Abstract
Oxidized low-density lipoprotein contributes to atherosclerotic plaque formation, and quercetin is expected to exert anti-atherosclerotic effects. We previously reported accumulation of conjugated quercetin metabolites in the aorta of rabbits fed high-cholesterol diets with quercetin glucosides, resulting in attenuation of lipid peroxidation and inhibition of lipid accumulation. Caveolin-1, a major structural protein of caveolae in vascular endothelial cells, plays a role in atherosclerosis development. Here we investigated effects of oxidized low-density lipoprotein, quercetin and its metabolite, quercetin 3-O-β-glucuronide, on caveolin-1 expression. Oxidized low-density lipoprotein significantly upregulated caveolin-1 mRNA expression. An oxidized low-density lipoprotein component, lysophosphatidylcholine, also induced expression of both caveolin-1 mRNA and protein. However, lysophosphatidylcholine did not affect the location of caveolin-1 proteins within caveolae structures. Co-treatment with quercetin or quercetin 3-O-β-glucuronide inhibited lysophosphatidylcholine-induced caveolin-1 expression. Quercetin and quercetin 3-O-β-glucuronide also suppressed expression of adhesion molecules induced by oxidized low-density lipoprotein and lysophosphatidylcholine. These results strongly suggest lysophosphatidylcholine derived from oxidized low-density lipoprotein contributes to atherosclerotic events by upregulating caveolin-1 expression, resulting in induction of adhesion molecules. Quercetin metabolites are likely to exert an anti-atherosclerotic effect by attenuating caveolin-1 expression in endothelial cells.
Keywords: caveolin-1, adhesion molecule, quercetin, quercetin metabolite, vascular endothelial cell
Introduction
Quercetin (3,3',4',5,7-pentahydroxyflavone; Q), a typical flavonol-type flavonoid, frequently presents as its glycoside form in fruits and vegetables. Epidemiological studies indicate dietary intake of flavonoids, including Q, is inversely associated with risk of cardiovascular diseases such as coronary heart disease(1,2) and stroke.(3) A Japanese study reported that intake of flavonoids, in particular Q, inversely correlated with plasma total cholesterol and low-density lipoprotein (LDL) concentrations.(4) In addition to reducing oxidative stress, dietary Q appears to exert effects on vascular function by elevating both endothelial nitric oxide synthase expression and blood glutathione redox ratio.(5)
Dietary Q is converted to glucuronidate and/or sulfate derivatives, or their O-methyl derivatives, during absorption into the body, and presents exclusively as these conjugated metabolites in circulating blood.(6) In a previous study utilizing high-cholesterol fed rabbits, we reported that dietary intake of Q glucoside resulted in accumulation of its conjugated metabolites within the aorta, leading to inhibition of aortic lipid peroxidation and accumulation.(7) We also demonstrated that Q 3-O-β-glucuronide (Q3GA), one of Q’s major conjugated metabolites, can return to its aglycone form to exert physiological functions in activated macrophages, an event expected to be involved in early atherosclerosis development.(8,9) Although oxidative damage to endothelial cells may profoundly affect vascular functions, effects of Q metabolites on vascular endothelial cells remain obscure.
Circulating LDL is oxidized in intima and resulting oxidized LDL (ox-LDL) plays diverse roles in atherosclerosis pathogenesis and progression.(10) In the early stages of atherosclerotic plaque formation, ox-LDL activates endothelial cells by inducing cell surface adhesion molecules, especially intracellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which mediate rolling and adhesion of monocytes.(11) Caveolae, a lipid raft subtype, are 50–100 nm flask-shaped invaginations of plasma membrane, which are abundant in endothelial cells. Caveolin-1 (CAV-1) is a major structural protein of caveolae.(12) Endothelial-specific over-expression of CAV-1 accelerates progression of atherosclerosis by reducing endothelial cell proliferation, migration and nitric oxide (NO) production leading to VCAM-1 expression.(13) Caveolae and CAV-1 mediate LDL uptake/transcytosis and regulate LDL entry into arterial walls.(14–16) Furthermore, it has been suggested that CAV-1 directly regulates expression of these adhesion molecules.(15) These facts suggest CAV-1 plays a critical role in regulation of endothelial cell activation. Therefore, we aimed to clarify effects of Q metabolites on ox-LDL-induced CAV-1 expression and adhesion molecule expression in human umbilical vein endothelial cells (HUVEC).
Materials and Methods
Reagents
Quercetin dehydrate, α-tocopherol, l-α-lysophosphatidylcholine (lysoPC) and rabbit polyclonal antibody to CAV-1 were purchased from Sigma-Aldrich (St. Louis, MO). Human LDL and human oxidized-LDL (low-thiobarbituric acid reactive) were obtained from Biomedical Technologies (Stoughton, MA). Q3GA was obtained from Extrasynthese (Genay, France). 13-Hydroperoxyoctadecadienoic acid (13-HPODE) was obtained from Larodan Fine Chemicals AB (Malmo, Sweden). 13(S)-Hydroxyoctadecadienoic acid (13-HODE) was obtained from Cayman Chemical (Ann Arbor, MI). 4-Hydroxynonenal (4-HNE) was obtained from Percipio Biosciences (Burlingame, CA). Rabbit polyclonal antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and anti-phospho-CAV-1 antibody were obtained from Cell Signaling Technology (Beverly, MA). Secondary antibody, polyclonal goat anti-rabbit immunoglobulin/horseradish peroxidase was obtained from Dako (Glostrup, Denmark).
Cells and culture
HUVEC (Lonza, Basel, Switzerland), were cultured in endothelial cell growth medium, EGM-2 (Lonza), and maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells were used in experiments from passages 3–10.
Real-time reverse-transcription polymerase chain reaction (RT-PCR)
HUVEC were plated into 24-well plates (4 × 104 cells/well) and incubated for 24 h. Cells were treated with ox-LDL or related compounds, as described in figure legends. To estimate effects of flavonoids, they were added to cells at the same time as lipids or related compounds. In 6 h pretreatment experiments, flavonoids were washed out with Hank’s balanced salt solution buffer, which was replaced with fresh medium containing lipids or related compounds. Total RNA was isolated from HUVEC using ISOGEN (Nippon Gene, Toyama, Japan). mRNA expression was determined by real-time RT-PCR, as previously described.(17) Primers used to examine genes are listed in Table 1. Primers for CAV-1 and GAPDH were obtained from Takara Bio, Inc. (Otsu, Japan). Primers for ICAM-1 and VCAM-1 were synthesized according to previous report.(18)
Table 1.
Primers used for real-time RT-PCR
| Gene | Forward | Reverse |
|---|---|---|
| CAV-1 | 5'-TTCTGGGCTTCATCTGGCAAC-3' | 5'-GCTAGCCCTATTGGTCC-3' |
| ICAM-1 | 5'-TCTGTGTCCCCCTCAAAAGTC-3' | 5'-GGGGTCTCTATGCCCAACAA-3' |
| VCAM-1 | 5'-GCTGCTCAGATTGGAGACTCA-3' | 5'-CGCTCAGAGGGCTGTCTATC-3' |
| GAPDH | 5'-GCACCGYCAAGGCTGAGAAC-3' | 5'-TGGTGAAGACGCCAGTGCA-3' |
Reaction conditions for RT and PCR were based on protocols provided by Applied Biosystems (Foster City, CA). Relative levels of gene expression for each sample were calculated using a comparative Ct method.(19) Expression of target genes (CAV-1, ICAM-1 and VCAM-1) in each sample was normalized to GAPDH Ct values. Data are expressed as mean ± SD of three separate experiments.
Cell viability assay
HUVEC were plated into 96-well plates (2 × 104 cells/well). After a 24 h incubation, culture medium was replaced with 100 µl of fresh medium. Ox-LDL-related lipids were dissolved in ethanol and added to culture medium, followed by incubation for an additional 24 h. Cells were treated with 5 µl of Cell Proliferation Reagent WST-1 (Roche, Cat No. 11644807001, Indianapolis, IN) and incubated for 4 h before absorbance at 450 nm was measured. Cell viability is expressed as a percentage of vehicle-treated control.
Western blotting
HUVEC were seeded into 60-mm dishes (4 × 105 cells/dish). After incubation for 24 h, cells were treated with lysoPC with or without flavonoid for an additional 24 h. Cell were washed twice with phosphate-buffered saline and lysed with lysis buffer [50 mM Tris-HCl (pH 8.0), 200 mM NaCl, 20 mM EDTA, 1% sodium dodecyl sulfate (SDS) polyacrylamide, 0.5% sodium deoxycholate, 0.01% Nonidet P-40, protease inhibitor mixture (Complete EDTA-free), and phosphatase inhibitor tablet (PhosSTOPTM)]. Protein concentration was determined by bicinchoninic acid protein assay (Thermo Fisher Scientific, Waltham, MA). Protein samples were boiled with reducing buffer (Nacalai Tesque, Kyoto, Japan) for 5 min.
Samples (10 µg) were separated by 10% SDS polyacrylamide gel electrophoresis. Proteins were transferred onto Immobilon®-P polyvinylidene fluoride transfer membranes (Millipore, Billerica, MA) followed by 1 h blocking of non-specific binding with a commercial blocking buffer (Blocking-One for CAV-1 or Blocking-One P for phospho-CAV-1, Nacalai Tesque, Kyoto, Japan). Membranes were incubated with an anti-CAV-1 antibody (1:5,000 dilution), anti-phospho-CAV-1 antibody (1:200) or anti-GAPDH antibody (1:3,000) for 1 h at room temperature. After washing with Tris-buffered saline containing 0.05% Tween® 20 (TBST), membranes were incubated for 1 h at room temperature with secondary antibody. After washing with TBST, membranes were visualized using Amersham Enhanced Chemiluminescence (ECLTM) Prime detection reagents (GE Healthcare, Buckinghamshire, UK). Images were captured with a Lumino Image Analyzer LAS-3000 mini (Fujifilm, Tokyo, Japan).
Partition of microdomains and non-microdomains in cell membranes
HUVEC were seeded into 60-mm dishes (4 × 105 cells/dish). After incubation for 24 h, cells were treated with lysoPC or methyl-beta-cyclodextrin (MβCD). Cell lysates were subjected to ultracentrifugation and fractionated as previously described.(20) Isolation of microdomains was confirmed by dot blots, and CAV-1 of each fraction was detected.
Statistical analyses
Data are expressed as mean ± SD from at least three independent experiments. Statistical analyses were performed using PASW Statistics 18.0 (IBM, Armonk, NY). Data were analyzed by one-way analysis of variance with Tukey multiple comparison test (p<0.05).
Results
Effect of ox-LDL on CAV-1 mRNA expression and suppression by Q and Q3GA
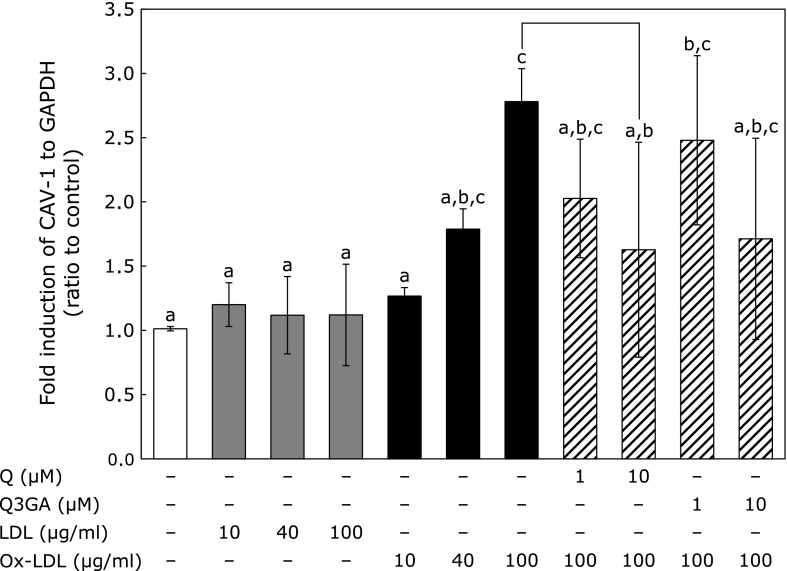
CAV-1 mRNA expression was upregulated by addition of ox-LDL in the range of 10–100 µg/ml, in a concentration-dependent manner (Fig. 1). In contrast, LDL itself did not exert such an effect. Next, HUVEC were exposed to ox-LDL (100 µg/ml) simultaneously with Q (1, 10 µM) or Q3GA (1, 10 µM) and incubated for 12 h to examine effects of Q and Q3GA on ox-LDL-induced CAV-1 mRNA expression. Significant suppression of ox-LDL-induced CAV-1 mRNA expression was observed with co-administration of 10 µM Q (Fig. 1). Q3GA also tended to suppress CAV-1 mRNA expression, although this effect was not significant.
Fig. 1.
Effect of Q and Q3GA on ox-LDL-induced CAV-1 mRNA expression in HUVECs. HUVECs were incubated with LDL or ox-LDL for 12 h or incubated with ox-LDL with Q or Q3GA for 12 h to assess effect of Q and Q3GA on CAV-1 expression. Values represent mean ± SD (n = 4). Letters represent significant differences among treatments.
Effect of lysoPC and ox-LDL-related lipid peroxidation products on CAV-1 mRNA expression
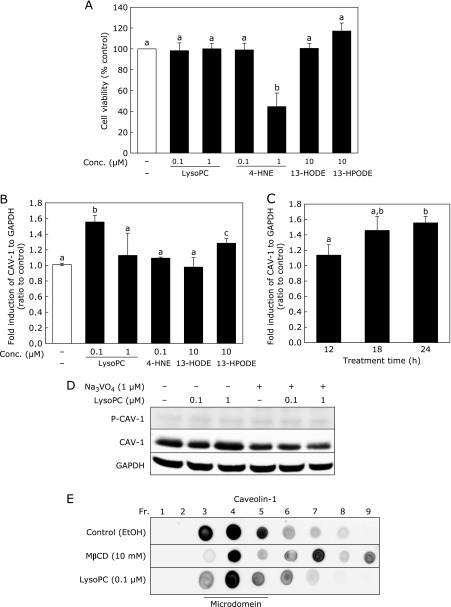
First, toxicity of lysoPC and lipid peroxidation products derived from ox-LDL was examined using a WST-1 assay (Fig. 2A). While administration of 0.1 or 1 µM lysoPC did not affect cell viability, 1 µM 4-HNE decreased cell viability by 55%. Neither 13-HPODE nor 13-HODE affected cell viability, even at 10 µM. For our treatment condition, we decided to use a concentration showing non-cytotoxicity. Therefore, concentrations of each compound varied in the following experiments. CAV-1 mRNA expression was upregulated by lysoPC at 0.1 µM, but not 1 µM, whereas 0.1 µM 4-HNE had no effect on expression. 13-HPODE, but not 13-HODE, upregulated CAV-1 mRNA expression at 10 µM (Fig. 2B). CAV-1 mRNA expression induced by 0.1 µM lysoPC increased in a time-dependent manner for 24 h (Fig. 2C); however, lysoPC did not induce phosphorylation of CAV-1 (Fig. 2D).
Fig. 2.
Effect of lysoPC and ox-LDL-related lipid peroxidation products on CAV-1 mRNA expression and effect of lysoPC on CAV-1 distribution in cell membranes. (A) Cell viability in lysoPC or ox-LDL-related lipid peroxidation products-treated HUVECs. HUVECs were incubated with lysoPC, 4-HNE, 13-HODE or 13-HPODE for 24 h. (B) CAV-1 mRNA expression in HUVECs. HUVECs were incubated with ox-LDL related lipid for 24 h. (C) Effect of lysoPC (0.1 µM) on CAV-1 expression at 12, 18, and 24 h. (A–C) Values represent mean ± SD (n = 3). Letters represent significant differences among treatment groups. (D) Effect of 24 h lysoPC treatment on CAV-1 phosphorylation in HUVECs in the absence (–) or presence (+) of sodium orthovanadate (Na3VO4). (E) Effect of lysoPC on CAV-1 distribution in microdomain fractions in HUVECs. After incubation of HUVECs with lysoPC for 24 h or MβCD for 30 min, cell lysates were split into nine fractions and CAV-1 distribution was determined by dot blot analysis.
Effect of lysoPC on CAV-1 distribution in cell membranes
Effect of lysoPC on CAV-1 distribution in cell membrane lipid rafts was examined using an ultracentrifugation technique (Fig. 2E). In control cells (only treated with vehicle), CAV-1 existed in the third to fifth fractions (lipid raft region). MβCD, which disrupts lipid rafts by removing cholesterol from cell membranes, broadened observance of CAV-1 through to the seventh fraction. After incubation of HUVEC with 0.1 µM lysoPC, distribution of CAV-1 remained unchanged in the lipid raft region (third to fifth fractions). Thus, it was confirmed that lysoPC did not affect location of CAV-1 in cellular membranes.
Effect of Q and Q3GA on lysoPC-induced CAV-1 expression
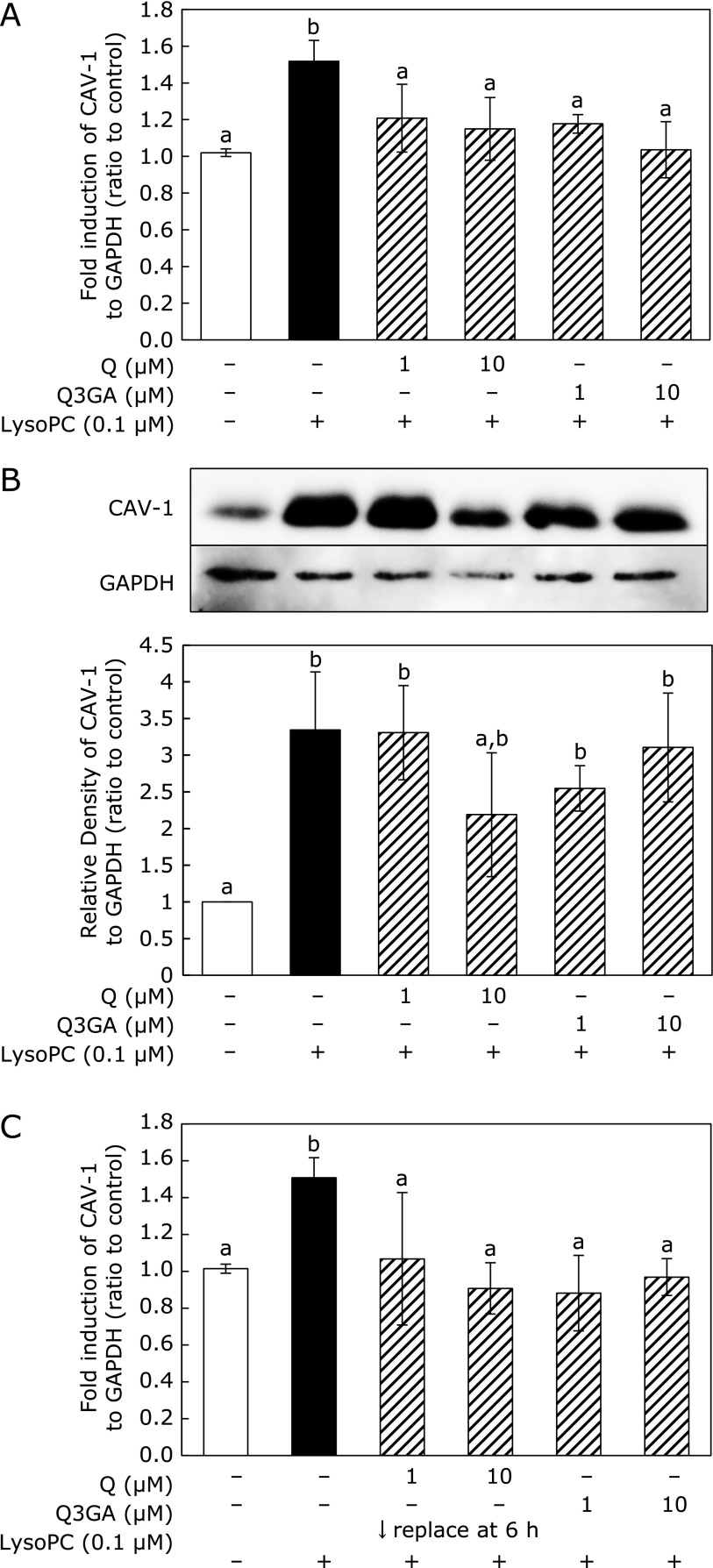
Both Q and Q3GA suppressed lysoPC-induced CAV-1 mRNA expression (Fig. 3A). CAV-1 protein levels were upregulated by addition of lysoPC for 48 h (Fig. 3B), whereas 10 µM Q suppressed upregulation of CAV-1 protein levels. Q3GA also tended to suppress CAV-1 protein upregulation, although this was not significant. To avoid direct interaction between Q/Q3GA and lysoPC in culture medium, Q or Q3GA were pre-incubated with cells for 6 h before addition of lysoPC. After removal of flavonoids from culture medium, cells were treated with lysoPC. Pre-incubation experiments also indicated that Q and Q3GA suppressed lysoPC-induced CAV-1 mRNA expression (Fig. 3C).
Fig. 3.
Effect of Q and Q3GA on lysoPC-induced CAV-1 expression in HUVECs. (A) CAV-1 mRNA expression is induced in HUVECs by lysoPC and co-addition of Q/Q3GA for 24 h. (B) Western blot of CAV-1 and density of each image analyzed. HUVECs were incubated with lysoPC with Q or Q3GA for 48 h. (C) CAV-1 mRNA expression induced in HUVECs by 6 h preincubation with Q/Q3GA and subsequent incubation with lysoPC for 24 h. (A–C) Values represent mean ± SD (n = 3). Letters represent significant differences among treatment groups.
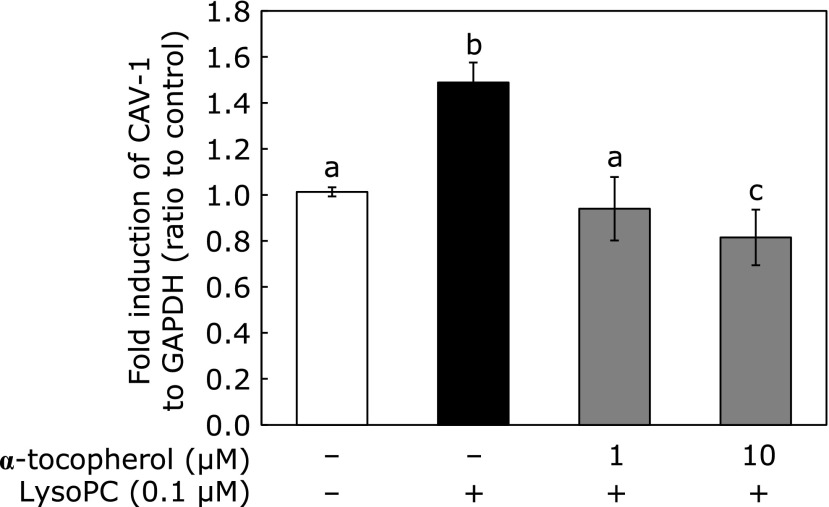
Effect of α-tocopherol on lysoPC-induced CAV-1 mRNA expression
We used α-tocopherol as a lipophilic antioxidant capable of exerting function in biomembranes. Fig. 4 shows co-administration of both 1 and 10 µM α-tocopherol suppressed lysoPC-induced CAV-1 mRNA expression.
Fig. 4.
Effect of α-tocopherol on lysoPC-induced CAV-1 expression in HUVECs. HUVECs were incubated with lysoPC with α-tocopherol for 24 h. Values represent mean ± SD (n = 3). Letters represent significant differences among treatment groups.
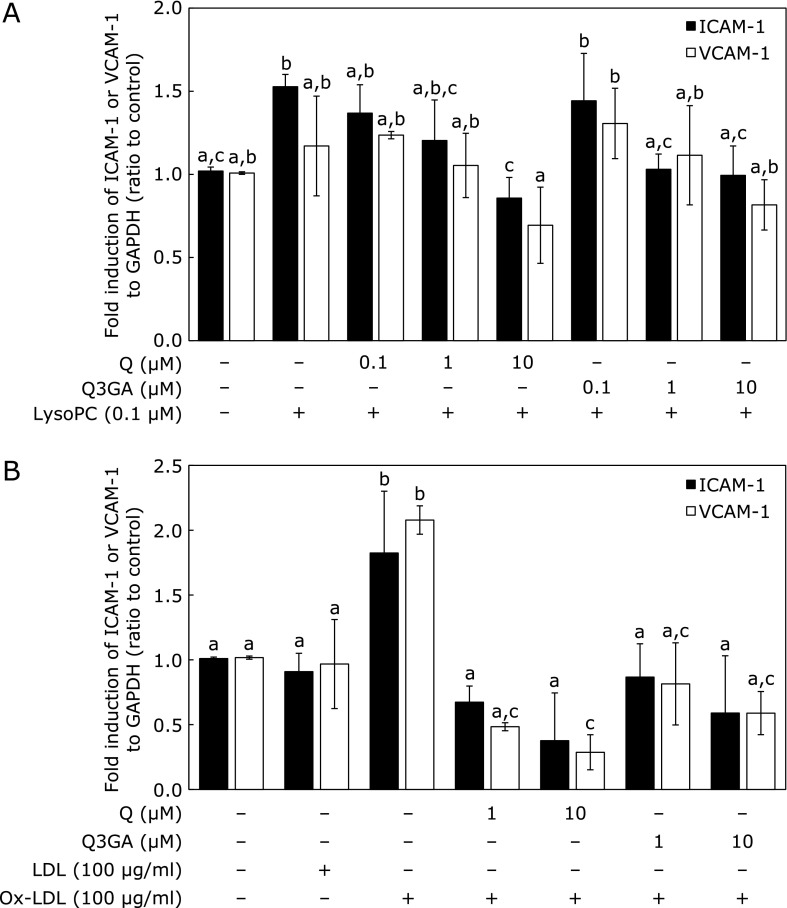
Effect of Q and Q3GA on lysoPC- or ox-LDL-induced ICAM-1 and VCAM-1 mRNA expression
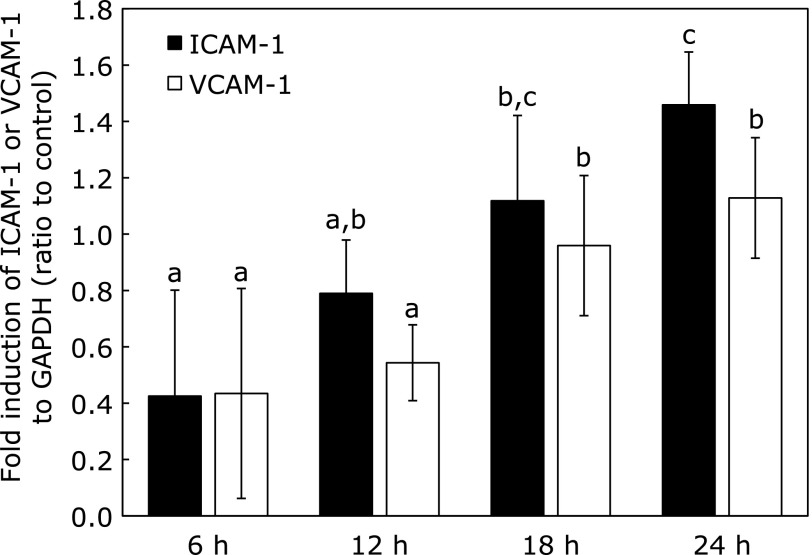
We first confirmed that mRNA expression of both ICAM-1 and VCAM-1 were upregulated by lysoPC in a time-dependent manner with 24 h treatment (Fig. 5). After 18 h of incubation with lysoPC, expression of both adhesion molecules was significantly upregulated. Treatment with lysoPC for 24 h significantly upregulated ICAM-1 mRNA expression (Fig. 6A), which was significantly suppressed by Q and Q3GA in a concentration-dependent manner in the range examined (between 0.1 and 10 µM; Fig. 6A). VCAM-1 mRNA expression tended to be upregulated by lysoPC, although no significant difference was observed compared with controls at 24 h (Fig. 6A).
Fig. 5.
Effect of lysoPC treatment on ICAM-1 and VCAM-1 mRNA expression in HUVECs. HUVECs were incubated with lysoPC for indicated time (6, 12, 18 or 24 h). Values represent mean ± SD (n = 3). Letters represent significant differences among treatment groups.
Fig. 6.
Effect of Q and Q3GA on lysoPC- or ox-LDL-induced ICAM-1 and VCAM-1 mRNA expression in HUVECs. (A) lysoPC-induced ICAM-1 and VCAM-1 expression. HUVECs were incubated with lysoPC with or without Q or Q3GA for 24 h. Values represent mean ± SD (n = 3). (B) Ox-LDL induced ICAM-1 and VCAM-1 expression. HUVECs were exposed to LDL or ox-LDL for 12 h to assess effects of Q and Q3GA. Values represent mean ± SD (n = 4). Letters represent significant differences among treatment groups.
Both ICAM-1 and VCAM-1 mRNA expression were upregulated by addition of ox-LDL, but LDL itself did not show an elevating effect (Fig. 6B). Both Q and Q3GA were found to significantly suppress expression of ICAM-1 and VCAM-1 induced by ox-LDL (Fig. 6B).
Discussion
Results from our previous study using cholesterol-fed rabbits suggested dietary Q accumulates as conjugated metabolites within the aorta, where it attenuates lipid peroxidation and hyperlipidemia.(7) Our immunohistochemical study using a monoclonal antibody demonstrated that Q metabolites target activated macrophages, resulting in selective deposition in human atherosclerotic arteries.(8) Nevertheless, human epidemiological and interventional studies(21,22) imply dietary Q exerts multiple mechanisms of action at diverse targets to protect vascular tissues from atherosclerosis. In this study, we focused on vascular endothelial cells as an alternative target of dietary Q. Endothelial activation and dysfunction are an initial event in atherosclerotic plaque formation, and endothelial CAV-1 plays a proatherogenic role. Furthermore, ox-LDL plays a critical role in pathogenesis and progression of atherosclerosis by several mechanisms including upregulation of CAV-1 expression.(23) However, little is known about the effects of dietary Q on ox-LDL-induced endothelial activation through CAV-1 expression. Therefore, we investigated the effect of ox-LDL on CAV-1 expression, as well as the modulating effect of Q and its major metabolite, Q3GA, on ox-LDL-induced CAV-1 expression in HUVEC.
CAV-1-knockout mice showed lower expression of adhesion molecules, VCAM-1(15,24) and ICAM-1.(15) Fernandez-Hernando et al.(13) observed transgenic mice overexpressing CAV-1 in endothelial cells have increased expression of VCAM-1. Fu et al.(25) suggested CAV-1 co-localizes with ICAM-1 in caveolae structures and modulates ICAM-1-dependent translocation of monocytes. In addition, an Src family kinase responsible for CAV-1 signaling activates downstream elements of the ICAM-1 pathway, resulting in ICAM-1 expression throughout caveolae structure.(26) Thus, it is likely CAV-1 expression plays an essential role in expression of adhesion molecules and their function in endothelial cells. A study in humans showed increased CAV-1 expression in endothelial cells from atherosclerotic patients.(27) CAV-1 expression was also elevated in smokers’ endothelial cells compared with non-smokers.(28)
Our in vitro study indicated expression of ICAM-1 and VCAM-1, along with CAV-1, was promoted by exposure to ox-LDL (Fig. 1 and 6B). That both Q and Q3GA suppressed expression of adhesion molecules and CAV-1 (Fig. 1 and 6B) strongly indicates Q metabolites accumulated within the aorta have the ability to attenuate CAV-1 expression to protect endothelial cells from overexpression of adhesion molecules. CAV-1 also promotes deposition of lipids into arterial walls,(14–16) by mechanisms of LDL uptake or transcytosis through direct binding to cholesterol and/or fatty acid moieties.(29) Suppression of aortic hyperlipidemia by intake of dietary Q(7) may be derived from attenuation of CAV-1 expression and following LDL uptake or transcytosis.
Oxidative modification of LDL produces various kinds of lipid peroxidation products, as well as their degradation products. Lipid hydroperoxides are lipid peroxidation products of ox-LDL that decompose into aldehydes such as 4-HNE. LysoPC is generated by an LDL-associated, platelet-activating factor-acetylhydrolase-dependent hydrolysis of oxidized phosphatidylcholine.(30) We previously reported that nonesterified fatty acid hydroperoxides released from phosphatidylcholine hydroperoxides can be reduced to hydroxyl derivatives by the action of apoA1 present in HDL.(31) Therefore, we selected lysoPC, 4-HNE, 13-HPODE and 13-HODE as representatives of ox-LDL-specific components and investigated their effects on CAV-1 expression in endothelial cells (Fig. 2A). Among them, 0.1 µM lysoPC and 10 µM 13-HPODE were found to induce CAV-1 expression without cell cytotoxicity. LysoPC is recognized to contribute to various atherosclerotic events.(32) In endothelial cells, lysoPC is known to participate in expression of adhesion molecules ICAM-1,(33) VCAM-1,(33) and P-selectin.(34) LysoPC appears to be a plausible endothelial cell activator, as lysoPC could induce CAV-1 expression at a lower concentration (0.1 µM) compared with 13-HPODE (10 µM). Thus, we deduced that lysoPC is at least partly responsible for the inductive effect of ox-LDL on CAV-1 expression.
Phosphorylation of CAV-1 is suggested to activate downstream signaling cascades associated with several endothelial functions.(35) However, lysoPC exposure did not induce phosphorylation of CAV-1 in endothelial cells (Fig. 2D). Further, lysoPC did not affect localization of CAV-1 in caveolae structures, as distribution of CAV-1 was hardly changed by addition of lysoPC to cells (Fig. 2E). LysoPC reportedly destabilizes cellular membranes and/or acts as a disruptor.(36,37) Thus, it was hypothesized that lysoPC affects function of CAV-1 by changing its location in caveolae structure. However, this hypothesis was ruled out and it can be concluded that lysoPC induces expression of CAV-1 without affecting caveolae.
LysoPC-induced gene expression of CAV-1 was effectively suppressed by simultaneous addition of Q or Q3GA (Fig. 3A). In vascular endothelial cells, lysoPC has been reported to promote O2•− production via enhanced NADPH oxidase activity(38,39) and induce mitochondrial reactive oxygen species (ROS) production.(40) Here, a lipophilic antioxidant, α-tocopherol, also suppressed CAV-1 expression (Fig. 4), indicating antioxidant activity relates to attenuation of CAV-1 expression and subsequent adhesion molecule expression. CAV-1 expression in fibroblasts was promoted by hydrogen peroxide through activation of the p38 MAPK pathway,(41) resulting from binding of specificity protein 1 (Sp1) to the promoter region, which increased transcription of CAV-1.(42) Interestingly, Q has been shown to suppress promoter region activation(42) and may suppress ROS-dependent signal transduction pathways by scavenging ROS generated during the incubation period. Pre-incubation of cells with Q or Q3GA before addition of lysoPC also resulted in effective inhibition of CAV-1 expression (Fig. 3C), suggesting a direct reaction of Q with lysoPC is not required to exert protective functions. It is noteworthy that both Q3GA and Q suppressed CAV-1 expression (Fig. 3A and C), although cellular uptake of Q3GA seems to barely occur because of its high hydrophilicity.(43) Q3GA may localize at the surface of cell membranes to affect signal transduction pathway at the initial site.
LysoPC stimulated ICAM-1 and VCAM-1 expression in a time-dependent manner (Fig. 5), although ICAM-1 increased more significantly than VCAM-1 after a 24 h incubation (Fig. 6A). LysoPC is known to promote expression of ICAM-1 and VCAM-1 at physiological concentrations,(44) resulting in rolling and adhesion of monocytes.(45) Activation of the PKC pathway may be involved in lysoPC-induced ICAM-1 expression in porcine coronary arterial endothelium.(46) Enhancement of ICAM-1 expression was effectively suppressed by Q and Q3GA (Fig. 6A). Q appears to be more effective than Q3GA with regard to lysoPC-induced adhesion molecule expression. Although Q is scarcely present in circulating blood in its aglycone form,(47) circulating conjugated Q metabolites may be deconjugated in the inflammatory site by efflux of β-glucuronidase from activated macrophages.(8,48) Resulting Q aglycone may act as a more effective suppressor of CAV-1 expression than Q metabolites upon exposure to lysoPC and other components present in ox-LDL. Koga and Meydani(49) also suggested the aglycone form is required for Q to exert effective suppression of monocyte cell adhesion to human aortic endothelial cells. Green tea polyphenols and one of their components, epigallocatechin-3-gallate, were reported to protect endothelial cells from CAV-1 expression by modulating MAPK signaling.(50,51) While it is expected that Q protects endothelial cells in a manner similar to green tea polyphenols, it should be noted that concentrations and structures required for effect vary greatly depending on each polyphenol. This study indicates effective concentrations of Q and Q3GA to modulate CAV-1 expression are around 1 µM, which seems to be within the physiological concentration of circulating blood.(52–54) Thus, dietary Q may act as a modulator of vascular endothelial functions leading to anti-atherosclerotic effects. This study may support results of previous interventional studies showing beneficial effects of flavonoid intake on reduction of cardiovascular disease risk and improvement of vascular endothelial function.(1–3,55)
In conclusion, ox-LDL increased expression of CAV-1 mRNA, as well as expression of mRNA for adhesion molecules ICAM-1 and VCAM-1. Among ox-LDL-related components, lysoPC significantly stimulated CAV-1 and ICAM-1 expression, indicating lysoPC is partly responsible for the role of ox-LDL in mediating CAV-1 expression. Both Q and Q3GA suppressed ox-LDL and lysoPC-induced CAV-1 and adhesion molecule expression. Therefore, Q metabolites may exert anti-atherosclerotic effects by targeting CAV-1 expression in endothelial cells and dietary Q is a promising food factor to prevent atherosclerosis.
Acknowledgments
Our research was supported in part by JSPS KAKENHI Grant Numbers 26892020 (R.M.) and 25292075 (J.T.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- CAV-1
caveolin-1
- GAPDH
glyceraldehyde-3-phosphate
- 4-HNE
4-hydroxynonenal
- 13-HODE
13(S)-hydroxyoctadecadienoic acid
- 13-HPODE
13-hydroperoxyoctadecadienoic acid
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intracellular cell adhesion molecule-1
- LDL
low-density lipoprotein
- LysoPC
lysophosphatidylcholine
- MβCD
methyl-beta-cyclodextrin
- NO
nitric oxide
- ox-LDL
oxidized low-density lipoprotein
- Q
quercetin
- Q3GA
quercetin-3-O-β-glucuronide
- ROS
reactive oxygen species
- RT-PCR
reverse-transcription polymerase chain reaction
- VCAM-1
vascular cell adhesion molecule-1
Conflict of Interest
No potential conflict of interest were disclosed.
References
- 1.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 2.Hertog MG, Kromhout D, Aravanis C, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 3.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- 4.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 5.Chirumbolo S. Role of quercetin in vascular physiology. Can J Physiol Pharmacol. 2012;90:1652–1657. doi: 10.1139/y2012-137. [DOI] [PubMed] [Google Scholar]
- 6.Terao J, Kawai Y, Murota K. Vegetable flavonoids and cardiovascular disease. Asia Pac J Clin Nutr. 2008;17 Suppl 1:291–293. [PubMed] [Google Scholar]
- 7.Kamada C, da Silva EL, Ohnishi-Kameyama M, Moon JH, Terao J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the aorta of high cholesterol-fed rabbit. Free Radic Res. 2005;39:185–194. doi: 10.1080/10715760400019638. [DOI] [PubMed] [Google Scholar]
- 8.Kawai Y, Nishikawa T, Shiba Y, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. J Biol Chem. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 9.Ishisaka A, Kawabata K, Miki S, et al. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages PLoS One20138e80843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiolino G, Rossitto G, Caielli P, Bisogni V, Rossi GP, Calò LA. The role of oxidized low-density lipoproteins in atherosclerosis: the myths and the facts. Mediators Inflamm. 2013;2013:714653. doi: 10.1155/2013/714653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frostegård J, Haegerstrand A, Gidlund M, Nilsson J. Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis. 1991;90:119–126. doi: 10.1016/0021-9150(91)90106-d. [DOI] [PubMed] [Google Scholar]
- 12.Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109 (23 Suppl 1):III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 13.Fernández-Hernando C, Yu J, Dávalos A, Prendergast J, Sessa WC. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2010;177:998–1003. doi: 10.2353/ajpath.2010.091287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank PG, Pavlides S, Lisanti MP. Caveolae and transcytosis in endothelial cells: role in atherosclerosis. Cell Tissue Res. 2009;335:41–47. doi: 10.1007/s00441-008-0659-8. [DOI] [PubMed] [Google Scholar]
- 15.Fernández-Hernando C, Yu J, Suárez Y, et al. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009;10:48–54. doi: 10.1016/j.cmet.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pavlides S, Gutierrez-Pajares JL, Iturrieta J, Lisanti MP, Frank PG. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res. 2014;356:147–157. doi: 10.1007/s00441-013-1767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukai R, Nakao R, Yamamoto H, Nikawa T, Takeda E, Terao J. Quercetin prevents unloading-derived disused muscle atrophy by attenuating the induction of ubiquitin ligases in tail-suspension mice. J Nat Prod. 2010;73:1708–1710. doi: 10.1021/np100240y. [DOI] [PubMed] [Google Scholar]
- 18.Khaidakov M, Wang X, Mehta JL. Potential involvement of LOX-1 in functional consequences of endothelial senescence. PLoS One. 2011;6:e20964. doi: 10.1371/journal.pone.0020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Noma A, Shimada S, et al. Non-selective distribution of isomeric cholesterol hydroperoxides to microdomains in cell membranes and activation of matrix metalloproteinase activity in a model of dermal cells. Chem Phys Lipids. 2013;174:17–23. doi: 10.1016/j.chemphyslip.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Vizcaino F, Duarte J. Flavonols and cardiovascular disease. Mol Aspects Med. 2010;31:478–494. doi: 10.1016/j.mam.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Hollman PC, Cassidy A, Comte B, et al. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J Nutr. 2011;141:989s–1009s. doi: 10.3945/jn.110.131490. [DOI] [PubMed] [Google Scholar]
- 23.Sun SW, Zu XY, Tuo QH, et al. Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacol Sin. 2010;31:1336–1342. doi: 10.1038/aps.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank PG, Lee H, Park DS, Tandon NN, Scherer PE, Lisanti MP. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:98–105. doi: 10.1161/01.ATV.0000101182.89118.E5. [DOI] [PubMed] [Google Scholar]
- 25.Fu C, He J, Li C, Shyy JY, Zhu Y. Cholesterol increases adhesion of monocytes to endothelium by moving adhesion molecules out of caveolae. Biochim Biophys Acta. 2010;1801:702–710. doi: 10.1016/j.bbalip.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Tilghman RW, Hoover RL. The Src-cortactin pathway is required for clustering of E-selectin and ICAM-1 in endothelial cells. FASEB J. 2002;16:1257–1259. doi: 10.1096/fj.01-0969fje. [DOI] [PubMed] [Google Scholar]
- 27.Voghel G, Thorin-Trescases N, Farhat N, et al. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev. 2007;128:662–671. doi: 10.1016/j.mad.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Farhat N, Thorin-Trescases N, Voghel G, et al. Stress-induced senescence predominates in endothelial cells isolated from atherosclerotic chronic smokers. Can J Physiol Pharmacol. 2008;86:761–769. doi: 10.1139/Y08-082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trigatti BL, Anderson RG, Gerber GE. Identification of caveolin-1 as a fatty acid binding protein. Biochem Biophys Res Commun. 1999;255:34–39. doi: 10.1006/bbrc.1998.0123. [DOI] [PubMed] [Google Scholar]
- 30.Marathe GK, Pandit C, Lakshmikanth CL, Chaithra VH, Jacob SP, D'Souza CJ. To hydrolyze or not to hydrolyze: the dilemma of platelet-activating factor acetylhydrolase. J Lipid Res. 2014;55:1847–1854. doi: 10.1194/jlr.R045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotosai M, Shimada S, Kanda M, et al. Plasma HDL reduces nonesterified fatty acid hydroperoxides originating from oxidized LDL: a mechanism for its antioxidant ability. Lipids. 2013;48:569–578. doi: 10.1007/s11745-013-3779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto T, Kobayashi T, Kamata K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr Med Chem. 2007;14:3209–3220. doi: 10.2174/092986707782793899. [DOI] [PubMed] [Google Scholar]
- 33.Khan BV, Parthasarathy SS, Alexander RW, Medford RM. Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J Clin Invest. 1995;95:1262–1270. doi: 10.1172/JCI117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White MC, Rastogi P, McHowat J. Lysoplasmenylcholine increases neutrophil adherence to human coronary artery endothelial cells. Am J Physiol Cell Physiol. 2007;293:C1467–1471. doi: 10.1152/ajpcell.00290.2007. [DOI] [PubMed] [Google Scholar]
- 35.Mastick CC, Sanguinetti AR, Cao H, Thakker S. Phosphorylation of caveolin and signaling from caveolae. In: Fielding CJ, editor. Lipid Rafts and Caveolae: From Membrane Biophysics to Cell Biology. Germany: Wiley-VCH Verlag GmbH & Co., KGaA; 2006. pp. 116–140. [Google Scholar]
- 36.Colles SM, Chisolm GM. Lysophosphatidylcholine-induced cellular injury in cultured fibroblasts involves oxidative events. J Lipid Res. 2000;41:1188–1198. [PubMed] [Google Scholar]
- 37.Golan DE, Brown CS, Cianci CM, Furlong ST, Caulfield JP. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986;103:819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeshita S, Inoue N, Gao D, et al. Lysophosphatidylcholine enhances superoxide anions production via endothelial NADH/NADPH oxidase. J Atheroscler Thromb. 2000;7:238–246. doi: 10.5551/jat1994.7.238. [DOI] [PubMed] [Google Scholar]
- 39.Kugiyama K, Sugiyama S, Ogata N, et al. Burst production of superoxide anion in human endothelial cells by lysophosphatidylcholine. Atherosclerosis. 1999;143:201–204. doi: 10.1016/s0021-9150(98)00288-3. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe N, Zmijewski JW, Takabe W, et al. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. Am J Pathol. 2006;168:1737–1748. doi: 10.2353/ajpath.2006.050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volonte D, Zhang K, Lisanti MP, Galbiati F. Expression of caveolin-1 induces premature cellular senescence in primary cultures of murine fibroblasts. Mol Biol Cell. 2002;13:2502–2517. doi: 10.1091/mbc.01-11-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasari A, Bartholomew JN, Volonte D, Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirai M, Yamanishi R, Moon JH, Murota K, Terao J. Effect of quercetin and its conjugated metabolite on the hydrogen peroxide-induced intracellular production of reactive oxygen species in mouse fibroblasts. Biosci Biotechnol Biochem. 2002;66:1015–1021. doi: 10.1271/bbb.66.1015. [DOI] [PubMed] [Google Scholar]
- 44.Kume N, Cybulsky MI, Gimbrone MA., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scalia R, Murohara T, Campbell B, Kaji A, Lefer AM. Lysophosphatidylcholine stimulates leukocyte rolling and adherence in rat mesenteric microvasculature. Am J Physiol. 1997;272 (6 Pt 2):H2584–2590. doi: 10.1152/ajpheart.1997.272.6.H2584. [DOI] [PubMed] [Google Scholar]
- 46.Sugiyama S, Kugiyama K, Ohgushi M, Fujimoto K, Yasue H. Lysophosphatidylcholine in oxidized low-density lipoprotein increases endothelial susceptibility to polymorphonuclear leukocyte-induced endothelial dysfunction in porcine coronary arteries. Role of protein kinase C. Circ Res. 1994;74:565–575. doi: 10.1161/01.res.74.4.565. [DOI] [PubMed] [Google Scholar]
- 47.Kawai Y, Saito S, Nishikawa T, Ishisaka A, Murota K, Terao J. Different profiles of quercetin metabolites in rat plasma: comparison of two administration methods. Biosci Biotechnol Biochem. 2009;73:517–523. doi: 10.1271/bbb.80516. [DOI] [PubMed] [Google Scholar]
- 48.O'Leary KA, Day AJ, Needs PW, Sly WS, O'Brien NM, Williamson G. Flavonoid glucuronides are substrates for human liver beta-glucuronidase. FEBS Lett. 2001;503:103–106. doi: 10.1016/s0014-5793(01)02684-9. [DOI] [PubMed] [Google Scholar]
- 49.Koga T, Meydani M. Effect of plasma metabolites of (+)-catechin and quercetin on monocyte adhesion to human aortic endothelial cells. Am J Clin Nutr. 2001;73:941–948. doi: 10.1093/ajcn/73.5.941. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Ying C, Zuo X, et al. Green tea polyphenols down-regulate caveolin-1 expression via ERK1/2 and p38MAPK in endothelial cells. J Nutr Biochem. 2009;20:1021–1027. doi: 10.1016/j.jnutbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Zheng Y, Lim EJ, Wang L, Smart EJ, Toborek M, Hennig B. Role of caveolin-1 in EGCG-mediated protection against linoleic-acid-induced endothelial cell activation. J Nutr Biochem. 2009;20:202–209. doi: 10.1016/j.jnutbio.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moon JH, Nakata R, Oshima S, Inakuma T, Terao J. Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am J Physiol Regul Integr Comp Physiol. 2000;279:R461–R467. doi: 10.1152/ajpregu.2000.279.2.R461. [DOI] [PubMed] [Google Scholar]
- 53.Egert S, Wolffram S, Bosy-Westphal A, et al. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 54.Tribolo S, Lodi F, Winterbone MS, et al. Human metabolic transformation of quercetin blocks its capacity to decrease endothelial nitric oxide synthase (eNOS) expression and endothelin-1 secretion by human endothelial cells. J Agric Food Chem. 2013;61:8589–8596. doi: 10.1021/jf402511c. [DOI] [PubMed] [Google Scholar]
- 55.Dower JI, Geleijnse JM, Gijsbers L, Schalkwijk C, Kromhout D, Hollman PC. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: a randomized double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145:1459–1463. doi: 10.3945/jn.115.211888. [DOI] [PubMed] [Google Scholar]