells initiated SPWs at latencies of 2–5 ms with probabilities of 0.07–0.76. Initiating pyramidal cells evoked field IPSPs (fIPSPs) at similar latencies when SPWs were not initiated. Similar spatial profiles for fIPSPs and middle components of SPWs suggested that SPW fields reflect repeated fIPSPs. Multiple extracellular records showed that the initiated SPWs tended to start near the stimulated pyramidal cell, whereas spontaneous SPWs could emerge at multiple sites. Single pyramidal cells could initiate two to six field IPSPs with distinct amplitude distributions, typically preceeded by a short‐duration extracellular action potential. Comparison of these initiated fields with spontaneously occurring inhibitory field motifs allowed us to identify firing in different interneurones during the spread of SPWs. Propagation away from an initiating pyramidal cell was typically associated with the recruitment of interneurones and field IPSPs that were not activated by the stimulated pyramidal cell. SPW fields initiated by single cells were less variable than spontaneous events, suggesting that more stereotyped neuronal ensembles were activated, although neither the spatial profiles of fields, nor the identities of interneurone firing were identical for initiated events. The effects of single pyramidal cell on network events are thus mediated by different sequences of interneurone firing.
Key points
The CA3 hippocampal region generates sharp waves (SPW), a population activity associated with neuronal representations. The synaptic mechanisms responsible for the generation of these events still require clarification.
Using slices maintained in an interface chamber, we found that the firing of single CA3 pyramidal cells triggers SPW like events at short latencies, similar to those for the induction of firing in interneurons.
Multi‐electrode records from the CA3 stratum pyramidale showed that pyramidal cells triggered events consisting of putative interneuron spikes followed by field IPSPs. SPW fields consisted of a repetition of these events at intervals of 4–8 ms. Although many properties of induced and spontaneous SPWs were similar, the triggered events tended to be initiated close to the stimulated cell.
These data show that the initiation of SPWs in vitro is mediated via pyramidal cell synapses that excite interneurons. They do not indicate why interneuron firing is repeated during a SPW.
Abbreviations
- DAMGO
(d‐Ala2 N‐MePhe4 Gly‐ol)‐enkephalin
- fIPSP
field inhibitory post‐synaptic potential
- SPW
sharp wave
Introduction
Single mammalian pyramidal cells are not considered to have major effects on the cortical networks to which they belong (Shadlen & Newsome, 1998). Even so, single pyramidal cells of layers 5 and 6 of the motor cortex can induce or affect whisker movements (Brecht et al. 2004), whereas stimulation of single layer 5 somatosensory pyramidal cells modifies behavioural responses during a detection task (Houweling & Brecht, 2008). Single neurones can also modify the collective activities of cortical neuronal populations. In the somatosensory or visual cortex, single cells can induce transitions between cortical up and down states (Li, Poo & Dan, 2009). The firing of a single GABAergic inhibitory cell can alter the timing of population events in the immature hippocampus (Bonifazi et al. 2009), whereas some pyramidal cells entrain or initiate epileptiform population events in the adult hippocampus (Miles & Wong, 1983; Prida et al. 2006).
Sharp waves (SPWs) are hippocampal EEG events with a duration of 30–60 ms (O'Keefe & Nadel, 1978; Buzsaki, Leung & Vanderwolf, 1983) that occur during behaviours including awake immobility and slow wave sleep. Buzsaki et al. (1992) showed that SPWs are accompanied by high frequency interneurone firing. They are initiated in CA3, spread into the CA1 region of the hippocampus, and pyramidal cell and GABAergic interneurones fire during SPWs of both regions (Csicsvari et al. 2000; Klausberger et al. 2003). SPWs are suggested to involve various forms of replay of previous sequences of spike discharge and so they have been associated with the consolidation of neuronal representations (Ji & Wilson, 2007; Girardeau et al. 2009; Jadhav et al. 2012).
SPW‐like events occur spontaneously in vitro (Kubota et al. 2003) and the mechanisms responsible for their generation have mostly been examined in slices. These mechanisms remain controversial and may differ for SPWs of the CA3 and CA1 regions. SPWs have been ascribed to electrotonic junctions between pyramidal cells (Draguhn et al. 1998; Bähner et al. 2011) or to interactions within recurrent circuits (Ellender et al. 2010) with predominant excitatory (Maier et al. 2011) or inhibitory synaptic signals (Ho, Zhang & Skinner, 2009; Aivar et al. 2014). Data on how single neurones affect the timing or initiation of SPWs could help discriminate between these possible mechanisms. Ellender et al. (2010) showed that stimulation of single interneurones increased the probability of SPW occurrence in slices with long (∼1 s) latencies, whereas stimulation of single pyramidal cells had no effect.
In the present study, we show, in vitro, that firing in single CA3 pyramidal cells could initiate SPW with latencies of 2–6 ms. This latency is similar to that between pyramidal cell firing and the discharge of post‐synaptic interneurones (Miles, 1990; Csicsvari et al. 1998). Extracellular records suggested that repeated firing of the same or different interneurones contributed to SPWs. SPWs induced by single cells were more stereotyped than SPWs that occurred spontaneously without stimulation. However, the identities and the timing of interneurone firing varied between successive initiated SPWs.
Methods
Slice preparation
Hippocampal slices were prepared from rats, aged 7–10 weeks and weighing 150–300 g, in accordance with EC Directive 08/120/EC and local INSERM guidelines. Protocols were approved by the Comité d'Ethique Darwin, Ministère de l'Enseignement Supérieur et de la Recherche; Paris. Twenty‐two animals were used to obtain the data reported in the present study. Rats were anaesthetized i.p. with ketamine (80 mg kg−1) and xylazine (12 mg kg−1) and perfused intracardially with a solution containing (in mm) 62 NaCl, 26 NaHCO3, 1 KCl, 10 MgCl2, 1 CaCl2, 122 sucrose and 10 d‐glucose and equilibrated with 5% CO in 95% O2 at 3–5°C. Both hippocampi were dissected free and transverse slices (thickness 500 μm) were cut with a vibratome (HM650V; Microm International GmbH, Walldorf, Germany) from their ventral portion. Slices were transferred to an interface recording chamber, where they were equilibrated with 5% CO2 in 95% O2, heated to 35–37°C and perfused with a solution containing (in mm) 124 NaCl, 26 NaHCO3, 3–5 KCl, 2 MgCl2, 2 CaCl2 and 10 glucose.
Drugs
In some experiments, GABAA receptor mediated signalling was suppressed by picrotoxin (100 μm). We also used the μ‐opioid receptor agonist (d‐Ala2, N‐MePhe4, Gly‐ol)‐enkephalin (20 μm; DAMGO), which is suggested to hyperpolarize perisomatic targeting interneurones and reduce release from inhibitory terminals (Svoboda et al. 1999; Gulyas et al. 2010). Drugs were obtained from Tocris Neuramin (Bristol, UK) or Ascent Scientific (Cambridge, UK).
Recordings
Intracellular records were made with glass electrodes filled with 4 m KAc (resistance 50–80 mΏ. Signals were amplified with an Axoclamp 2B amplifier (Molecular Devices, Sunnyvale, CA, USA) operated in current‐clamp mode. Intracellular records from neurons with overshooting action potentials, an input resistance larger than 20 MΩ and a time constant longer than 10 ms were retained. Extracellular records were made with arrays of eight to 12 nichrome electrodes (diameter 50 μm) positioned to contact slices from above (Bazelot et al. 2010). In some experiments, linear arrays with a separation of ∼100 μm between electrodes were placed along the CA3 pyramidal cell somatodendritic axis, orthogonal to the stratum pyramidale, In other experiments, curved arrays of separation ∼200 μm between electrodes were used to record from sites along the CA3 stratum pyramidale. Signals were amplified and filtered (pass‐band 0.1 Hz to 20 kHz) with a 16 channel amplifier (Dr F. Dubois; Dipsi, Châtillon, France). Intracellular and extracellular voltage signals were digitized using a 12 bit, 16 channel analog‐to‐digital converter (Digidata 1200A; Molecular Devices) and visualized on a PC (Axoscope; Molecular Devices).
Signal analysis
Intracellular and multiple (8–12) extracellular records were analysed with laboratory‐written routines (Matlab, MathWorks Inc., Natick, MA, USA; Python, https://www.python.org). The amplitude of SPWs and unitary inhibitory synaptic fields (fIPSPs) was measured at their peak on any recording site. Extracellular spikes were detected from signals filtered above 600 Hz using a threshold of 5× the SD of baseline fluctuations. Field IPSPs (fIPSPs) were detected from low pass‐filtered (80 Hz) signals as single positive‐going waves of amplitude exceeding 5 SDs. SPWs were detected (Fig. 1) from low pass‐filtered (80 Hz) signals obtained from eight sites of the CA3 stratum pyramidale. An amplitude threshold was adjusted to detect events similar to user‐identified SPWs defined as fields generated at three or more sites, with at least three waves (Fig. 1 A).
Figure 1. Detection and measurement of SPWs .
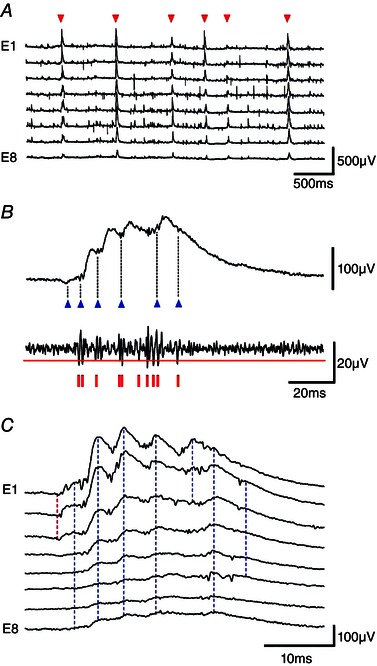
A, SPWs (red triangles) were recorded extracellularly from the CA3 stratum pyramidale with eight electrodes, E1–E8 separated by ∼200 μm in a curved array. SPWs were defined as events recorded from at least three electrodes, comprising three or more waves, and exceeding a user‐defined amplitude threshold. B, multi‐unit and wave components of SPW fields. Upper trace, SPW field (band pass filtered, 1–80 Hz); lower trace, unit activity (high‐pass filtered, 600 Hz). Blue triangles indicate the start of six detected waves and red vertical lines indicate 10 detected spikes. C, SPW recorded by eight extracellular electrodes (E1–E8) from the CA3 stratum pyramidale. The onset of the SPW was detected on electrodes E1–E3 (red dotted line). Seven waves were detected (blue dotted lines). The first, fifth and seventh waves were recorded by some (but not all) electrodes. An index of spatial coherence was used to define the spatial variability of SPWs: (summed number of waves detected from all sites)/(the number of recording sites × number of waves). The index has a value of 1 for an SPW where each wave is detected at each site. For this event, it was 0.77 = (3 + 8 + 8 + 8 + 3 + 8 + 5)/(8 × 7).
Wave components of SPWs (Fig. 1 B) were detected from zero‐crossings of the second derivative of voltage in low‐pass filtered records (80 Hz). Spikes associated with SPWs (Fig. 1 B) were detected from high‐pass filtered traces (600 Hz). The start of an SPW was defined as the shortest latency spikes and/or waves across all recording sites in the stratum pyramidale (Fig. 1 C). Sequential wave components of SPWs were defined from their start and peak, as well as the timing of firing, in comparisons of records from all sites in the CA3 stratum pyramidale. An index of the spatial coherence of SPWs, their similarity at different recording sites, was derived as: (summed number of waves detected from all sites)/(the number of recording sites × number of waves).
Spontaneously occurring and induced fIPSPs and associated spikes were sorted by unsupervised clustering of extracellular signals (n = 8) recorded from the stratum pyramidale. Events with overlaps of spikes or fIPSPs were excluded. Traces were measured at multiple time points chosen to provide a good discrimination of extracellular spikes and fIPSPs. The time points, with respect to the peak of the largest extracellular spike, were typically at −1.0, −0.5, 0.1, 0 (spike peak), 0.1 and 2.0 ms. Subtracting values for each trace from the value at −1.0 ms gave five parameters per trace and with eight channels a string of 40 numbers. Strings were analysed using k‐mean clustering procedures as described previously (Bazelot et al. 2010). Reliability of the clustering was confirmed by visual matching of the form of spikes and fIPSPs at all recording sites on sets of eight traces aligned to the peak of the largest extracellular spike. Current source densities were estimated from eight to 12 extracellular records made along the CA3 pyramidal cell somatodendritic axis as described previously (Bazelot et al. 2010) using the approximation of Nicholson and Freeman (1975).
Initiated and spontaneous SPWs were selected from all events detected in records with a duration of 10–45 min. SPWs occurring with latencies <5 ms after a pyramidal cell action potential induced by current injection were classed as evoked events. Other SPWs were considered to occur spontaneously. The initiation of spontaneous and evoked SPWs was compared for events aligned at their start, defined from both waves and unit spikes. Extracellular firing at SPW initiation was compared for all spikes detected from all electrodes within 1 ms of the start of the SPW. A cumulative sum procedure was used to compare the variability of spontaneous and single‐cell initiated SPWs. A running sum was made from each point, of root‐mean‐square differences between the voltage trajectory of each event on each electrode, and the mean event from that electrode for all spontaneous or initiated SPWs. Cumulative variability from all electrodes was then added to derive summed values for spontaneous and initiated events. The significance of differences was explored using a bootstrap test, which compared sums of the squared differences from the means of either spontaneous or triggered events with values derived identically from 1000 randomized groups. The amplitude of fIPSPs and SPWs were compared using the peak amplitude detected at any site.
Statistical analysis
Values are reported as the mean ± SD. Statistical analyses were conducted using Student's t test in SigmaStat, version 3.0 (Systat Software Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Single pyramidal cells initiate SPWs and field IPSPs
We observed action potentials in some CA3 pyramidal cells affected the timing of SPWs (Fig. 2). Pyramidal cells were made to fire single action potentials by current injection at intervals of 1–5 s. In 10 of 30 CA3b‐c pyramidal cells tested, SPWs followed action potentials with latencies of ≤5 ms (Fig. 2 A–C) and a probability of 0.07–0.76 (from 250 or more trials). The probability of SPW occurrence by chance was estimated to be in the range 0.002–0.012, as a result of dividing the latency of 5 ms by the mean interval between SPWs for each slice (Prida et al. 2006). The mean delay from the pyramidal cell spike to the first wave of the SPW was 2.7 ± 0.5 ms (20% of peak; n = 10) (Fig. 2 B and C). Initiated SPWs were accompanied by an increase in multi‐unit activity. The duration and pattern of this firing is shown in Fig. 2 D, which plots all the extracellular spikes from SPWs initiated by single pyramidal cells (n = 10 cells; 38–252 SPWs per cell).
Figure 2. Single CA3 pyramidal cells trigger SPWs .
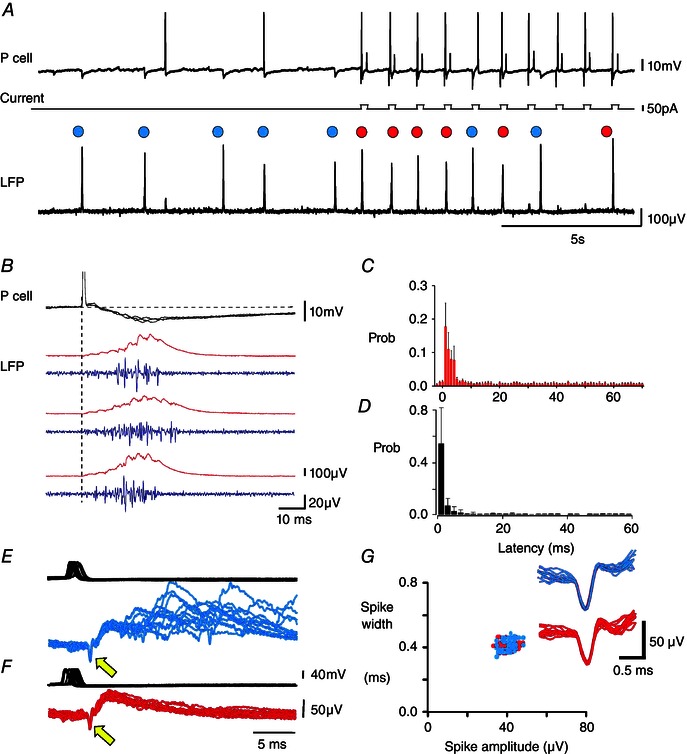
A, traces are CA3 pyramidal cell membrane potential, injected current and local field potential (LFP). Blue circles indicate spontaneously occurring SPWs at intervals of 1.5–3.0 s. Current pulses (50 pA, duration 200 ms, interval 1000 ms) were injected to induce single pyramidal cell action potentials almost half‐way along the traces. Red circles indicate six of 10 action potentials followed at short latency by a SPW. Overall, 172 of 626 spikes induced in this pyramidal cell were followed by a SPW at a latency shorter than 5 ms. In total, 99 spikes elicited no response, 128 spikes evoked a single fIPSP and 227 spikes elicited events intermediate between a single fIPSP and an SPW. B, three SPWs initiated by pyramidal cell firing. Intracellular potential, field (red) and multi‐unit firing (blue, 0.5–5 kHz band‐pass filtered). C, interval distribution between the intracellular action potential and the start of detected SPWs (n = 10 experiments, 1145 SPWs, mean ± SD of probabilities). D, normalized probability distribution of latencies from single pyramidal action potentials to extracellular spikes associated with the next SPW (mean ± SD of probabilities, n = 1726 action potentials from 10 pyramidal cells). E–G, SPWs, fIPSPs and unit firing. E, overlay of 20 SPWs (blue) initiated by single pyramidal cell action potentials (upper trace) and preceeded by an extracellular spike (yellow arrow). F, overlay of 20 fIPSPs (red), initiated by single pyramidal cell spikes (upper trace) and preceeded by an extracellular spike (yellow arrow). Traces of (E) and (F) triggered on the extracellular spike. G, plot of width against amplitude for spikes preceding fIPSPs (red) and SPWs (blue). The inset shows overlays of extracellular spikes preceeding SPWs (n = 20, blue) and fIPSPs (n = 20, red).
A delay of 2–3 ms is similar to that between pyramidal cell firing and discharge of a post‐synaptic interneurone (Miles, 1990; Csicsvari et al. 1998). Field IPSPs are an extracellular sign of the activation of all the inhibitory synapses established by a single interneurone (Glickfeld et al. 2009; Bazelot et al. 2010). We found the same pyramidal cells induced either SPWs (Fig. 2 E) or fIPSPs (Fig. 2 F) with similar latency. Both fIPSPs and SPWs could be preceded by an extracellular action potential typically of short duration (0.3–0.6 ms; n = 10) as associated with interneurone firing (Henze et al. 2000). The probability of inducing a fIPSP was 0.08–0.42 (n = 10 cells; 250 or more trials). The observation that a single pyramidal cell could initiate either a fIPSP or a SPW suggested that the same circuits might be involved. Similar latencies (Fig. 2 E and F) and spike shapes (Fig. 2 G) suggest that the same extracellular unit may have fired when a single pyramidal cell initiated a fIPSP or a SPW.
fIPSPs from perisomatic interneurones are repeated in SPW fields
We therefore examined the role of interneurones and inhibitory synaptic circuits in SPW generation. Most recorded pyramidal cells were inhibited both during spontaneous SPWs and those that they initiated (24 of 30 neurons) (Figs 2 B and 3 A). Three were depolarized and three others received mixed excitatory–inhibitory synaptic events (not shown). Inhibitory events occurring during a SPW were correlated in time and in amplitude with fields recorded from the stratum pyramidale. By contrast, all (n = 4) fast‐spiking interneurones recorded close to the stratum pyramidale received depolarizing events correlated with successive waves of SPWs (Fig. 3 B)
Figure 3. Synaptic events corresponding to SPWs and fIPSPs .
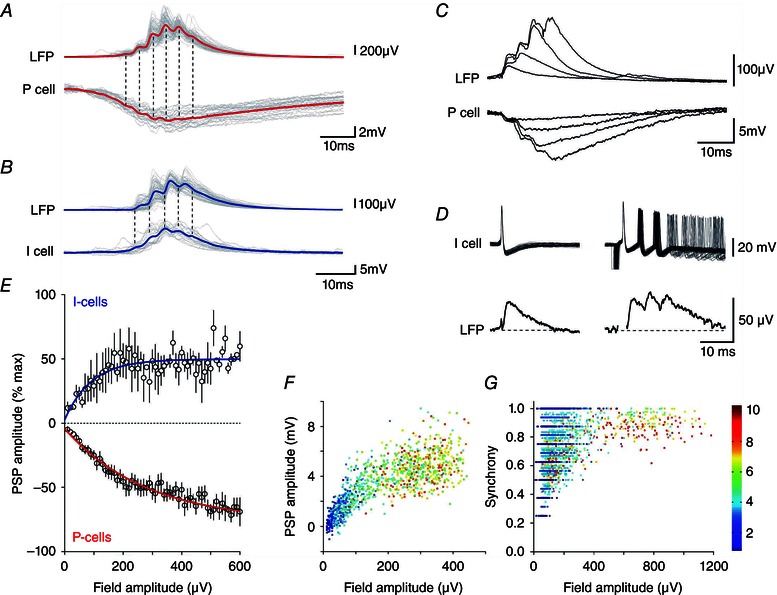
Correlates of SPWs in pyramidal cells (red) (A) and in interneurones (blue) (B) showing the averaged local field and intracellular membrane potential with overlays of 20 traces (grey). C, overlaid field and pyramidal cell membrane potentials for a fIPSP and for SPWs of up to four waves; same records as in (A). D, field potentials (average of 30) induced by single and multiple action potentials in an interneurone. E, peak SPW field amplitude plotted against membrane potential changes in interneurones (blue, n = 4, r 2 = 0.22) and pyramidal cells (red, n = 16, r 2 = 0.96). F, hyperpolarizing change in pyramidal cell membrane potential (n = 16) plotted against the amplitude of fields associated with events from single fIPSPs (blue) to eight to 10 wave SPWs (red). G, spatial coherence for fIPSPs (blue) through to eight to 10 wave SPWs (red) plotted against field potential amplitude (all events from 10 slices).
There was a continuum between single inhibitory events and multi‐component SPWs in field records and intracellular traces from pyramidal cells. Each wave of a SPW field was similar in form to a fIPSP of time to peak 2–5 ms (Glickfeld et al. 2009; Bazelot et al. 2010). Typically, three to 10 waves were repeated at intervals of 4–10 ms. Figure 3 C shows a superimposition of field and pyramidal cell membrane potential records for an isolated fIPSP and SPWs of up to four waves. Figure 3 D shows that, although one interneurone action potential evoked a fIPSP, repeated firing elicited field events similar to those at the start of a SPW. Relationships between peak field amplitude and the amplitude of depolarizations in interneurones (n = 4) or hyperpolarizations in pyramidal cells (n = 8) are summarized in Fig. 3 E (n = 300–800 events at resting potential). Peak SPW field amplitude and membrane hyperpolarizations in pyramidal cells increased together with the number of waves in a SPW (Fig. 3 F). Figure 3 G shows how the spatial coherence of SPWs (see Methods) recorded from multiple electrodes also co‐varied with field amplitude and the number of waves. These data suggest that a continuum exists between fIPSPs consisting of a single wave and SPWs of up to eight to 10 waves.
We next attempted to compare the identity of inhibitory synapses contributing to fIPSPs and to SPWs using current source density analysis. Fields were recorded with multiple electrodes (n = 12) from sites along the somatodendritic axis of CA3 pyramidal cells (n = 5 slices). Comparisons of the spatial profiles of fIPSPs (Fig. 4 A) and an intermediate wave of SPWs (Fig. 4 B) revealed a current source in the stratum pyramidale. These data suggest that pyramidal cells initiating SPWs also excite interneurones (Csicsvari et al. 1998). Repeated firing in the same or different interneurones is associated with succeeding waves of the SPW field.
Figure 4. Evidence for activation of perisomatic inhibitory synapses during SPWs .
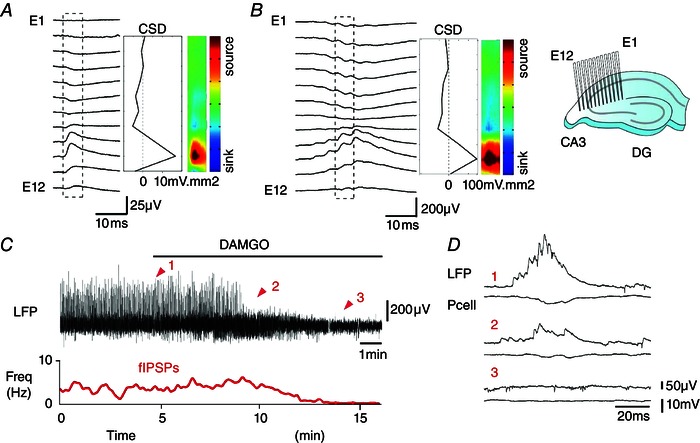
Comparison of CSD profiles for a fIPSP (A) and an intermediate wave of a SPW (B) recorded with 12 electrodes (E1–E12, separation ∼100 μm) placed along the CA3 pyramidal cell somatodendritic axis (inset). A current source (red) was apparent near the stratum pyramidale for both fIPSPs (n = 200) and SPWs (n = 200). C and D, DAMGO (20 μm) suppressed both SPWs and fIPSPs. Traces are the LFP (upper) and fIPSP frequency (red, lower). D, field and pyramidal cell potentials during DAMGO application as indicated by red arrows (1–3).
Confirmation that interneurones are involved in SPW generation was obtained by showing that SPWs were suppressed by the GABAA receptor antagonist picrotoxin (20 μm; n = 5, data not shown). The opiate DAMGO, which hyperpolarizes and reduces transmitter release from perisomatic interneurones (Svoboda et al. 1999; Gulyas et al. 2010), also suppressed SPWs (20 μm, n = 4) (Fig. 4 C and D). Together with the current profile data (Fig. 4 A and B), these data suggest that interneurons forming perisomatic synapses contribute to SPW fields. Pyramidal cell initiation of SPWs involves the excitation of one or several perisomatic interneurones.
Excitation of interneurones by single pyramidal cells
We estimated the number and spatial distribution of interneurones discharged by single pyramidal cells by making multiple extracellular records of fIPSPs from sites along the CA3 stratum pyramidale with eight electrodes separated by ∼200 μm (Fig. 5). Inhibitory fields were typically recorded from three to six of these electrodes (Fig. 5 A–D), which is consistent with the dimensions of axonal arbors of perisomatically‐terminating inhibitory cells in this region (Gulyas et al. 2010). fIPSPs were often preceded, at two to four recording sites, by a short‐duration extracellular spike (Figs 2 E and F and 5 A–D). These two features, a local spike, presumably generated by an interneurone, and a more widespread fIPSP of distinct amplitude distribution across different sites, could define multiple, spatially different inhibitory field motifs.
Figure 5. Single pyramidal cells induce firing in multiple interneurones .
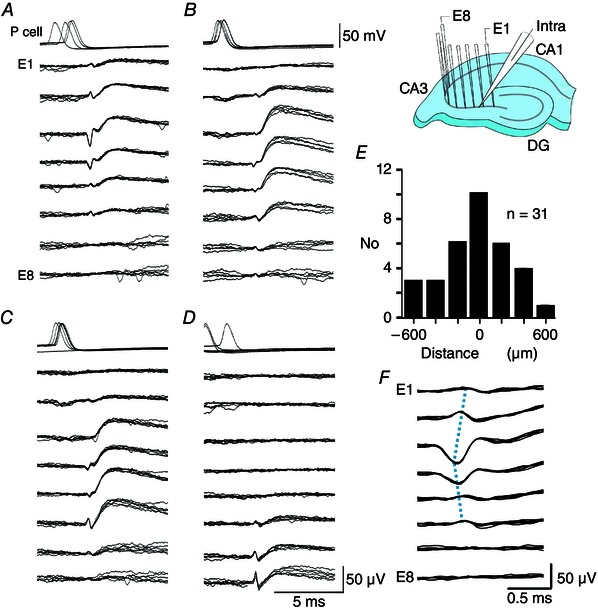
A to D, single action potentials of the same CA3 pyramidal cell initiated four spatially distinct combinations of an extracellular spike followed by a field IPSP. Overlays of six traces for pyramidal cell potential and extracellular potentials at eight sites in the stratum pyramidale (inset, E1–E8, electrode separation 200 μm). All sets of traces are aligned on the largest extracellular spike. Spikes were detected at three to six sites and fIPSPs were recorded by three to seven electrodes. In total, 436 action potentials of this pyramidal cell triggered 90 fIPSPs, 130 events intermediate between fIPSPs and SPWs, and 178 SPWs, and 38 spikes elicited no response. A, the largest spike amplitude was ∼55 μV on E3 (13 of 90 initiated fIPSPs). B, the largest spike was ∼25 μV on E4 (18 of 90 fIPSPs). C, the largest spike was ∼40 μV on E6 (43 of 90 fIPSPs). D, the largest spike amplitude was ∼55 μV on E8, (16 of 90 fIPSPs). E, distance between the initiating pyramidal cell and the site of the maximal extracellular spike (n = 31 spikes of amplitude >20 μV; duration <0.6 ms, initiated by 10 pyramidal cells). F, enlarged extracellular spikes from (A), detected over distances of 4–800 μm, suggest that interneurone axonal action potentials may propagate at ∼1 mm ms–1 (blue dotted lines aligned to spike peaks).
Pyramidal cells that initiated SPWs also activated multiple distinct fIPSP motifs. Overlays of traces selected after clustering and template matching show (Fig. 5 A–D) that a single pyramidal cell initiated distinct fIPSPs with maximal amplitude at different recording sites preceded by a short‐duration extracellular spike. Nine of 10 single pyramidal cells that initiated SPWs (Fig. 2) initiated at least two (2–6) spatially distinct fIPSP motifs. The other initiating cell evoked fIPSPs, although no extracellular spike was reliably detected. Plotting distances between stimulated pyramidal cells (n = 10) and all detected spikes (31 spikes of amplitude larger than 20 μV) revealed a distribution clustered around the initiating neurone (Fig. 5 E). Extracellular spikes preceding fIPSPs were typically recorded on several electrodes (Fig. 5 F). This is unexpected because the amplitude of extracellular spikes generated by pyramidal cells decays to undetectable levels at distances of ∼100 μm (Cohen & Miles, 2000; Henze et al. 2000). The extracellular spike shown in Fig. 5 F, propagated at ∼1 mm ms−1, which is similar to the speed of action potential conduction in interneurone axons (Hu & Jonas, 2014).
Comparison of spontaneous SPWs and SPWs initiated by single cells
If pyramidal cells tend to discharge nearby interneurones, then evoked SPWs might also be initiated at sites clustered around a stimulated pyramidal cell. We examined this by comparing initiation sites for initiated and spontaneously occurring SPW field potentials in the stratum pyramidale. The initial wave of initiated SPW fields always began close to the initiating cell (Fig. 6 A and C). By contrast, spontaneous SPWs were typically initiated at multiple sites in CA3 (Fig. 6 B and C). Thus, although SPWs appear to be initiated via the firing of interneurones near the stimulated pyramidal cell, spontaneous SPWs may depend on similar processes at multiple, distinct sites.
Figure 6. Differences between initiated and spontaneous SPW fields .
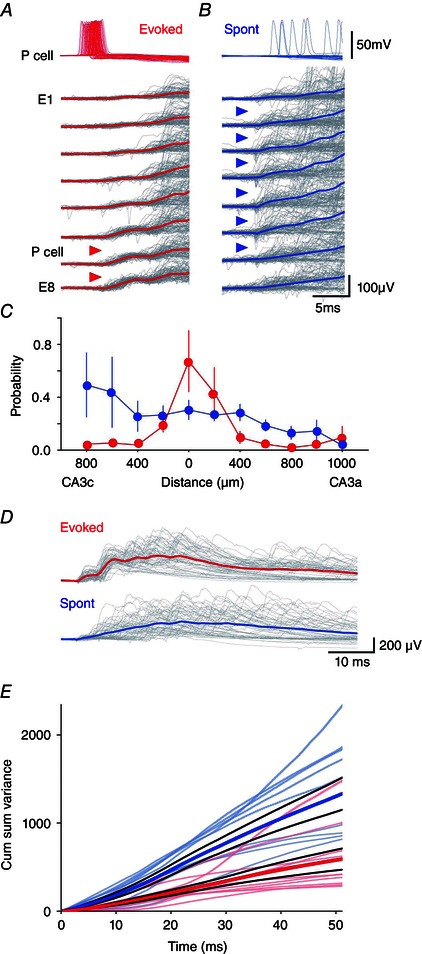
The site of initiation for evoked SPWs (red triangles) (A) varied less than that of spontaneous SPWs (blue triangles) (B). Overlay (grey) and averages of 100 initiated (red) and 100 spontaneous SPWs recorded over the same period from electrodes E1–E8 in the CA3 stratum pyramidale. SPWs were aligned at their initiation site defined from spikes and field. The stimulated pyramidal cell was situated between E6 and E7. C, the spatial distribution of extracellular unit activity at the start of initiated SPWs (red) was more restricted than that preceding spontaneous events (blue). Data from 10 slices, with distance 0 μm corresponding to the extracellular electrode closest to the initiating cell. D, cumulative variability of SPW fields was less for initiated than spontaneous events (P = 0.016, bootstrap). Above: overlays of 50 evoked SPWs (grey, mean shown in red) and 50 spontaneous SPWs (grey, mean in blue). E, time course of cumulative variability for initiated (red, n = 10 slices) and spontaneous SPWs (blue; n = 10), with mean ± SE (bold lines). A lower variability at initiation was maintained through SPW time course.
We compared several characteristics of spontaneous SPWs and those initiated by single pyramidal cells. The duration of SPWs was measured as the delay between the start of the first and the last detected fIPSP. For initiated events, the mean duration was 23.8 ± 5.3 ms and, for spontaneous events, it was 21.6 ± 4.0 ms (paired t test, P = 0.15, n = 1233 initiated and 1233 spontaneous events from 10 different slices). The mean number of waves or fIPSPs was counted across eight recording sites, with events simultaneous at multiple sites counted as one event. There were 6.7 ± 1.4 waves for initiated events and
6.3 ± 1.2 waves for spontaneous events (paired t test, P = 0.29, n = 2466). The mean interval between fIPSPs, from all electrodes, was 6.7 ± 1.4 for initiated events and 7.1 ± 1.0 for spontaneous events (paired t test, P = 0.17, n = 2466). On the basis of these criteria, initiated events did not differ from spontaneously occurring SPWs.
Finally, we investigated whether later phases of initiated SPW fields followed a more stereotyped time course than spontaneous SPW fields (Fig. 6 D). As an index of field variability, we used a cumulative sum of root‐mean‐square differences between each field potential and the mean field from each electrode (see Methods). Identical numbers of initiated and spontaneous SPWs from the same time period for each recording were analysed (n = 10). This index of cumulative field variability was always lower near the start of initiated SPWs, as expected, because the initiation site tended to be more stereotyped. Figure 6 D and E shows that the lower variability for initiated SPWs was maintained throughout their time course. If SPW fields in the stratum pyramidale largely reflect fIPSPs, then the set of interneurones firing during evoked SPWs may be more stereotyped than those active during spontaneous SPWs.
Patterns of SPW spread and the activity of identified interneurones
We attempted to identify interneurones that fired during SPWs from the involvement of distinct spike and fIPSP motifs in SPW fields. We searched for spatially distinct events involving large extracellular spikes (>20 μV) as shown in Fig. 5. In seven of 10 records, we could distinguish (1) a motif triggered by a pyramidal cell that initiated SPWs and (2) a second inhibitory motif not evoked by that pyramidal cell. Figure 7 A shows an example where the initiated motif consisted of a maximal spike on electrode E3 and a fIPSP on electrodes E1–E6. By contrast, the inhibitory motif shown in Fig. 7 B, consisting of a maximal spike on electrode E6 and a fIPSP on electrodes E3–E8, was not initiated by the recorded pyramidal cell.
Figure 7. Field inhibitory motifs during SPWs .
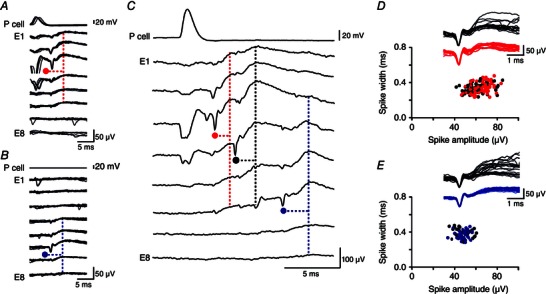
A, pyramidal cell spikes induce a presumed interneurone spike (red, maximum on electrode E3) and associated fIPSP. In total, 377 pyramidal cell action potentials initiated 130 single fIPSPs and 118 SPWs. Traces aligned on the extracellular spike. B, a spatially distinct interneurone spike (blue, maximum on E6) and fIPSP was never initiated by pyramidal cell firing. A and B, pyramidal cell and eight extracellular records from the stratum pyramidale. C, spike and fIPSP motifs were evident during SPWs initiated by the stimulated pyramidal cell. One interneurone spike (red, E3; A) occurs at ∼2 ms and the other (blue, E6; B) at ∼10 ms after pyramidal cell firing in this example. Another putative interneurone spike of amplitude >20 μV (black, maximum on E4) occurs at a latency of ∼5 ms. D, amplitude plotted against width of spikes initiated by the pyramidal cell and recorded by electrode E3 before fIPSPs (red) or SPWs (black). Examples are overlain in the inset (fIPSPs, n = 20, red; SPWs, n = 20, black). E, amplitude plotted against width for spikes not directly initiated by the pyramidal cell and recorded on electrode E6 before fIPSPs (blue) or SPWs (black). Overlaid examples in the insets (fIPSPs, n = 20, blue; SPWs, n = 20, black).
Both extracellular units (Fig. 7 A and C) appeared to participate in initiated SPWs (Fig. 7 C–E). Isolated motifs and those embedded in SPWs were compared on the basis of spike amplitude and shape, as well as on the amplitude and form of fIPSPs recorded from all electrodes. In this way, the initiated motif of Fig. 7 A appeared to be involved in 81 of 118 (69%) and the non‐initiated fIPSP motif of Fig. 7 B in 37of 118 (31%) of triggered SPWs. FIPSP motifs triggered by initiating pyramidal cells were detected in SPWs with probabilities of 38–82% (n = 7). Identified fIPSP motifs that were not elicited by pyramidal cell firing were detected with probability of 16–62% (n = 7). These data suggest that, as SPWs spread, previously silent interneurons were recruited at longer latencies than directly excited interneurones. The occurrence and the timing of firing in identified interneurones during SPWs initiated by the same pyramidal cell varied between trials (Fig. 6).
Discussion
Single identified neurones of invertebrates and fish can initiate motor behaviours (Ikeda & Wiersma, 1964; Getting & Deakin 1985; Eaton, Bombardieri & Mayer, 1977). Single mammalian pyramidal cells can affect movement (Brecht et al. 2004), sensory perception (Houweling & Brecht, 2008), volition (Fried, Mukamel & Kreiman, 2011), entrain or initiate population activities (Miles & Wong, 1983; Prida et al. 2006; Bonifazi et al. 2009), and alter EEG activities between patterns associated with different brain states (Li, Poo & Dan, 2009). These effects depend on the identity and numbers of neurones driven to discharge by firing in the single initiating cell (Kwan & Dan, 2012).
Data are reported in the present study showing that ∼30% of CA3 pyramidal cells triggered SPW‐like events in vitro. We further show that these pyramidal cells evoked firing at similar latencies in several (2–6) perisomatic interneurones. Comparison of inhibitory fields and SPW fields suggested that, during a SPW, different interneurones fire repeatedly at intervals of 3–8 ms.
Advantages of an in vitro study
The work in the present study was facilitated by employing an in vitro approach. Accurate placement of linear electrode arrays orthogonal to the CA3 stratum pyramidale permitted field profile analyses of current profiles associated with SPWs and fIPSPs. Curved arrays placed along the stratum pyramidale allowed us to discriminate between the firing of different interneurones and the fIPSPs that they generated, revealing distinct fIPSP motifs. SPW‐like fields in vitro have a form and duration similar to those recorded in the intact animal. Equally, single pyramidal cells discharge post‐synaptic interneurones at comparable latencies and probabilities in vivo (Csicsvari et al. 1998), as well as in slices kept in an interface chamber (Miles, 1990). Inhibitory field motifs consisting of an interneurone spike followed mono‐synaptically by a spatially extended fIPSP have not yet been detected in vivo, possibly as a result of higher levels of background field fluctuations. Lower levels of fluctuation in fields in slices may have facilitated attempts to follow the firing of specific interneurones during SPWs.
We used a clustering approach to sort inhibitory motifs identified by measurements of spike and inhibitory field waveforms, followed by visual comparison of aligned traces. To validate this approach, multiple field records from stratum pyramidale could be compared with responses to single action potentials of intracellularly recorded, anatomically identified interneurons (Bazelot et al. 2010). This would provide data on the variability and spatial distribution of extracellular spikes and inhibitory fields initiated by an identified interneuron and also permit comparison with other extracellularly recorded motifs.
Population activities involving interneurones may be detected more easily when slices are maintained at a liquid–gas interface or when precautions are taken to enhance oxygenation of submerged slices (Hajos et al. 2009) The amplitude of field IPSPs recorded from slices in interface chambers is several times larger than similar events recorded from submerged slices (Glickfeld et al. 2009; Bazelot et al. 2010). These factors may have contributed to the data reported by Ellender et al. (2010) suggesting that single pyramidal cell firing does not influence SPWs in submerged slices.
Initiating pyramidal cells excite perisomatic interneurones
Evidence suggesting that SPW initiation involves pyramidal cell excitation of interneurones (Figs 5 and 7) is based on the latency of initiated events and signs of interneurone firing at the start of SPWs (Hájos et al. 2013; Sasaki, Matsuki & Ikegaya, 2014; Schlingloff et al. 2014). The intervals between pyramidal cell firing and SPW initiation are comparable to the delays between pyramidal cell firing and spikes discharged by a post‐synaptic interneurone. By contrast, transmission of firing between mono‐synaptically coupled pyramidal cells requires multiple pre‐synaptic action potentials and occurs at latencies of 10–15 ms or more (Miles & Wong, 1987; Kwan & Dan, 2012; Ikegaya et al. 2013). Extracellular spikes detected at SPW initiation possessed characteristics of interneurone spikes (Henze et al. 2000). When SPWs were not triggered, these spikes could be followed by unitary extracellular inhibitory fields (Glickfeld et al. 2009; Bazelot et al. 2010). Recorded from the stratum pyramidale, SPW fields apparently correspond to repeated, summed fIPSPs at intervals of 3—8 ms, as first suggested by Buzsaki et al. (1992). Our comparison of current sources for fIPSPs and SPWs, as well as the suppression of SPWs by the opiate DAMGO, suggests that the interneurones involved synapse with pyramidal cells at perisynaptic sites as inferred from studies performed in vitro (Hájos et al. 2013; Aivar et al. 2014) and in vivo (Klausberger et al. 2003).
Continuation, spread and cellular components of SPWs
Although our data suggest that interneurone firing, which may be induced by pyramidal cells, should precede SPWs, they do not clarify the mechanisms ensuring repeated firing of the same or different interneurones as SPWs continue. Records from interneurones (Fig. 3 B) show repeated, fast depolarizations aligned with each wave of a SPW. Possibly, these events reflect excitatory synaptic inputs from pyramidal cells. However, few pyramidal cells fired during SPW‐like events and the second EPSP in interneurones appears to occur too soon (at 5–10 ms after SPW initiation) for it to depend on synaptically induced firing in pyramidal cells recruited by the initiating cell. Figure 5 shows that single initiating pyramidal cells can induce firing in multiple interneurones. Firing is probabilistic. Not all innervated interneurones fire in response to the same pyramidal cell action potential, and we found no evidence for a delayed firing by different interneurones that could sculpt successive waves of a SPW (Sasaki et al. 2014). Possibly, interactions between interneurones (Fukuda & Kosaka, 2000) ensure that SPWs continue after their initiation. If so, such interactions should be able to generate the repeated depolarizations recorded from interneurones during a SPW (Fig. 3 B). Alternatively repeated events emerging from supralinear dendritic electrogenesis may ensure that SPWs continue (Memmesheimer, 2010)
Records with electrodes placed along the CA3 stratum pyramidale demonstrate how SPW‐like events spread in a slice. SPWs are known to propagate from CA3 into the CA1 region (Csicsvari et al. 2000; Maier, Nimmrich & Draguhn, 2003) and macroscopic array records show that some SPWs are spatially restricted in the intact animal, whereas others spread longitudinally throughout the CA3 region (Patel et al. 2013). At the smaller scale of a transverse slice, our data suggest that previously silent, distant interneurones fire as later waves of a SPW field spread to new sites. Because these interneurones are not excited by the initiating pyramidal cell (Fig. 7), they must be recruited in another way. Their activity generates a spatially distinct fIPSP and so underlies, in part, propagation of the SWP field.
Field potentials of SPWs initiated by single pyramidal cells are more stereotyped than those associated with spontaneous SPWs (Fig. 6 A and D). However, even if only some participating neurones were recognized, our data show variation with respect to the occurrence and identity of directly triggered interneurone firing and those of interneurones indirectly recruited during the later stages of SPWs (Figs 2, 5 and 7). Presumably, more complex mechanisms control the apparently precise time sequences of pyramidal cell firing replay during SPWs in the intact animal (Lee & Wilson, 2002; Diba & Buzsaki, 2007; Stark et al. 2014).
In summary, the results of the present study reveal a continuum between single fIPSPs and SPWs. Both events were triggered by some (∼30%) recorded pyramidal cells. Latencies were consistent with those for the transmission of firing at synapses that excite interneurones. Multiple extracellular records allow us to separate spatially distinct spikes of interneurones and the resulting inhibitory fields. In this way, pyramidal cells that initiate SPWs were shown to excite several interneurones. The identification of different interneurones and the fields that they produced revealed (1) fluctuations in the composition of SPWs initiated by the same pyramidal cell and (2) the recruitment of previously silent inhibitory cells as SPWs spread through the CA3 region.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
MB carried out the experiments. MB, MT and RM analysed data. RM advised on the experiments and the analysis. MB, MT and RM wrote the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
We gratefully acknowledge financial support from INSERM, UPMC, ANR (08MNP006), NIH (MH054671) and ERC (322721), as well as a graduate studentship from ENP and DIM‐Ile de France to MT.
Acknowledgements
We wish to thank A. Bacci, M. Capogna, D. Dupret, A. Gulyas, J. C. Poncer and L. M. Prida for their comments on versions of the manuscript, as well as B. Telenczuk for help with analysis.
References
- Aivar P, Valero M, Bellistri E & Prida LM (2014). Extracellular calcium controls the expression of two different forms of ripple‐like hippocampal oscillations. J Neurosci 34, 2989–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähner F, Weiss EK, Birke G, Maier N, Schmitz D, Rudolph U, Frotscher M, Traub RD, Both M & Draguhn A (2011). Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc Natl Acad Sci USA 108, E607–E616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazelot M, Dinocourt C, Cohen I & Miles R (2010). Unitary inhibitory field potentials in the CA3 region of rat hippocampus. J Physiol 588, 2077–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, Represa A, Ben‐Ari Y & Cossart R (2009). GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424. [DOI] [PubMed] [Google Scholar]
- Brecht M, Schneider M, Sakmann B & Margrie TW (2004). Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature 427,704–710. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Leung LW & Vanderwolf CH (1983). Cellular bases of hippocampal EEG in the behaving rat. Brain Res 287, 139–171. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J & Wise K (1992). High‐frequency network oscillation in the hippocampus. Science 256, 1025–1027. [DOI] [PubMed] [Google Scholar]
- Cohen I & Miles R (2000). Contributions of intrinsic and synaptic activities to the generation of neuronal discharges in in vitro hippocampus. J Physiol 524, 485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A & Buzsáki G (1998). Reliability and state dependence of pyramidal cell‐interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron 21, 179–189. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Mamiya A & Buzsáki G (2000). Ensemble patterns of hippocampal CA3‐CA1 neurons during sharp wave‐associated population events. Neuron 28, 585–594. [DOI] [PubMed] [Google Scholar]
- Diba K & Buzsáki G (2007). Forward and reverse hippocampal place‐cell sequences during ripples. Nat Neurosci 10, 1241–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Schmitz D & Jefferys JG (1998). Electrical coupling underlies high‐frequency oscillations in the hippocampus in vitro. Nature 394, 189–192. [DOI] [PubMed] [Google Scholar]
- Eaton RC, Bombardieri RA & Meyer DL (1977). The Mauthner‐initiated startle response in teleost fish. J Exp Biol 66, 65–81. [DOI] [PubMed] [Google Scholar]
- Ellender TJ, Nissen W, Colgin LL, Mann EO & Paulsen O (2010). Priming of hippocampal population bursts by individual perisomatic‐targeting interneurons. J Neurosci 30, 5979–5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried I, Mukamel R & Kreiman G (2011). Internally generated preactivation of single neurons in human medial frontal cortex predicts volition. Neuron 69, 548–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T & Kosaka T (2000). Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J Neurosci 20, 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getting PA & Dekin MS (1985). Mechanisms of pattern generation underlying swimming in Tritonia. IV. Gating of central pattern generator. J Neurophysiol 53, 466–480. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G & Zugaro MB (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12, 1222–1223. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Roberts JD, Somogyi P & Scanziani M (2009). Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci 12, 21–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás AI, GG Szabó, Ulbert I, Holderith N, Monyer H, Erdélyi F, G Szabó, Freund TF & Hájos N (2010). Parvalbumin‐containing fast‐spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci 30, 15134–15145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häjos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, Freund TF & Paulsen O (2009). Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci 29, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hájos N, Karlócai MR, Németh B, Ulbert I, Monyer H, G Szabó, Erdélyi F, Freund TF & Gulyás AI (2013). Input‐output features of anatomically identified CA3 neurons during hippocampal sharp wave/ripple oscillation in vitro. J Neurosci 33, 11677–11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, Csicsvari J, Mamiya A, Harris KD & Buzsáki G (2000). Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol 84, 390–400. [DOI] [PubMed] [Google Scholar]
- Ho EC, Zhang L & Skinner FK (2009). Inhibition dominates in shaping spontaneous CA3 hippocampal network activities in vitro. Hippocampus 19, 152–165. [DOI] [PubMed] [Google Scholar]
- Houweling AR & Brecht M (2008). Behavioural report of single neuron stimulation in somatosensory cortex. Nature 451, 65–68. [DOI] [PubMed] [Google Scholar]
- Hu H & Jonas P (2014). A supercritical density of Na+ channels ensures fast signalling in GABAergic interneuron axons. Nat Neurosci 17, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K & Wiersma CAG (1964). Autogenic rhythmicity in the abdominal ganglia of the crayfish: the control of swimmeret movements. Comp Biochem Physiol 12, 107–115. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Sasaki T, Ishikawa D, Honma N, Tao K, Takahashi N, Minamisawa G, Ujita S & Matsuki N (2013). Interpyramid spike transmission stabilizes the sparseness of recurrent network activity. Cereb Cortex 23, 293–304. [DOI] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW & Frank LM (2012). Awake hippocampal sharp‐wave ripples support spatial memory. Science 336, 1454–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D & Wilson MA (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10, 100–107. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Márton LF, Roberts JD, Cobden PM, Buzsáki G & Somogyi P (2003). Brain‐state‐ and cell‐type‐specific firing of hippocampal interneurons in vivo. Nature 421, 844–848. [DOI] [PubMed] [Google Scholar]
- Kubota D, Colgin LL, Casale M, Brucher FA & Lynch G (2003). Endogenous waves in hippocampal slices. J Neurophysiol 89, 81–89. [DOI] [PubMed] [Google Scholar]
- Kwan AC & Dan Y (2012). Dissection of cortical microcircuits by single‐neuron stimulation in vivo. Curr Biol 22, 1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Poo MM & Dan Y (2009). Burst spiking of a single cortical neuron modifies global brain state. Science 324, 643–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK & Wilson MA (2002). Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194. [DOI] [PubMed] [Google Scholar]
- Maier N, Nimmrich V & Draguhn A (2003). Cellular and network mechanisms underlying spontaneous sharp wave‐ripple complexes in mouse hippocampal slice. J Physiol 550, 873–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier N, Tejero‐Cantero A, Dorrn AL, Winterer J, Beed PS, Morris G, Kempter R, Poulet JF, Leibold C & Schmitz D (2011). Coherent phasic excitation during hippocampal ripples. Neuron 72, 137–152. [DOI] [PubMed] [Google Scholar]
- Memmesheimer RM (2010) Quantitative prediction of intermittent high‐frequency oscillations in neural networks with supralinear dendritic interactions. PNAS (USA) 107, 11092–11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R & Wong RKS (1983). Single neurones can initiate synchronized population discharges in the hipoocampus Nature 306, 371–373. [DOI] [PubMed] [Google Scholar]
- Miles R & Wong RKS (1987). Inhibitory control of local excitatory circuits in the guinea‐pig hippocampus. J Physiol 388, 611–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R (1990). Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea‐pig in vitro. J Physiol 428, 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C & Freeman JA (1975). Theory of current source‐density analysis and determination of conductivity tensor for anuran cerebellum. J Neurophysiol 38, 356–368. [DOI] [PubMed] [Google Scholar]
- O'Keefe J & Nadel L (1978). The Hippocampus as a Cognitive Map, pp. 150–153. Oxford University Press, Oxford. [Google Scholar]
- Patel J, Schomburg EW, Berényi A, Fujisawa S & Buzsáki G (2013). Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J Neurosci 33, 17029–17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prida LM, Huberfeld G, Cohen I & Miles R (2006). Threshold behaviour in the initiation of hippocampal population bursts. Neuron 49, 131–142. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Matsuki N & Ikegaya Y (2014). Interneuron firing precedes sequential activation of neuronal ensembles in hippocampal slices. Eur J Neurosci 39, 2771–2783. [DOI] [PubMed] [Google Scholar]
- Schlingloff D, Káli S, Freund TF, Hájos N & Gulyás AI (2014). Mechanisms of sharp wave initiation and ripple generation. J Neurosci 34, 11385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN & Newsome WT (1998). The variable discharge of cortical neurons: implic‐ations for connectivity, computation, and information coding. J Neurosci 18, 3870–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark E, Roux L, Eichler R, Senzai Y, Royer S & Buzsáki G (2014). Pyramidal cell‐interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda KR, Adams CE & Lupica CR (1999). Opioid receptor subtype expression defines morphologically distinct classes of hippocampal interneurons. J Neurosci 19, 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


