Abstract
Photodynamic therapy is useful for the treatment of cancer because it is minimally invasive for patients. Certain porphyrin compounds and their derivatives have been used as the photosensitizer because they accumulate specifically in cancerous tissues. However, the detailed mechanism of this phenomenon has not been clarified. We previously reported that a proton-coupled folate transporter, HCP1, transported porphyrins and that regulation of the protein was associated with cancer-specific reactive oxygen species from mitochondria (mitROS). Therefore, over-generation of mitROS could increase HCP1 expression and the effect of photodynamic therapy. We investigated whether pretreatment with indomethacin influenced photodynamic therapy by using a rat normal gastric mucosal cell line, RGM1, its cancer-like mutated cell line, RGK1, and a manganese superoxide dismutase (MnSOD)-overexpressing RGK cell line, RGK-MnSOD. Indomethacin promotes the generation of cellular mitROS by inhibiting the electron transport chain, and MnSOD scavenges the mitROS. We elucidated that indomethacin enhanced cancer-specific mitROS generation and increased HCP1 expression. Furthermore, RGK1 cells showed higher cellular incorporation of hematoporphyrin and better therapeutic effect with indomethacin treatment whereas RGK-MnSOD cells did not show a difference. Thus, we concluded that indomethacin improved the effect of photodynamic therapy by inducing increased mitROS generation in cancer cells.
Keywords: mitROS, indomethacin, gastric epithelial cell, HCP1, PDT
Introduction
Aging is a severe social problem, particularly in developed countries. In these nations, neoplasia is one of the most common causes of death. Many treatment modalities such as molecularly targeted drugs have been proposed, but no single agent delivers complete remission. Although combined surgical intervention and radiation may facilitate achievement of this goal, old-age patients are often unable to tolerate these treatments because of impaired function of important organs such as the heart, lung, liver, and kidneys, and they succumb to the disease. Therefore, aged patients desire minimally invasive therapy.
Photodynamic therapy (PDT) is a treatment for cancer patients that uses photosensitizers that accumulate preferentially in neoplastic cells.(1) Radiation of the intracellular photosensitizers with a laser of ideal wavelength causes generation of singlet oxygen, which induces oxidative stress-induced apoptosis of cancer cells.(2) Because of less bleeding and damage in normal tissues, this therapy is now attracting attention as a cancer treatment for aged persons. A key factor in the success of this therapy is the accumulation of the cancer-specific photosensitizer.(3) Therefore, a reagent that accelerates this phenomenon is desired.
We have previously reported that a heme-transport protein, heme carrier protein 1 (HCP1), played an important role in inducing cancer-specific porphyrin accumulation.(4,5) Moreover, we have also reported that PDT effects were accelerated by high concentrations of cancer-specific reactive oxygen species from mitochondria (mitROS), which involved overexpression of HCP1.(6) This association with the signaling pathways occurs because the mitROS are involved in the activation of transcriptional factors.(7) Thus, a reagent that accelerates mitROS concentration may increase the efficacy of PDT via overexpression of HCP1.
We have also reported that non-steroidal anti-inflammatory drugs (NSAIDs), especially indomethacin (IND), cause uncoupling in the electron transport system to generate mitROS in normal gastric epithelial cells.(8,9) IND has been widely used as an antipyretic analgesic, and its properties are well understood. Although the drug is known for causing gastrointestinal damage, this side effect can be prevented because the pharmaceutical mechanisms of NSAIDs have been completely revealed.(10) Therefore, IND may be pre-administered to induce upregulation of HCP1 by excess generation of mitROS to enhance PDT effects in gastric cancer with few complications. In this study, we thus investigated whether IND may be a useful agent for enhancing PDT effects in gastric cancer with four gastric epithelial cell-lines.
Materials and Methods
Cell lines
We established previously and used the following four cell lines: a rat normal gastric mucosal cell line, RGM1, its cancer-like mutated cell line, RGK1, a manganese superoxide dismutase (MnSOD)-overexpressing RGK cell line, RGK-MnSOD and a plasmid without MnSOD cDNA transfected (vector alone) cell line, RGK-vector.(11,12) Because MnSOD expresses in the mitochondrion to specifically scavenge mitROS, MnSOD-overexpressing cells enable investigation of the influence of mitROS.
Cell culture
RGM1 was cultured in DMEM/F12 with l-glutamine (Life Technologies Co., Carlsbad, CA) and RGK cell lines were cultured in DMEM/F12 without l-glutamine. These media included 10% inactivated fetal bovine serum (Biowest LLC, Kansas City, MS) and 1% penicillin and streptomycin (Life Technologies). All cells were cultured in atmospheric air with 5% CO2 at 37°C.
Cell viability assay
Cytotoxicity of IND was examined using a Cell Counting Kit 8 (DOJINDO LABORATORIES, Kumamoto, Japan) colorimetric assay, a water-soluble tetrazolium (WST)-8 assay. Cells were seeded on a 96-well cell culture plate at a density of 1 × 104 cells/well and incubated overnight. The medium was replaced with fresh medium containing 0.1, 0.2, 0.5, 1, 2, 5, or 10 mM IND dissolved in a final concentration of 1% dimethyl sulfoxide (DMSO) and incubated for 6 h in an incubator. After incubation, cells were washed twice with PBS and the medium was replaced with fresh medium containing 10% Cell Counting Kit 8 reagent and further incubated. The absorbance at 450 nm was measured using a DTX880 multi-mode micro-plate reader (Beckman Coulter Inc., Brea, CA).
Intracellular ROS measurement using electron spin resonance
ROS generation in cells was measured using electron spin resonance (ESR) according to previous study.(13) Cells were seeded on a glass cover slide (49 × 5 × 0.2 mm) and incubated overnight. Cells were exposed to the medium containing 1 mM IND for 1 h. Cells were immersed in respiratory buffer containing 5 mM succinate (Sigma-Aldrich Japan K.K., Tokyo, Japan), 5 mM malate (Wako Pure Chem. Ind., Ltd., Osaka, Japan), 5 mM glutamate (Sigma-Aldrich Japan K.K.), 5 mM nicotinamide adenine dinucleotide (NADH) (Sigma-Aldrich Japan K.K.), and 10 mM 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) (DOJINDO). The cell-attached glass cover slide was placed on a tissue glass, and the ESR spectra were obtained by inserting the tissue glass into the device. All ESR spectra were obtained using a JEOL-TE X-band spectrometer (JEOL Ltd., Tokyo, Japan) under the following conditions: 20 mW incident microwave power, 9.42 GHz frequency, and 0.1 mT field modulation amplitude.
Immunostaining of HCP1 after IND exposure
Cells were seeded on a Lab-Tek II chamber slide (Nalge Nunc International, Rochester, NY) at a density of 2 × 105 cells/well. After cells were attached, the medium was changed to fresh medium containing 1 mM IND and incubated for 1 h. Cells were washed twice with PBS and further incubated for 24 h. Cells were washed twice with PBS and 4% paraformaldehyde (Sigma-Aldrich Japan K.K.) was added for fixation of cells. After 20 min incubation at room temperature, cells were washed twice and incubated in 0.25% Triton X-100 (Sigma-Aldrich Japan K.K.) prepared with PBS for 10 min. Cells were washed again with PBS, and immunostaining of HCP1 was performed using a VECTASTAIN ABC-AP Kit and Vector® Red Alkaline Phosphatase Substrate (VECTOR LABORATORIES, INC., Burlingame, CA) according to manufacturer protocol. Briefly, cells were treated using a blocking reagent for 20 min to avoid non-specific adsorption of the primary antibody. The blocking reagent was removed, and PBS containing HCP1 antibody (Santa Cruz Biotechnology Inc., Dallas, TX), which was diluted 50-fold, was added and incubated for 30 min. Cells were washed and incubated in PBS for 5 min twice. Cells were treated with secondary antibody solution for 30 min and washed with PBS again. Then, cells were treated with the signal amplification solution for 30 min and washed with PBS. To color cells, Vector® Red Alkaline Phosphatase Substrate reagent in 100 mM Tris-HCl buffer (pH 8.3) was added for 30 min, followed by washing with distilled water. The cells were observed and the images were obtained using an Eclipse Ti automated inverted microscope (NIKON CORPORATION, Tokyo, Japan).
HP uptake study
The amounts of hematoporphyrin (HP) incorporated in cells after exposure to IND were measured as follows. A 12-well plate was seeded with 2 × 105 cells per well and incubated overnight. The medium was changed to fresh medium containing 1 mM IND dissolved in DMSO, at a final concentration of 1%, and incubated for 1 h. After incubation, cells were washed twice with PBS and further incubated for 24 h. The medium was changed to fresh medium containing 20 µM HP, which was dissolved in ethanol, and incubated for 6 h. Cells were washed twice with PBS and dissolved in 100 µl self-prepared cell lysis buffer, which was constituted with 25 mM Tris-HCl solution (pH 7.6), 150 mM NaCl, 1% (v/v) Triton X-100 (Sigma-Aldrich Japan K.K.), 0.1% (w/v) sodium dodecyl sulfate (SDS) (Wako Pure Chem. Ind., Ltd.), and 0.2% (w/v) deoxycholic acid (Wako Pure Chem. Ind., Ltd.). The cell lysis solution was transferred into a 96-well plate and the fluorescence intensities at Ex. 415 nm and Em. 625 were measured using a Varioskan micro-plate reader (Thermo Fisher Scientific K.K., Kanagawa, Japan).
Evaluation of PDT effect
PDT effect after exposure to IND was evaluated according to a previous report.(6) Cells were seeded on a 96-well plate at a density of 1 × 104 cells/well and incubated overnight. The medium was changed to fresh medium containing 1 mM IND and incubated for 1 h. After incubation, cells were washed twice with PBS and further incubated for 24 h. The medium was changed to the above-mentioned hematoporphyrin-containing medium and incubated for 6 h. Cells were washed twice with PBS, and fresh medium without phenol red (Life Technologies) was added. Cells were irradiated with excimer dye laser light (630 nm, 1 J/cm3) using a PDT EDL-1 device (Hamamatsu Photonics K.K., Hamamatsu, Japan). After irradiation, cells were incubated for a further 24 h. The medium containing 10% Cell Counting Kit 8 was added to each well and further incubated. The absorbance at 450 nm was measured by a DTX880 multi-mode micro-plate reader.
Statistical analysis
All data are expressed as mean ± SD. Statistical analysis was performed using SPSS statistics 21 software (IBM Corporation, Armonk, NY). Tukey’s test was used for comparison of more than two data sets and Student’s t test for comparison of two data sets; p<0.05 and p<0.01 were considered statistically significant.
Results
Cytotoxicity of IND
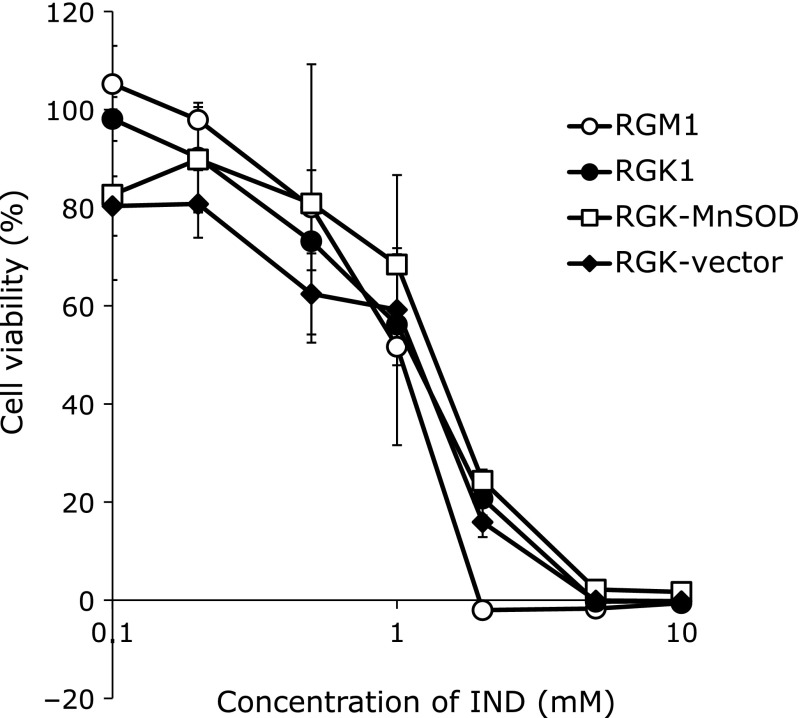
Cytotoxicity of IND was examined using a cell viability assay, a WST-8 assay (Fig. 1). Six hours exposure to IND decreased viability of all types of cells in a dose-dependent manner. Viability of all types of cells gradually decreased with exposure to IND at concentrations up to 1 mM, and acute cell death was observed at subsequent concentrations. Exposure to 1 mM IND resulted in cell damage in about 30% to 40% of cells, and there was no significant difference among all types of cells. Exposure to IND at concentrations over 2 mM resulted in obvious cellular damage, and almost all cells were dead at concentrations of IND greater than 5 mM.
Fig. 1.
Cytotoxicity of indomethacin (IND) was evaluated using a water-soluble tetrazolium (WST) assay. Cells at a density of 1 × 104 cells/well were incubated in culture medium containing IND at various concentrations for 6 h. The absorbance at 450 nm was measured and viability was calculated against cells treated with medium containing 1% DMSO. n = 4, error bar: SD.
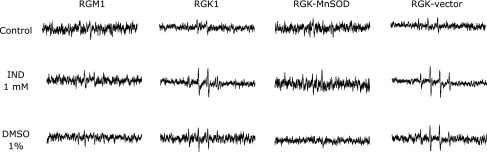
Transformation of ESR spectra by IND
ROS generation in cells with and without 1 mM IND exposure was analyzed using ESR (Fig. 2). The IND treatment obviously enhanced the ESR spectral intensity in cancer cells. Although exposure to DMSO also increased the spectral intensity, the difference was negligible in comparison with that in 1 mM IND treated cells. In RGK1 cells, the value of the intensity in the treatments decreased in the following order: 1 mM IND, DMSO, and control. In normal gastric cells, the intensity was moderately increased with IND treatment and showed no increase with DMSO treatment. In RGK-MnSOD cells, there was no enhancement of intensity with IND, unlike in both RGK1 and RGK-vector cells. This result suggested that the increased ROS generation with the IND treatment mainly originated from mitochondria.
Fig. 2.
Electron spin resonance spectra of respective living cells after incubation with 1 mM IND for 1 h were measured. Reactive oxygen species (ROS) generated by cells were trapped using 5,5-dimethyl-1-pyrroline 1-oxide (DMPO), and the signals of DMPO-adducts were detected.
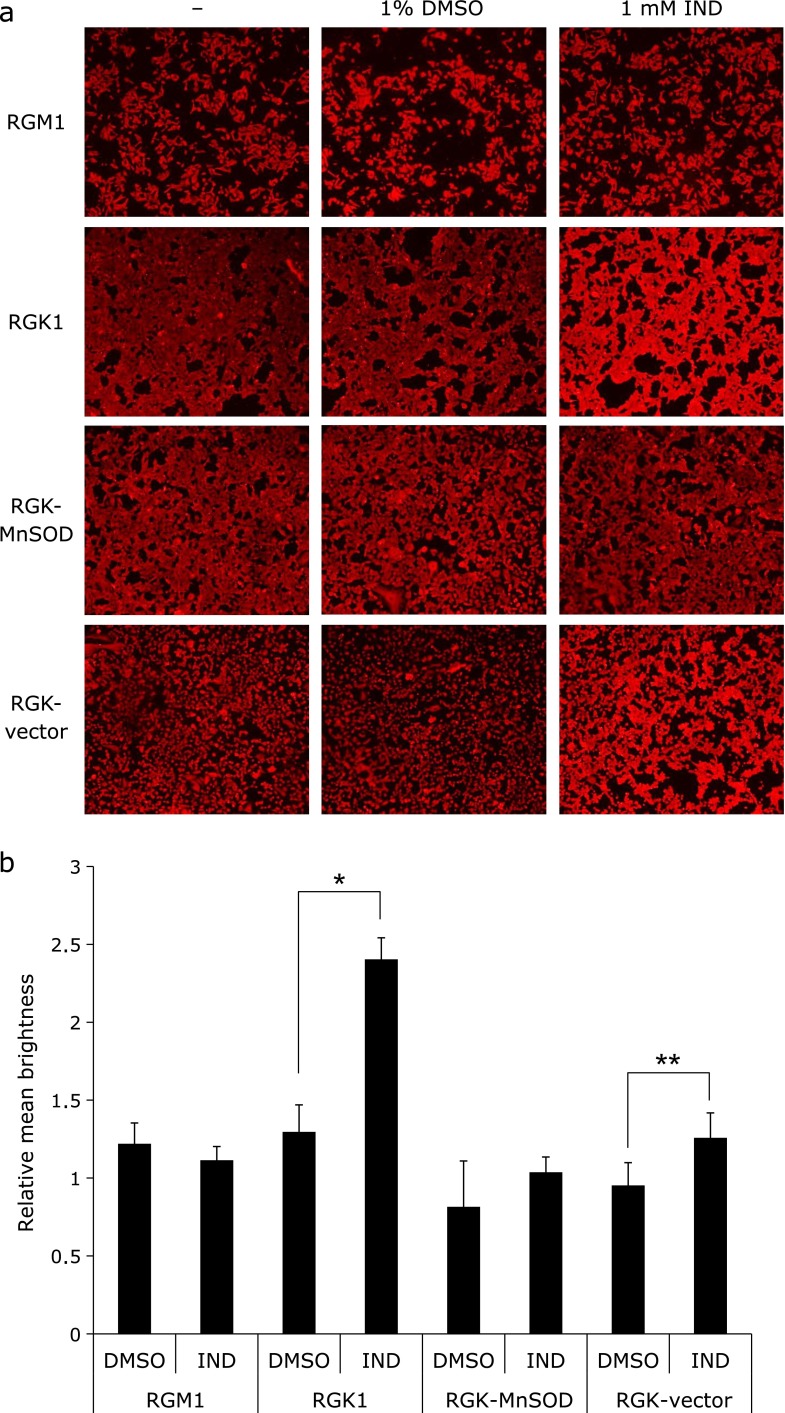
Enhancement of HCP1 expression by IND
HCP1 expression after exposure to 1 mM IND was investigated using immunostaining methods. Pictures of each cell type after staining (Fig. 3a) and the relative intensities of brightness against non-treated cells (Fig. 3b) are shown. These figures show that the intensity of brightness was significantly greater in IND-treated RGK1 cells. In addition, RGK-vector cells showed a similar result. In MnSOD-overexpressing cells, the intensities were virtually the same with both the IND treatment and DMSO treatment. These results indicated that the IND treatment induced overexpression of HCP1, particularly in cancer cells, and that ROS scavenging by MnSOD overexpression restrained the phenomenon.
Fig. 3.
Immunohistochemical analysis of heme carrier protein 1 (HCP1) in each cell type. (a) Images of cellular immunostaining of HCP1 after exposure to 1 mM IND or 1% dimethyl sulfoxide (DMSO) or no treatment for 1 h. (b) Relative mean brightness in comparison with untreated cells was calculated in each cell type. n = 4, error bar: SD; *p<0.01, **p<0.05, Student’s t test.
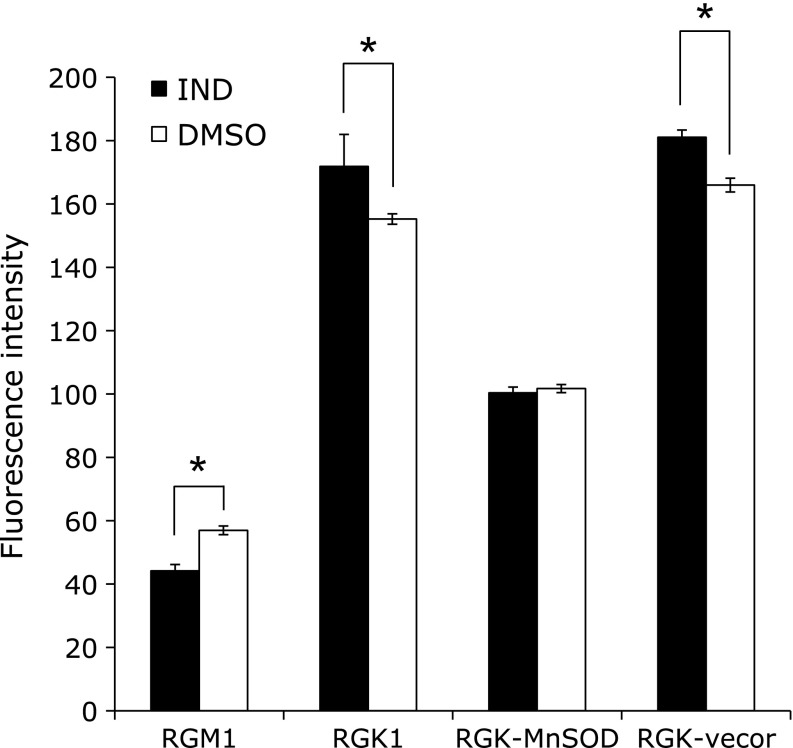
Effect of IND on cellular HP uptake
The amounts of incorporated HP in cells after IND exposure were determined in all cell types. Fig. 4 shows that the fluorescence intensities of HP increased significantly in cancer cells after IND exposure, compared with that after DMSO treatment. On the other hand, in MnSOD-overexpressing cancer cells, the IND treatment showed no significant difference from the DMSO treatment. In addition, normal cells showed higher intensities with the DMSO treatment than the IND treatment. These results indicated that IND accelerated the incorporation of HP into cancer cells and expression of MnSOD attenuated the incorporation. Furthermore, the IND treatment resulted in less acceleration of HP accumulation in normal cells.
Fig. 4.
The amounts of hematoporphyrin (HP) incorporated in cells after treatment with 1 mM IND or 1% DMSO were measured by detecting the fluorescence intensity at Ex. 415 nm and Em. 625 nm. n = 4, error bar: SD; *p<0.05, Student’s t test.
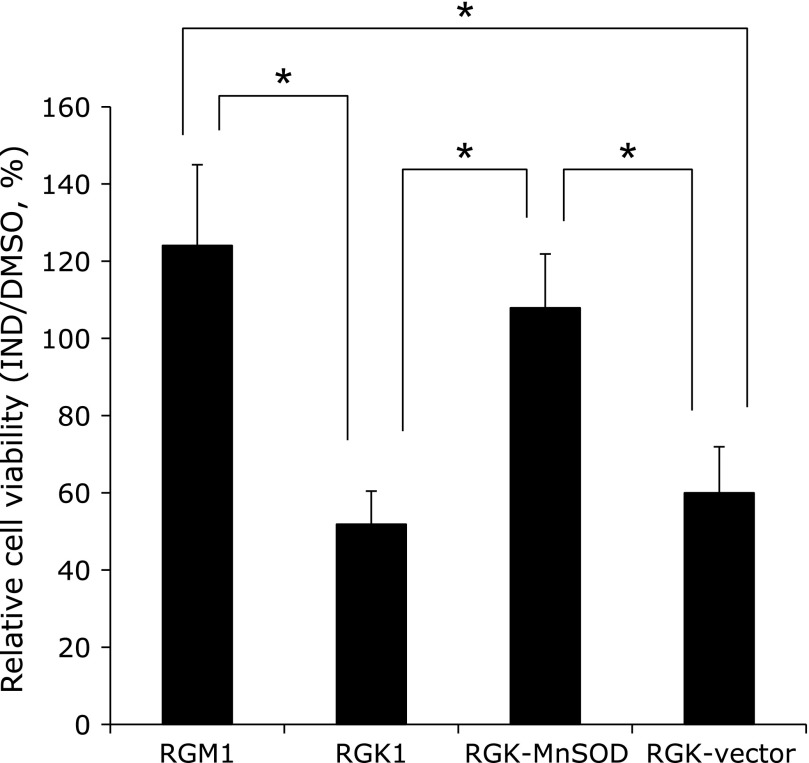
Influence of IND on PDT effect
To evaluate the effect of IND on PDT, cells were exposed to HP after IND treatment, and then irradiated with the laser light; thereafter, viabilities were investigated. Fig. 5 shows the relative cell viabilities after treatment with IND against treatment with DMSO, and that cell viabilities were significantly decreased in RGK1 and RGK-vector cells, compared with RGM1 cells. Of note, the reduction of viability in cancer cells was inhibited by overexpression of MnSOD.
Fig. 5.
Relative cell viabilities after administering HP-PDT to cells treated with IND or DMSO were calculated. n = 4, error bar: SD; *p<0.01, Tukey HSD.
Discussion
In this study, we found for the first time that a non-steroidal anti-inflammatory drug, IND, increased the targeted effects of PDT in cancer cells. Because use of IND is widespread and its properties well known, pretreatment with IND is a possible means to improving the efficacy of PDT.
IND exposure significantly increased ESR signal intensities of superoxide anion in cancer cells, whereas the increase of the intensities was less in normal epithelial cells. Although the reason for this discrepancy between the effects of IND on superoxide anion concentrations in cancer cells and normal cells remains unknown, we propose that IND may be an ideal drug for enhancing PDT effects because this reagent appears to have differing effects on cancer and normal epithelial porphyrin accumulation.
Overexpression of MnSOD suppressed the cancer-cell-specific ESR intensity increase of superoxide anion. Cells with transfection of the MnSOD gene overproduce the protein, and MnSOD proteins accumulate in the mitochondrial matrix and scavenge mitROS.(14) Therefore, comparison of cellular ROS levels between wild-type cells and MnSOD-overexpressing cells determines whether the origin of ROS is mitochondrial or not. Thus, we concluded that the increased ROS production after IND administration likely originated from mitochondria in cancer cells.
There have been several reports that dying cells generate ROS.(15,16) The ROS detected in this study might have originated from dying cells. However, cellular viability was reduced by about 50% in all the cell types, while definite ESR spectra were observed with exposure to 1 mM IND. We thus concluded that these ESR signals originated from living cells, not dying cells.
The ROS from mitochondria are associated with the expression of HCP1. HCP1 is a solute carrier, SLC46A1, that transports both heme and folate.(17) We have previously reported that cancer-specific HCP1 expression induced by high concentrations of ROS was associated with cancer-specific porphyrin accumulation, which presented us an ideal cancer therapy, PDT.(6) We evaluated the relationship between increased mitROS after IND treatment and HCP1 expression by using immunostaining assays. HCP1 was upregulated in RGK1 cells by pretreatment with IND, whereas this obvious difference was not observed in RGK-MnSOD cells. These results indicate that over-generation of mitROS induced by IND resulted in increased HCP1 expression and HP uptake. Of note, upregulation of HCP1 induced by mitROS derived from IND is likely to cause intensification of cancer cellular HP incorporation. As a result of this larger amount of HP accumulation in cancer cells, subsequent PDT effects may be increased.
In RGM1 cells, expressions of HCP1, uptakes of HP, and PDT effects with DMSO treatment were significantly greater without than with IND. In our previous study, IND treatment was associated with changes in MnSOD levels; thus, these phenomena could be explained by the enhancement of MnSOD expression or the induction of other defense mechanisms in response to oxidative stress.(8) On the contrary, DMSO treatment with IND showed significantly higher values than that without IND treatment in the expressions of HCP1, uptakes of HP, and PDT effects in cancer cells. As mentioned above, RGK1 cellular intensity of ESR spectra after IND treatment showed a definite increase in comparison with that without IND. This phenomenon suggests that the cancer cells were unable to upregulate MnSOD or other defense factors against oxidative stress because these mechanisms had already been maximally induced. In this study, we concluded that cancer cells cannot contend with the oxidative stress induced by IND treatment.
Shayeghi et al.(18) reported that hypoxic conditions upregulated HCP1. In hypoxia, hypoxia inducible factor (HIF) was stabilized; thereafter, this protein, especially HIF-1, was associated with abundant signaling of genes and expression of proteins, including HCP1 expression.(18,19) Furthermore, Chandel et al.(20) reported that generation of mitROS was accelerated during hypoxia and the overproduced ROS were necessary to stabilize HIF-1α, a subunit of HIF-1. Thus, HCP1 is likely to be mainly expressed in cancer cells because neoplastic environments tend to be hypoxic and cancer cells generate more mitROS than do normal cells.(6,13) In addition, reinforcement of mitROS generation by IND may further stabilize HIF-1 and contribute to overexpression of HCP1.
Meanwhile, Mateo et al.(21) reported that high concentrations of nitric oxide (NO) generated both exogenously and endogenously affect stabilization of HIF-1α, even in normoxic conditions. In addition, Weidinger et al.(22) have elucidated that although endogenous NO is generated by inducible NO synthase (iNOS), mitROS are involved in expression of iNOS and the NO production cycle. Accordingly, upregulation of iNOS by mitROS may promote a larger amount of NO production, resulting in overexpression of HCP1 because of stabilization of HIF-1α induced by NO. IND may contribute to the expression of HCP1 by such a signaling pathway.
In conclusion, we clarified that mitochondrial ROS induced by IND activate the expression of HCP1, a transporter of porphyrin compounds, and enhance the cancer-specific effects of PDT. We are currently studying the details of the signaling pathway involved in IND treatment in cancer cells.
Acknowledgments
This study was partially supported by JSPS Grant-in-Aid for Scientific Research (KAKENHI) #15K15188 and #23106005A.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hirohara S, Obata M, Ogata S, et al. Cellular uptake and photocytotoxicity of glycoconjugated chlorins in HeLa cells. J Photochem Photobiol B. 2005;78:7–15. doi: 10.1016/j.jphotobiol.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Mroz P, Yaroslavsky A, Kharkwal GB, Hamblin MR. Cell death pathways in photodynamic therapy of cancer. Cancers (Basel) 2011;3:2516–2539. doi: 10.3390/cancers3022516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirohara S, Kawasaki Y, Funasako R, et al. Sugar and heavy atom effects of glycoconjugated chlorin palladium complex on photocytotoxicity. Bioconjug Chem. 2012;23:1881–1890. doi: 10.1021/bc300223j. [DOI] [PubMed] [Google Scholar]
- 4.Hiyama K, Matsui H, Tamura M, et al. Cancer cells uptake porphyrins via heme carrier protein 1. J Porph Phtal. 2013;17:36–43. [Google Scholar]
- 5.Takada T, Tamura M, Yamamoto T, Matsui H, Matsumura A. Selective accumulation of hematoporphyrin derivative in glioma through proton-coupled folate transporter SLC46A1. J Clin Biochem Nutr. 2014;54:26–30. doi: 10.3164/jcbn.13-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito H, Matsui H, Tamura M, Majima HJ, Indo HP, Hyodo I. Mitochondrial reactive oxygen species accelerate the expression of heme carrier protein 1 and enhance photodynamic cancer therapy effect. J Clin Biochem Nutr. 2014;55:67–71. doi: 10.3164/jcbn.14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo L, Li L, Wang W, Pan Z, Zhou Q, Wu Z. Mitochondrial reactive oxygen species mediates nicotine-induced hypoxia-inducible factor-1α expression in human non-small cell lung cancer cells. Biochem Biophys Acta. 2012;1822:852–861. doi: 10.1016/j.bbadis.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Nagano Y, Matsui H, Shimokawa O, et al. Rebamipide attenuates nonsteroidal anti-inflammatory drugs (NSAID) induced lipid peroxidation by the manganese superoxide dismutase (MnSOD) overexpression in gastrointestinal epithelial cells. J Physiol Pharmacol. 2012;63:137–142. [PubMed] [Google Scholar]
- 9.Saito R, Tamura M, Matsui H, et al. Qing Dai attenuates nonsteroidal anti-inflammatory drug-induced mitochondrial reactive oxygen species in gastrointestinal epithelial cells. J Clin Biochem Nutr. 2015;56:8–14. doi: 10.3164/jcbn.14-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motawi TK, Abd Elgawad HM, Shahin NN. Modulation of indomethacin-induced gastric injury by spermine and taurine in rats. J Biochem Mol Toxicol. 2007;21:280–288. doi: 10.1002/jbt.20194. [DOI] [PubMed] [Google Scholar]
- 11.Ito H, Tamura M, Matsui H, Majima HJ, Indo HP, Hyodo I. Reactive oxygen species involved cancer cellular specific 5-aminolevulinic acid uptake in gastric epithelial cells. J Clin Biochem Nutr. 2014;54:81–85. doi: 10.3164/jcbn.13-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Indo HP, Davidson M, Yen HC, et al. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Tamura M, Matsui H, Tomita T, et al. Mitochondrial reactive oxygen species accelerate gastric cancer cell invasion. J Clin Biochem Nutr. 2014;54:12–17. doi: 10.3164/jcbn.13-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirai F, Motoori S, Kakinuma S, et al. Mitochondrial signal lacking manganese superoxide dismutase failed to prevent cell death by reoxygenation following hypoxia in a human pancreatic cancer cell line, KP4. Antioxid Redox Signal. 2004;6:523–535. doi: 10.1089/152308604773934288. [DOI] [PubMed] [Google Scholar]
- 15.Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–141. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 16.Sakon S, Xue X, Takekawa M, et al. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiu A, Min SH, Jansen M, et al. Rodent intestinal folate transporters (SLC46A1): secondary structure, functional properties, and response to dietary folate restriction. Am J Physiol Cell Physiol. 2007;293:C1669–C1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- 18.Shayeghi M, Latunde-Dada GO, Oakhill JS, et al. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 19.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 20.Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 21.Mateo J, García-Lecea M, Cadenas S, Hernández C, Moncada S. Regulation of hypoxia-inducible factor-1α by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376 (Pt 2):537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weidinger A, Müllebner A, Paier-Pourani J, et al. Vicious inducible nitric oxide synthase-mitochondrial reactive oxygen species cycle accelerates inflammatory response and causes liver injury in rats. Antioxid Redox Signal. 2015;22:572–586. doi: 10.1089/ars.2014.5996. [DOI] [PubMed] [Google Scholar]