Abstract
Caffeic acid phenethyl ester (CAPE), one of the major polyphenols, exhibits anti-oxidative, anti-bacterial, and anti-cancer properties. Atherosclerosis is a chronic inflammatory disease, the progression of which is closely related to the accumulated adhesion of inflammatory monocytes/macrophages to the endothelium. We herein determined whether CAPE and its derivatives suppressed THP-1 cell adhesion to human umbilical vein endothelial cells (HUVEC). Of the four polyphenols tested, CAPE significantly suppressed the 12-O-tetradecanoylphorbol 13-acetate (TPA)-elicited expression of cluster for differentiation (CD) 11b, 14, and 36, and this was accompanied by the inhibition of THP-1 cell adhesion to HUVEC. CAPE also suppressed the activation of TPA-elicited nuclear factor-κB (NF-κB) and accumulation of NADPH oxidase 2 (NOX2)-derived reactive oxygen species (ROS), but did not affect extracellular signal-regulated kinase (ERK) phosphorylation. Taken together, these results demonstrated that CAPE suppressed THP-1 cell adhesion to HUVEC through, at least in part, the NF-κB, NOX2, and ROS-derived signaling axis.
Keywords: caffeic acid phenethyl ester, cluster for differentiation, monocyte adhesion, nuclear factor-κB, NADPH oxidase 2
Introduction
Atherosclerosis is a chronic inflammatory disease that is initiated by the activation of endothelial cells following monocyte/macrophage adhesion to endothelial cells.(1,2) A previous study reported that some adhesion molecules including vascular adhesion molecule-1 (VCAM-1) and intracellular adhesion molecule-1 (ICAM-1), which are expressed in activated endothelial cells, played critical roles during the initiation step of atherosclerosis.(3) Other adhesion molecules such as cluster for differentiation (CD) 11b, 14, and 36, which are expressed in monocytes/macrophages, were also shown to be involved in the progression of monocyte/macrophage adhesion to endothelial cells following the formation of foaming cells.(4–6) Previous findings demonstrated that intracellular reactive oxygen species (ROS) such as superoxide and hydrogen peroxide induced VCAM-1, ICAM-1, and CDs, and these processes exacerbated atherogenesis.(7,8) Therefore, scavenging the excess production of ROS may suppress atherogenesis.
Caffeic acid phenethyl ester (CAPE) is a major polyphenol commonly contained in fruits, vegetables, wine, propolis, and coffee, and its analogues, such as caffeic acid (CA), ferulic acid (FA), and chlorogenic acid (CGA), exist in abundant amounts in coffee.(9–12) These polyphenols include the plural phenolic hydroxyl group in their molecules, which is known to have various health benefits as well as anti-oxidative properties.(13–15) Moreover, it has been reported that CAPE has the ability to inhibit nuclear factor-κB (NF-κB) in human leukemic U937 cells.(16) The prevention of arteriosclerosis through the intake of coffee has already been reported and has been attributed to the polyphenols in coffee.(17,18)
In this study, we examined the effects of CA, FA, CGA, and CAPE on 12-O-tetradecanoylphorbol 13-acetate (TPA)-elicited THP-1 cell adhesion to human umbilical vein endothelial cells (HUVEC). Of these polyphenols, CAPE markedly suppressed TPA-elicited adhesion and was accompanied by the inhibited induction of CDs. Furthermore, the inhibitory effects of CAPE were, at least in part, due to the suppressed activation of NF-κB and NADPH oxidase 2 (NOX2)-derived ROS signaling.
Materials and Methods
Reagents
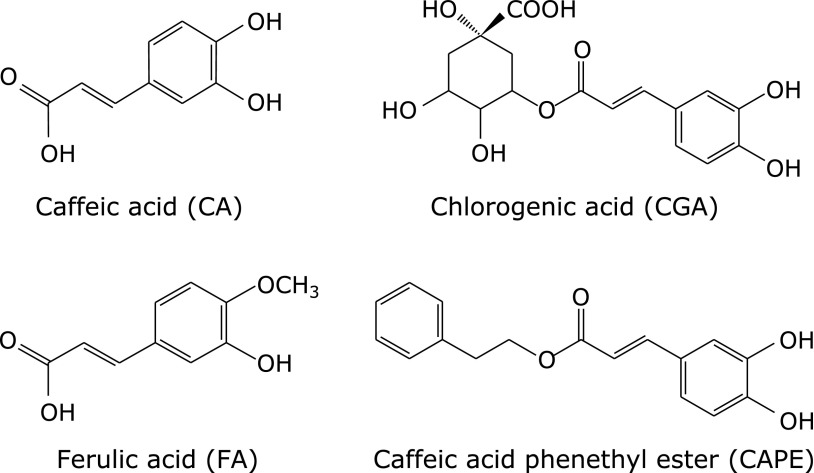
TPA was purchased from Sigma-Aldrich Co. (St. Louis, MO). 5-(and-6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) was purchased from Molecular Probes (Eugene, OR). Anti-mitogen-activated protein kinase kinase (MEK) rabbit polyclonal antibody, anti-phospho-MEK rabbit polyclonal antibody, anti-extracellular-signal regulated kinase (ERK) rabbit monoclonal antibody, anti-phospho-ERK mouse monoclonal antibody, and anti-Lamin B1 rabbit polyclonal antibody were purchased from Cell Signaling Technology (Danvers, MA). An anti-p47phox rabbit polyclonal antibody and anti-actin mouse monoclonal antibody were purchased from Millipore Co. (Billerica, MA). Biotin-conjugated goat anti-rabbit and -mouse IgG (H + L) antibodies were purchased from Invitrogen (Carlsbad, CA). CA, FA, and CGA were purchased from Sigma-Aldrich Japan Ltd. (Tokyo, Japan), (Fig. 1) and CAPE, U0126 and Calcein-AM were purchased from Wako Pure Chem. Ind., Co., Ltd. (Osaka, Japan). BAY11-7082 was purchased from Calbiochem (San Diego, CA).
Fig. 1.
Structures of four coffee polyphenols: caffeic acid (CA), ferulic acid (FA), chlorogenic acid (CGA) and caffeic acid phenethyl ester (CAPE).
Cell culture
THP-1 cells were cultured in RPMI1640 medium containing 10% (v/v) heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, and 100 µg/ml streptomycin at 37°C in a humidified 5% CO2 incubator. Regarding the differentiation of THP-1 cells, they were seeded at 2 × 106 cells in 3.5-cm dishes, and 100 nM TPA was added. After differentiation, the cells were scraped and washed with cold phosphate-buffered saline (PBS), followed by the extraction of total RNA, a flow cytometric analysis, and Western blotting.
Reverse-transcriptional polymerase chain reaction (RT-PCR) analysis
After THP-1 cells had been treated with the various reagents, they were lysed in 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA). The preparation of cDNA and RT-PCR were performed by a previously described method with minor modifications.(19) The primer sequences used in the present study were as follows: CD11b, sense 5'-CCC CCA GGT CAC CTT CTC CG-3'; antisense 5'-GCT CTG TCG GGA AGG AGC CG-3' [525 base pair (bp)]; CD14, sense 5'-CGA GGA CCT AAA GAT AAC CGG C-3'; antisense 5'-GTT GCA GCT GAG ATC GAG CAC-3' (510 bp); CD36, sense 5'-TGC CTC TCC AGT TGA AAA CCC-3'; antisense 5'-GCA ACA AAC ATC ACC ACA CCA-3' (484 bp); NOX2, sense 5'-GGA GTT TCA AGA TGC GTG GAA CTA-3'; antisense 5'-GCC AGA CTC AGA GTT GGA GAT GCT-3' (550 bp); p47phox, sense 5'-ATG AGC CTG CCC ACC AAG AT-3'; antisense 5'-CGT CGT CTT TCC TGA TGA CC-3' (508 bp); 18s ribosomal RNA (rRNA), sense 5'-CGG CTA CCA CAT CCA AGG AA-3', antisense 5'-GCT GGA ATT ACC GCG GCT-3' (187 bp). These PCR products were loaded on a 2% (w/v) agarose gel for electrophoresis, and a densitometric analysis of the PCR products was performed with Multi Gauge V3.0 (Fuji Film, Tokyo, Japan).
Western blotting
Whole cell extracts and membrane fractions were prepared with lysis buffers as described previously.(19) Nuclear fraction was prepared described below. The cells were lysed in lysis buffer A (10 mM HEPES, pH 7.8 containing 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, proteinase inhibitors, and 0.1% Triton X-100) and incubated on ice for 5 min. After centrifugation at 1,300 g for 5 min, soluble fraction was removed and the remaining pellets were further washed with lysis buffer A followed by additional centrifugation at 1,300 g for 5 min. After removing supernatant, the remaining pellets were dissolved in lysis buffer B (10 mM HEPES, pH 7.8 containing 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, proteinase inhibitors, and 1% Triton X-100) and sonicated briefly using an ultrasonic homogenizer Vivracell VC100 (Sonic & Materials, Danbury, CT). After centrifugation at 15,000 g for 10 min, soluble fraction was collected as nuclear fraction. Extracts containing 20 µg of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% (w/v) polyacrylamide gel followed by electrophoretic transferal onto polyvinylidene difluoride (PVDF) membranes. The membranes were then incubated with the respective specific primary antibodies (1:3,000). The blots were incubated with a biotin-conjugated goat anti-rabbit or -mouse IgG antibody (1:3,000) and then with ABC reagents (Vector Laboratories, Inc., Burlingame, CA) (1:5,000). Bands were detected using SuperSignal West Pico (Thermo Scientific, Rockford, IL) or ImmunoStar LD (Wako Pure Chem.) and imaged using an LAS-3000 UV mini apparatus (Fuji Film).
Measurement of intracellular ROS accumulation
Intracellular ROS accumulation was measured according to a previously described method with minor modifications.(19,20) Briefly, cells were suspended followed by staining with 10 µM carboxy-H2DCFDA in PBS containing 1% paraformaldehyde (PFA) for 20 min in a 5% CO2 incubator. After being incubated, the cells were washed with ice-cold PBS three times and resuspended in 1% PFA-PBS. The fluorescence intensities of DCF were analyzed using FACS Verse (BD Biosciences, San Jose, CA). Analyses were performed using BD CellQuest Pro Software.
THP-1 cell adhesion to human umbilical vein endothelial cells (HUVECs)
HUVECs were grown in CS-C medium containing 10% FCS on 4-well plates. When the cells reached 80% confluence, this experience was carried out. THP-1 cells treated with 50 µM CAPE and/or 100 nM TPA for 24 h were labeled with 0.67 µM calcein-AM in RPMI 1640 medium containing 10% heat-inactivated FCS for 30 min. After washing twice with PBS, the labeled THP-1 cells were seeded at a density of 1.0 × 105 cells/well onto HUVECs and incubated for 4 h in a CO2 incubator. After being incubated, non-adherent cells were removed by gentle washing twice with PBS and images of random fields were captured using the HS All In One fluorescence microscope BZ-9000 (Keyence, Osaka, Japan).
Statistical Analysis
Data are expressed as the means ± SD of three independent experiments. Statistical evaluations of the data were performed using ANOVA followed by a post hoc Bonferroni test. A p value of less than 0.05 was considered significant.
Results
Effects of coffee polyphenols on TPA-elicited expression of CD11b, CD14, and CD36 in THP-1 cells
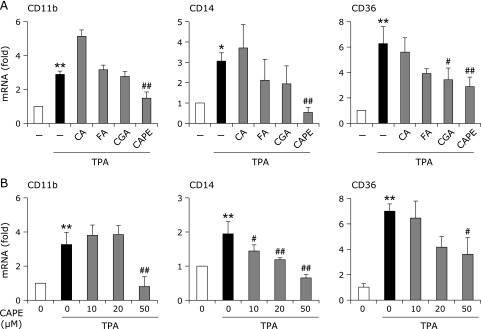
The treatment of THP-1 cells with TPA significantly induced CD11b, CD14, and CD36, which are well-known monocyte differentiation markers and adhesion molecules. Of the 4 polyphenols tested, CA and FA did not affect the TPA-elicited CDs expression, whereas CGA significantly suppressed the induction of CD36 (Fig. 2A). On the other hand, the pretreatment with CAPE markedly suppressed these inductions in a dose-dependent manner (Fig. 2B).
Fig. 2.
Effects of coffee polyphenols on TPA-elicited expression of CD11b, CD14, and CD36 in THP-1 cells. (A) THP-1 cells were pretreated with 50 µM CA, FA, CGA, or CAPE for 1 h, and then treated with or without 100 nM TPA for 24 h. (B) Cells were pretreated with the indicated concentrations of CAPE for 1 h, and then treated with 100 nM TPA for 24 h. RT-PCR was performed after the treatment. RT-PCR data were normalized using 18s rRNA levels (*p<0.05, **p<0.01 vs untreated cells, #p<0.05, ##p<0.01 vs TPA-treated cells).
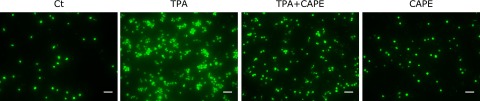
The pretreatment with CAPE suppressed TPA-induced THP-1 cell adhesion to HUVECs
It has been well recognized that monocyte/macrophage adhesion to the endothelium is the initiation step for the progression of atherosclerosis. Therefore, we next investigated whether CAPE suppressed TPA-elicited THP-1 cell adhesion to HUVEC using calcein-AM. As shown in Fig. 3, TPA markedly induced THP-1 cell adhesion, and the pretreatment with CAPE suppressed this adhesion.
Fig. 3.
The pretreatment with CAPE suppressed TPA-induced THP-1 cell adhesion to HUVECs. THP-1 cells were pretreated with 50 µM CAPE for 1 h, and then treated with 100 nM TPA for 24 h. After collecting THP-1 cells, the cells were stained with calcein-AM for 30 min. Cells were then seeded onto HUVEC and incubated for 4 h, and cell adhesion to HUVEC was detected using a fluorescence microscope. The scale bar shows 50 µm.
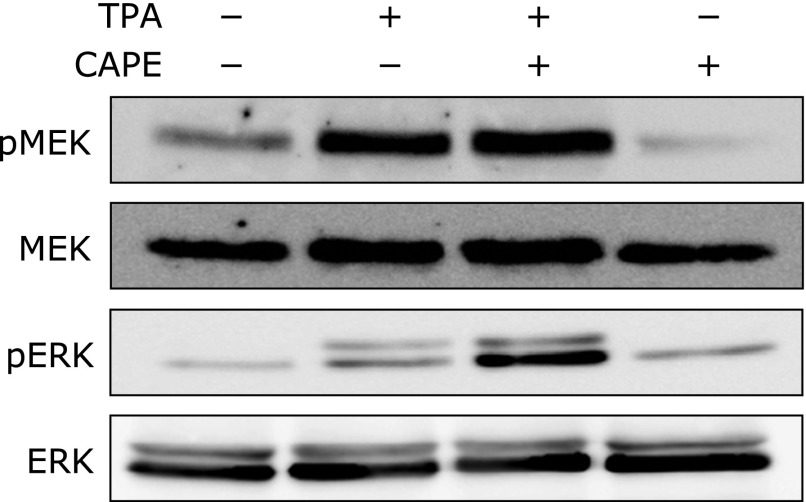
Effects of CAPE on TPA-elicited MEK and ERK phosphorylation
We previously reported that MEK/ERK signaling functions as key molecules, which regulate CD expression in THP-1 cells and U937 cells. Accordingly, we hypothesized that the suppression of the TPA-triggered expression of CD and THP-1 cell adhesion to HUVEC may be mediated through MEK/ERK pathways. As shown in Fig. 4, the TPA treatment induced the phosphorylation of MEK and ERK; however, CAPE did not affect this phosphorylation, suggesting that CAPE suppressed the TPA-elicited expression of CD in a MEK/ERK-independent manner.
Fig. 4.
Effects of CAPE on TPA-elicited MEK and ERK phosphorylation. THP-1 cells were pretreated with (+) or without (–) 50 µM CAPE for 1 h, and then treated with (+) or without (–) 100 nM TPA for 15 min. After the cells were treated, phosphorylated-and total MEK or ERK levels were determined by Western blotting.
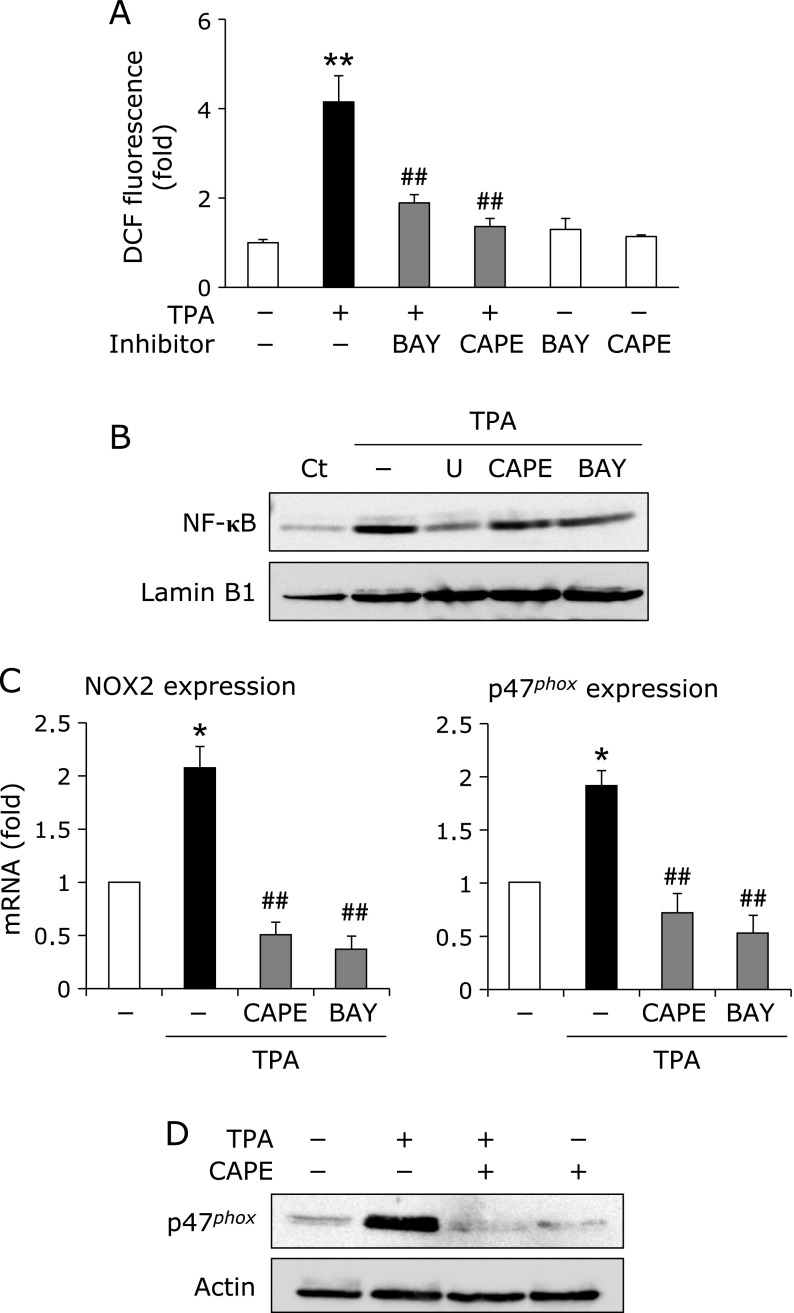
CAPE suppressed TPA-elicited ROS generation by inhibiting NF-κB and NOX2 activation in THP-1 cells
We previously reported that luteolin, a major flavonoid, suppresses TPA-elicited CD expression through the inhibition of p47phox induction and its membrane translocation. Therefore, it was speculated that CAPE suppressed THP-1 cell adhesion through the inhibition of ROS generation systems as well. The treatment with TPA for 24 h induced, while the pretreatment with CAPE significantly suppressed the intracellular ROS accumulation (Fig. 5A). Moreover, pretreatment with BAY11-7082, an inhibitor of NF-κB, also suppressed TPA-elicited intracellular ROS accumulation (Fig. 5A). The pretreatment with U0126, an inhibitor of MEK/ERK, BAY11-7082, or CAPE blocked the TPA-elicited nuclear translocation of NF-κB, and also reduced mRNA levels of NOX2 and p47phox, a main component of NOX2 (Fig. 5B and C). Furthermore, the pretreatment with CAPE completely blocked TPA-induced p47phox translocation to the membrane (Fig. 5D), suggesting that the inhibitory effects of CAPE on the TPA-induced expression of CD, and THP-1 cell adhesion may be related to the suppressed activation of NF-κB and intracellular ROS accumulation.
Fig. 5.
CAPE suppressed TPA-elicited ROS generation by inhibiting NF-κB and NOX2 activation in THP-1 cells. (A) ROS accumulation. THP-1 cells were pretreated with (+) or without (–) 10 µM BAY11-7082 (BAY) or 50 µM CAPE for 1 h, and then treated with (+) or without (–) 100 nM TPA for 24 h. After this treatment, intracellular ROS accumulation was measured by flow cytometry (**p<0.01 vs vehicle, ##p<0.01 vs TPA-treated cells). (B) Nuclear translocation of NF-κB. Cells were pretreated with 5 µM U0126 (U), 50 µM CAPE, or 10 µM BAY for 1 h, and then treated with 100 nM TPA for 4 h. After this treatment, the nuclear translocation of NF-κB was determined by Western blotting. (C) mRNA analysis. Cells were pretreated with 50 µM CAPE or 10 µM BAY for 1 h, and then treated with 100 nM TPA for 6 h. The mRNA expression of NOX2 and p47phox was then determined. RT-PCR data were normalized using 18s rRNA levels (*p<0.05 vs untreated cells, ##p<0.01 vs TPA-treated cells). (D) Membrane translocation of p47phox. Cells were pretreated with (+) or without (–) 50 µM CAPE for 1 h, and then treated with (+) or without (–) 100 nM TPA for 12 h. After this treatment, the membrane translocation of p47phox was determined by Western blotting.
Discussion
Atherosclerosis, a chronic inflammatory disease, is characterized by the excess accumulation of activated and foamed macrophages in the intima.(21,22) Foamed macrophages generate ROS, secrete inflammatory cytokines such as tumor necrosis factor-α and transforming growth factor-β, and advance vascular smooth muscle cell proliferation and migration, leading to neointima hypertrophy and infarction.(7,8) Therefore, the regulation of monocyte adhesion to endothelial cells as well as blockade of foaming cell formation may suppress the progression of atherosclerosis.
CAPE is a polyphenol that exhibits several physiological properties such as anti-oxidative, anti-bacterial, and anti-cancer effects. We previously reported that the administration of CAPE induced human breast cancer MCF-7 cell apoptosis by inducing endoplasmic reticulum stress.(23) On the other hand, previous studies demonstrated that CAPE exhibited potent radical scavenging activity and the ability to induce heme oxygenase-1.(24,25) Hence, CAPE may suppress the progression of atherosclerosis by inhibiting ROS-related signaling. As expected, of the 4 polyphenols tested, CAPE markedly suppressed TPA-elicited CD induction (Fig. 2). Moreover, we also determined that CAPE suppressed TPA-elicited THP-1 cell adhesion to HUVEC (Fig. 3). Based on our previous findings,(20) the inhibitory effects of CAPE on the induction of CD was considered to depend on ROS signaling mediated by the TPA-elicited activation of NOX2. The anti-oxidative properties of polyphenols have generally been attributed to the catechol ring in its structure;(26) however, its hydrophobicity also determines physiological activities. We previously reported that luteolin, one of the major flavonoids with a catechol ring, suppressed the TPA-elicited expression of CD in THP-1 cells. On the other hand, tricetin, another flavonoid with a 3 hydroxyl group in ring B, did not affect the induction of CD.(27) A previous study reported differences in the absorption and accumulation of nobiletin, which has a methoxy group, and luteolin in rat tissues.(28) Although it currently remains unknown whether polyphenols are taken up by THP-1 cells effectively, differences in the cell permeabilities of the 4 polyphenols examined may be involved in their inhibitory effects against the TPA-elicited CD induction.
Previous studies showed that MAPK, including ERK, c-jun N-terminal kinase, and p38-MAPK, played critical roles in physiological processes such as cell proliferation, differentiation, and apoptosis.(29,30) Of these, ERK-derived signal transduction was found to induce the expression of CD during monocytic differentiation.(19,20) However, CAPE rather increased TPA-elicited MEK/ERK phosphorylation (Fig. 4), and this finding is same to our previous report.(27) Recently, it has been reported that CAPE induces heme oxygenease-1, an anti-oxidative enzyme, through the activation of NF-E2-related factor 2 (Nrf2) in HepG2 cells.(31) Accordingly, it raises the possibility that CAPE increases a resistance against intracellular oxidative stress, and this leads to suppress TPA-elicited CD induction; however, some additional experiments will be necessary to determine the meaning of CAPE-elicited ERK activation in the regulation of CD expression.
It has been well recognized that ROS act as signaling molecules in various physiological processes including monocytic differentiation to macrophages,(32–35) and TPA-elicited ROS generation is derived from the activation of NOX2, a multisubunit enzymatic complex comprising two membrane-bound subunits, gp91 and p22phox.(36,37) The activation of NOX is regulated by cytoplasmic subunits such as p47phox, p67phox, and the small G protein Rac1. On the other hand, the TPA-elicited activation of NF-κB has been shown to up-regulate some inflammatory cytokines such as tumor necrosis factor-α and is involved in NOX2-derived ROS generation.(38,39) As shown in Fig. 5, pretreatment with CAPE completely blocked NF-κB activation following intracellular ROS accumulation. It might be possible that CAPE directly scavenges TPA-elicited intracellular ROS generation, because it has catechol ring. However, pretreatment with BAY11-7082 also significantly suppressed TPA-elicited intracellular ROS accumulation as well as CAPE (Fig. 5A), suggesting that CAPE suppressed TPA-elicited ROS accumulation through the inhibition of NF-κB pathways. Hence, the inhibitory effects of CAPE on the TPA-induced expression of CD, and THP-1 cell adhesion might be closely related to the suppressed activation of NF-κB and intracellular ROS accumulation.
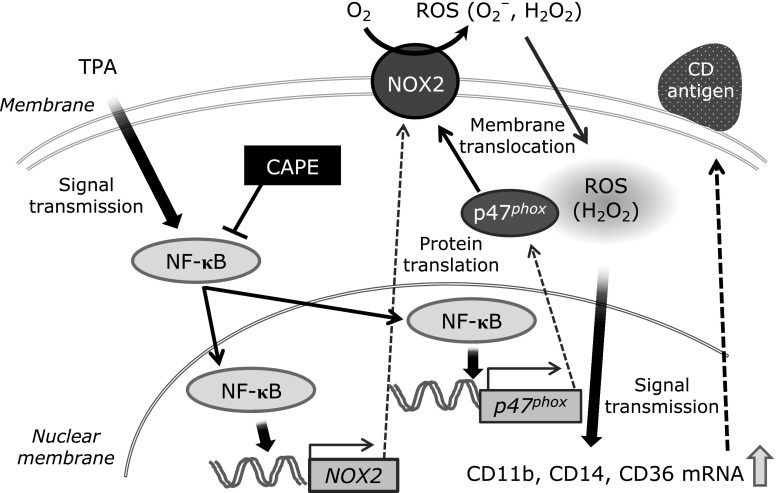
In the present study, we demonstrated that CAPE affected TPA-elicited THP-1 cell adhesion to HUVEC by inhibiting the activation of NF-κB and NOX2-derived intracellular ROS accumulation (Fig. 6). These results show the usefulness of CAPE in the treatment of the initial arteriosclerotic condition and provide information for improving the quality of life of patients by preventing atherosclerosis.
Fig. 6.
Proposed model for the mechanism underlying the inhibitory effect of CAPE on TPA-elicited CD induction and THP-1 cell adhesion to HUVEC. CAPE blocks the TPA-elicited induction of CD by suppressing NF-κB nuclear translocation and NOX2-derived ROS generation. This axis may be involved in the suppression of TPA-elicited THP-1 cell adhesion to HUVEC.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion for Science (T. K.: No. 26460070), a grant for the encouragement of young scientists from Gifu Pharmaceutical University (T. K.), All Japan Coffee Association (T. A.), and Api Co., Ltd. (T. A.).
Abbreviations
- bp
base pair
- CA
caffeic acid
- CAPE
caffeic acid phenethyl ester
- carboxy-H2DCFDA
5-(and-6)-carboxy-2',7'-dichlorodihydrofluorescein diacetate
- CD
cluster for differentiation
- CGA
chlorogenic acid
- ERK
extracellular signal-regulated kinase
- FA
ferulic acid
- FCS
fetal calf serum
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intracellular adhesion molecule-1
- MEK
mitogen-activated protein kinase kinase
- NF-κB
nuclear factor-κB
- NOX
NADPH oxidase
- Nrf2
NF-E2-related factor 2
- PBS
phosphate-buffered saline
- PFA
paraformaldehyde
- PVDF
polyvinylidene difluoride
- ROS
reactive oxygen species
- rRNA
ribosomal RNA
- RT-PCR
reverse-transcriptional polymerase chain reaction
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TPA
12-O-tetradecanoylphorbol 13-acetate
- VCAM-1
vascular adhesion molecule-1
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Cybulsky MI, Iiyama K, Li H, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis--an inflammatory disease. New Eng J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Duan J, Yu Y, Yu Y, et al. Silica nanoparticles enhance autophagic activity, disturb endothelial cell homeostasis and impair angiogenesis. Part Fibre Toxicol. 2014;11:50. doi: 10.1186/s12989-014-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pathak MK, Hu X, Yi T. Effects of sodium stibogluconate on differentiation and proliferation of human myeloid leukemia cell lines in vitro. Leukemia. 2002;16:2285–2291. doi: 10.1038/sj.leu.2402692. [DOI] [PubMed] [Google Scholar]
- 5.Miranda MB, McGuire TF, Johnson DE. Importance of MEK-1/-2 signaling in monocytic and granulocytic differentiation of myeloid cell lines. Leukemia. 2002;16:683–692. doi: 10.1038/sj.leu.2402400. [DOI] [PubMed] [Google Scholar]
- 6.Barbieri SS, Eligini S, Brambilla M, Tremoli E, Colli S. Reactive oxygen species mediate cyclooxygenase-2 induction during monocyte to macrophage differentiation: critical role of NADPH oxidase. Cardiovasc Res. 2003;60:187–197. doi: 10.1016/s0008-6363(03)00365-1. [DOI] [PubMed] [Google Scholar]
- 7.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JG, Oh GT. The role of peroxidases in the pathogenesis of atherosclerosis. BMB Rep. 2011;44:497–505. doi: 10.5483/bmbrep.2011.44.8.497. [DOI] [PubMed] [Google Scholar]
- 9.Cliford MN. Chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. J Sci Food Agric. 2000;80:1033–1043. [Google Scholar]
- 10.Manash C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 11.Marques V, Farah A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009;113:1370–1376. [Google Scholar]
- 12.Genaro-Mattos TC, Maurício ÂQ, Rettori D, Alonso A, Hermes-Lima M. Antioxidant activity of caffeic acid against iron-induced free radical generation--a chemical approach. PLoS One. 2015;10:e0129963. doi: 10.1371/journal.pone.0129963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaliora AC, Dedoussis GV, Schmidt H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Garcia E, van Dam RM, Willett WC, et al. Coffee consumption and coronary heart disease in men and women: a prospective cohort study. Circulation. 2006;113:2045–2053. doi: 10.1161/CIRCULATIONAHA.105.598664. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi T, Chikama A, Mori K, et al. Hydroxyhydroquinone-free coffee: a double-blind, randomized controlled dose-response study of blood pressure. Nutr Metab Cardiovasc Dis. 2008;18:408–414. doi: 10.1016/j.numecd.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukushima Y, Ohie T, Yonekawa Y, et al. Coffee and green tea as a large source of antioxidant polyphenols in the Japanese population. J Agric Food Chem. 2009;57:1253–1259. doi: 10.1021/jf802418j. [DOI] [PubMed] [Google Scholar]
- 18.Sirota R, Gorelik S, Harris R, Kohen R, Kanner J. Coffee polyphenols protect human plasma from postprandial carbonyl modifications. Mol Nutr Food Res. 2013;57:916–919. doi: 10.1002/mnfr.201200557. [DOI] [PubMed] [Google Scholar]
- 19.Kamiya T, Makino J, Hara H, Inagaki N, Adachi T. Extracellular-superoxide dismutase expression during monocytic differentiation of U937 cells. J Cell Biochem. 2011;112:244–255. doi: 10.1002/jcb.22917. [DOI] [PubMed] [Google Scholar]
- 20.Makino J, Kamiya T, Hara H, Adachi T. TPA induces the expression of EC-SOD in human monocytic THP-1 cells: involvement of PKC, MEK/ERK and NOX-derived ROS. Free Radic Res. 2012;46:637–644. doi: 10.3109/10715762.2012.664841. [DOI] [PubMed] [Google Scholar]
- 21.Gu BJ, Saunders BM, Petrou S, Wiley JS. P2X7 is a scavenger receptor for apoptotic cells in the absence of its ligand, extracellular ATP. J Immunol. 2011;187:2365–2375. doi: 10.4049/jimmunol.1101178. [DOI] [PubMed] [Google Scholar]
- 22.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamiya T, Nishihara H, Hara H, Adachi T. Ethanol extract of Brazilian red propolis induces apoptosis in human breast cancer MCF-7 cells through endoplasmic reticulum stress. J Agric Food Chem. 2012;60:11065–11070. doi: 10.1021/jf303004n. [DOI] [PubMed] [Google Scholar]
- 24.Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 25.Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 26.Kessler M, Ubeaud G, Jung L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J Pharmacy Pharmacol. 2003;55:131–142. doi: 10.1211/002235702559. [DOI] [PubMed] [Google Scholar]
- 27.Makino J, Nakanishi R, Kamiya T, et al. Luteolin suppresses the differentiation of THP-1 cells through the inhibition of NOX2 mRNA expression and the membrane translocation of p47phox. J Nat Prod. 2013;76:1285–1290. doi: 10.1021/np400224w. [DOI] [PubMed] [Google Scholar]
- 28.Murakami A, Koshimizu K, Ohigashi H, et al. Characteristic rat tissue accumulation of nobiletin, a chemopreventive polymethoxyflavonoid, in comparison with luteolin. BioFactors. 2002;16:73–82. doi: 10.1002/biof.5520160303. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 30.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 31.Kim JK, Jang HD. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int J Mol Sci. 2014;15:12149–12165. doi: 10.3390/ijms150712149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J Clin Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiningham KK, Cardozo ZA, Cook C, et al. All-trans-retinoic acid induces manganese superoxide dismutase in human neuroblastoma through NF-κB. Free Radic Biol Med. 2008;44:1610–1616. doi: 10.1016/j.freeradbiomed.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 35.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 36.Rada B, Hably C, Meczner A, et al. Role of Nox2 in elimination of microorganisms. Semin Immunopathol. 2008;30:237–253. doi: 10.1007/s00281-008-0126-3. [DOI] [PubMed] [Google Scholar]
- 37.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pennington KN, Taylor JA, Bren GD, Paya CV. IκB kinase-dependent chronic activation of NF-κB is necessary for p21(WAF1/Cip1) inhibition of differentiation-induced apoptosis of monocytes. Mol Cell Biol. 2001;21:1930–1941. doi: 10.1128/MCB.21.6.1930-1941.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crews FT, Vetreno RP. Addiction, adolescence, and innate immune gene induction. Front Psychiatry. 2011;2:19. doi: 10.3389/fpsyt.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]