Abstract
Although immunoassays in measuring 25-hydroxyvitamin D [25(OH)D] have been improved recently, relatively large differences are still seen between results of 25(OH)D measured by immunoassays and by liquid chromatography-tandem mass spectrometry (LC-MS/MS). In the present studies, we compared two immunoassays with LC-MS/MS for measuring 25(OH)D concentrations. Concentrations of 25-hydroxyvitamin D2 [25(OH)D2] and 25-hydroxyvitamin D3 [25(OH)D3] in serum samples from 59 healthy subjects were measured by two immunoassays including Siemens ADVIA Centaur Vitamin D Total (Centaur) and Roche Elecsys Vitamin D Total (Elecsys) and LC-MS/MS. To determine the cross reactivity of Elecsys and Centaur toward 25(OH)D2, a dosage of 200,000 IU vitamin D2 was given after first sampling. Serum samples were obtained 30 days later and concentrations of 25(OH)D2 and 25(OH)D3 were measured again. The results showed poor agreement between the immunoassays and LC-MS/MS in 25(OH)D2 and 25(OH)D3 measurements. The percentage of 25(OH)D2 cross-reactivity was 45.3% for Centaur and 41.2% for Elecsys and there was no significant difference between Centaur and Elecsys. In conclusion, Centaur and Elecsys perform unsatisfactorily in measuring 25(OH)D levels, especially for 25(OH)D2 cross-reactivity. Therefore, clinicians need to be aware of the underestimation of vitamin D status when using these immunoassays for measuring individuals supplemented with vitamin D2.
Keywords: 25(OH)D, immunoassay, LC-MS/MS, Centaur, Elecsys
Introduction
The active metabolite of vitamin D is widely known to play a critical role in maintaining calcium and phosphorus homeostasis. Severe vitamin D deficiency manifests as rickets in children and osteomalacia in adults.(1) Recent studies have indicated that physiological roles which vitamin D played are not only in the bone but also in a wide variety of tissues.(2,3) Vitamin D deficiency has been shown to be associated with increased risk of many diseases, such as cardiovascular diseases, certain types of cancer, diabetes, and immune diseases.(4–7)
There are two forms of vitamin D in the circulation: vitamin D3 or cholecalciferol and vitamin D2 or ergocalciferol. Vitamin D3 is derived endogenously from skin exposure to the sunshine and exogenously from vitamin D3 supplements and certain animal foods such as fatty fish. Vitamin D2 is derived mainly from vitamin D2 supplements and certain plant foods such as irradiated mushrooms. Vitamin D2 and vitamin D3 are converted to 25(OH)D2 and 25(OH)D3, respectively, in the liver and then to the active form 1.25-dihydroxyvitamin D2 [1,25(OH)2D2] and 1.25-dihydroxyvitamin D3 [1,25(OH)2D3] mainly in the kidney. Among all the related metabolites, 25(OH)D is regarded as the best indicator of vitamin D nutritional status.(8)
Vitamin D deficiency has become a worldwide issue.(9) Studies have shown that vitamin D deficiency or insufficiency is prevalent in almost all age groups if individuals are not taking vitamin D supplements or are lacking sufficient sunshine exposure.(10,11)
There has been a technical improvement in measuring 25(OH)D and a massive rise in 25(OH)D testing all over the world. In the competitive market, new methods emerge in an endless stream and typical ones of noted brands have been committed them to modification. Current methods for measuring 25(OH)D includes immunoassay that use antibodies or vitamin D binding protein (DBP) against the D2 and/or D3 form of 25(OH)D and chromatographic assay [including high performance liquid chromatography (HPLC) and liquid chromatography tandem mass spectrometry (LC-MS/MS)] that separate 25(OH)D2 and 25(OH)D3 based on their respective chemical properties.(12) This means higher precision for chromatographic methods and makes them as the gold standard for measuring 25(OH)D. However, high cost of instrument, large sample volume and lack of operating expertise restrict their use in hospitals and laboratories. High-throughput, easy practicality and reduced operator error contribute to the immunoassays popularity in markets. However, immunoassays which do not separate 25(OH)D from DBP thoroughly are affected by competition between the analyte capture antibodies or DBP and are more flexible to matrix effects. Inability of fully recovering 25(OH)D2 is a major cause for the variation between immunoassays and chromatography methods according to previous reports.(13)
Recently several diagnostic manufacturers have claimed to be able to align their assays to a truth in 25(OH)D testing. To determine whether manufacturers’ efforts in modifying technical issues in recent years exhibiting actual effects in improving the performance of 25(OH)D2 immunoassays, we compared two automated immunoassays including Simens ADVIA Centaur Vitamin D Total assay (Siemens Healthcare Diagnostics Inc, Tarrytown, NY) and Roche Elecsys Vitamin D Total assay (Roche Diagnostics, Mannheim, Germany) with LC-MS/MS which is regarded as the gold-standard reference for their precision of detecting the total level of 25(OH)D and the ability of recovering 25(OH)D2.
Materials and Methods
Study design
Fifty nine healthy adults worked in the same corporation were recruited in May, 2014. Blood were collected by routine venipuncture and then centrifuged at 3,000 rotate per minute for 5 min. No visible sign of hemolysis or lipemia was observed in these samples. Serum was isolated and divided into 3 aliquots and frozen at –20°C until transported on dry ice to designated laboratories. Storage time for analysis did not exceed 3 weeks and all aliquots thawed only once. All subjects received a single dosage of 200,000 IU vitamin D2 by intramuscular route immediately after the first sampling. Six subjects were withdrawn from the study during the interval. Fifty three subjects had repeated serum samples collected 30 days later. The samples were processed as described above. During the interval, none of the subjects had taken any foods or supplements containing vitamin D. Measurements were performed in the same analytical run as singletons in a blinded fashion in 3 different laboratories. The Centaur measurements were performed in the laboratory of Institute of Metabolism and Endocrinology, the Second Xiangya Hospital of Central South University (Changsha, Hunan, China). The Elecsys measurements were performed in the laboratory of Chengdu Division of KingMed Diagnostics (Chengdu, Sichuan, China). The LC-MS/MS measurements were performed in the laboratory of Guangzhou Division of KingMed Diagnostics (Guangzhou, Guangdong, China). The LC-MS/MS was used as a gold-standard reference. The ethics committee of the Second Xiangya Hospital approved this study (project number MK0217A-356) and written, full-informed consent was obtained from all trial subjects before participating in the study.
The LC-MS/MS analysis
The LC-MS/MS analysis was performed on an API 4000 ABSCIEX triple quadrupole tandem mass analyzer (ABSciex, Framingham, MA) with an atmospheric pressure chemical ionization (APCI) source at KingMed Diagnostics (Guangzhou, Guangdong, China). Briefly, 200 µl serum was mixed with protein precipitant and internal standard (IS)[25(OH)D3-d6] (Sigma Aldrich, St. Louis, MO). Liquid-liquid extraction was used to separate analytes from its binding proteins. After centrifugation, the supernatant was pipetted into glass vials and dried under nitrogen, and redissolved. The analyte was separated thoroughly from interfering components in the liquid chromatography system with a quaternary gradient pump. The quantification of 25(OH)D2, 25(OH)D3 and IS, a stable vitamin D derivative, was performed on the tandem mass spectrometer. The LC-MS/MS method was calibrated using commercially available calibrators traceable to standard reference materials (SRM) 972 from National Institute of Standards and Technology (NIST).
Siemens ADVIA Centaur Vitamin D total assay
Siemens ADVIA Centaur Vitamin D Total assay (Centaur) is a one-step, 18-min competitive chemiluminescent immunoassay. The assay employs an anti-fluorescein monoclonal mouse antibody covalently bound to paramagnetic particles, an anti-25(OH)D monoclonal mouse antibody labeled with acridinium ester, and a vitamin D analog labeled with fluorescein. There was an inverse relationship between the resulting chemiluminescent signal detected by the system and the amount of vitamin D present in the patient sample. The measurements with Siemens ADVIA Centaur Vitamin D Total kits were performed on the ADVIA Centaur XP Immunoassay System according to the manufacturer’s protocol.
Roche Elecsys Vitamin D total assay
Roche Elecsys Vitamin D Total assay (Elecsys) is a competitive, 27-min electrochemiluminescence binding assay. The assay uses a ruthenium labeled vitamin D binding protein as capture agent. Similar to Centaur, there was an inverse relationship between the resulting chemiluminescent signal detected by the Roche system and the amount of vitamin D present in the patient sample. The measurements with Roche Elecsys Vitamin D Total kits were performed on the Modular Cobas E601 Analyzer according to the manufacturer’s protocol.
Precision
Precision studies were performed at three different laboratories. Between-run CVs were obtained by measuring two separate pools of the control sample (BIO-RAD, Hercules, CA) once every day for 7 consecutive days. Within-run CVs were determined by measuring 7 replicates of two separate pools of the control sample.
Accuracy
Accuracy studies for LC-MS/MS were performed by using calibrators traceable to SRM 972 from NIST to ensure its correctness (data not provided). In addition, accuracy studies for LC-MS/MS were evaluated and validated by participating in Endocrinology Proficiency Testing Program of New York State Department of Health. The same recovery test was used to evaluate the accuracy of the LC-MS/MS, Centaur and Elecsys. Briefly, the standard serum containing a low or high concentration of 25(OH)D was added to the serum sample from a healthy subject at a dilution of 1:9 and then the background and spikes were measured twice to calculate their recovery rates.
25(OH)D3 cross-reactivity
25(OH)D3 is the predominant form in circulation to evaluate vitamin D nutritional status. The concentration of 25(OH)D2 is usually minimal unless vitamin D2 containing supplements are taken. Therefore, the analysis of 25(OH)D3 cross-reactivity was achieved by directly measuring the total 25(OH)D in serum samples before treatment as the samples contained almost exclusively 25(OH)D3. The levels of 25(OH)D3 were measured with Siemens’ kits, Roche’s kits and LC-MS/MS.
25(OH)D2 cross-reactivity
Separate quantification of 25(OH)D2 and 25(OH)D3 can be acquired by LC-MS/MS, but not immunoassays, which can not differentiate 25(OH)D2 from 25(OH)D3. For this reason, the analysis of 25(OH)D2 cross-reactivity was performed indirectly by calculating 25(OH)D2 concentrations from the total concentration of 25(OH)D measured by the two immunoassays. The concentrations of 25(OH)D2 was calculated from results of the total 25(OH)D obtained from each immunoassay using equations as previously described.(14–16) Firstly, we established the linear regression equation for samples containing exclusively 25(OH)D3 before treatment: Y = a + bX3, where Y was values measured by immunoassays before treatment and X3 was values measured by LC-MS/MS before treatment. Then, we used the following equation to calculate 25(OH)D3 after treatment: Y’ = a + bX3’, where Y’ was the estimated 25(OH)D3 for immunoassays and X3’ was the 25(OH)D3 concentrations measured by LC-MS/MS after treatment. The concentration of 25(OH)D2 for an immunoassay was calculated by subtracting the estimated 25(OH)D3 value from the total 25(OH)D value after treatment. The percentage 25(OH)D2 cross-reactivity for each immunoassay was computed by dividing the 25(OH)D2 values from immunoassays by the 25(OH)D2 values from LC-MS/MS after treatment.
Statistical analysis
The non-parametric Friedman M test was used to compare the results of the three methods before or after treatment and paired Student-test was used to compare the results of each method before and after treatment. Serum 25(OH)D concentrations determined by LC-MS/MS were arbitrarily defined as a reference to which the other results were compared using Passing–Bablok regression, concordance correlation coefficient and Bland–Altman plots analysis. The slope and intercept of the Passing–Bablok regression were referred to in the text as systemic and constant bias, respectively. The cumulative sum linearity test (cusum linearity test) was used to confirm the linear relationship between two methods. The Concordance Correlation Coefficient (CCC) was used to evaluate the degree to which pairs of observations fall on the 45° line through the origin and contains a measurement of precision (Pearson correlation coefficient r) and accuracy (bias correction factor Cb). Interpretation of CCC results was as follows: >0.99, excellent agreement; 0.99–0.95, substantial agreement; 0.90–0.94, moderate agreement; <0.9, poor agreement. The former methods were applied to determine agreement of all the commercial assays against LC-MS/MS. Assay biases were calculated by the Bland–Altman plot. In addition, the Pearson regression equation was applied for estimated 25(OH)D2 calculation. The analyses were performed using SPSS19.0 and Medcalc ver. 11.4.2.0. A p value <0.05 was considered statistically significant.
Results
The results of precision studies for LC-MS/MS and the two immunoassays were shown in Table 1. The LC-MS/MS method showed that within-run and between-run CVs were less than 5% for both 25(OH)D2 and 25(OH)D3 at each of two different levels. The Centaur assay showed that within-run and between-run CVs were 10.3% and 11.0% at one level, and 9.2% and 5.1% at the other level. The Elecsys assay showed that within-run CVs were less than 5%, but between-run CVs were more than 10% at two different levels.
Table 1.
Precision studies for LC-MS/MS and two immunoassays performed using same two serum pools with different 25(OH)D concentrations
| Within-run | Between-run | |||||
|---|---|---|---|---|---|---|
| Mean ± SD (nmol/L) | CV (%) | Mean ± SD (nmol/L) | CV (%) | |||
| Centaur | pool 1 | 132.0 ± 13.6 | 10.3 | 130.4 ± 14.3 | 11 | |
| pool 2 | 338.0 ± 31.1 | 9.2 | 340.2 ± 17.3 | 5.1 | ||
| Elecsys | pool 1 | 14.0 ± 0.2 | 1.4 | 14.3 ± 3.6 | 25.2 | |
| pool 2 | 33.6 ± 0.5 | 1.5 | 36.2 ± 7.1 | 19.6 | ||
| LC-MS/MS 25(OH)D2 | pool 1 | 18.7 ± 0.5 | 2.7 | 19.3 ± 1.1 | 5.7 | |
| pool 2 | 44.4 ± 1.0 | 2.3 | 46.5 ± 2.4 | 5.2 | ||
| LC-MS/MS 25(OH)D3 | pool 1 | 17.9 ± 0.4 | 2.2 | 18.3 ± 0.8 | 4.4 | |
| pool 2 | 38.9 ± 1.3 | 3.3 | 37.9 ± 1.7 | 4.5 | ||
The accuracy of LC-MS/MS was validated by participating in Endocrinology Proficiency Testing Program of New York State Department of Health. The results were shown in Supplemental Table 1*. In our laboratory, the accuracy of LC-MS/MS, Centaur and Elecsys was tested by the recovery test. The results showed a good recovery efficiency for LC-MS/MS (124.1% for low level of additive 25(OH)D and 93.6% for the high level), but a modest recovery efficiency for Centaur (75.9% and 51.7%) and Elecsys (82.4% and 62.9%) (Supplemental Table 2*).
The mean (±SD) total 25(OH) D concentrations measured with Centaur kits, Elecsys kits and LC-MS/MS before and after vitamin D2 supplement are shown in Table 2. Friedman M test analysis revealed that the concentrations of 25(OH)D3 obtained from three different methods before treatment were not significantly different from each other. The results showed after treatment the highest values of total 25(OH)D measured by LC-MS/MS (56.0 nmol/L), followed by Elecsys (46.7 nmol/L) and then Centaur (43.9 nmol/L). There was an apparent elevation in total 25(OH)D concentration 30 days after vitamin D2 treatment. As expected, the LC-MS/MS method revealed a marked increase in 25(OH)D2 concentrations from 0.6 nmol/L to 15.6 nmol/L in average after 25(OH)D2 treatment, and a subtle but statistically significant increase in 25(OH)D3 concentrations from 37.8 nmol/L to 40.6 nmol/L after treatment probably due to the slightly increased sun exposure.
Table 2.
25(OH)D values in nmol/L by each immunoassay and LC-MS/MS before and 30 days after a single dosage of 200,000 IU vitamin D2
| Before treatment (nmol/L) | After treatment (nmol/L) | |
|---|---|---|
| Centaur | 35.4 ± 6.2 | 43.9 ± 8.7†,‡ |
| Elecsys | 37.6 ± 11.8 | 46.7 ± 13.4†,‡ |
| LC-MS/MS 25(OH)D2 | 0.6 ± 2.2§ | 15.6 ± 7.6‡ |
| LC-MS/MS 25(OH)D3 | 37.8 ± 10.2 | 40.6 ± 12.7‡ |
| LC-MS/MS total | 38.3 ± 10.7 | 56.0 ± 14.7†,‡ |
†p<0.05, comparing the three assays in the same before or after treatment group, using Friedman M test; ‡p<0.05, comparing the corresponding before and after treatment value, using paired t test; §most 25(OH)D2 levels before treatment were undetectable (<5.5 nmol/L), which we regarded as 0.
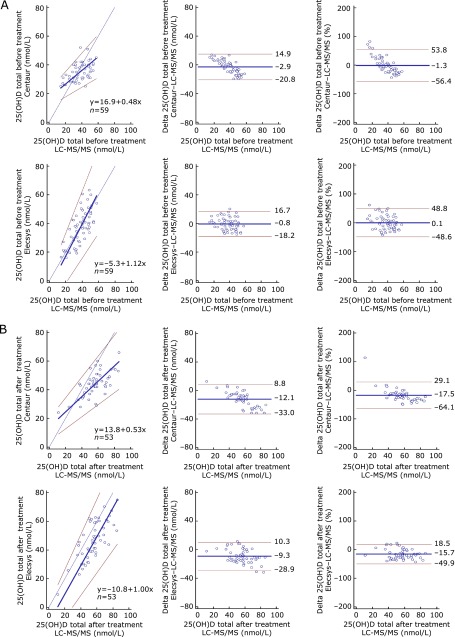
Having shown that there was no difference in the mean values of 25(OH)D measured by three different methods before treatment, we wanted to know whether the results of immunoassays correlated with that of LC-MS/MS. To address this issue, we did Passing–Bablok regression coeffecient and CCC analyses. Table 3 shows the results of the Passing–Bablok regression coeffecients, CCCs, and their 95% confidence intervals. The scatters (Passing–Bablok regression) and Bland–Altman plots for the total 25(OH)D before and after treatment are shown in Fig. 1. Although Elecsys demonstrated better CCCs than Centaur before and after treatment, CCCs of the two immunoassays against LC-MS/MS, varying from 0.39 to 0.69, were all below 0.9. The Passing–Bablok regression analysis revealed the slopes for Centaur were 0.48 (95% CI of 0.34 to 0.65) before treatment and 0.53 (95% CI of 0.42 to 0.73) after treatment. However, the slopes for Elecsys were 1 before and after treatment. The intercepts for Centaur were 16.9 (95% CI of 10.8 to 22.1) before treatment and 13.8 (95% CI of 4.4 to 19.8) after treatment. The intercepts for Elecsys before and after treatment were 0. In addition, the best fit lines for Centaur before and after treatment crossed the line of identity, suggesting a low bias at the concentration above 35 nmol/L and a high bias below this value. These data indicate a poor agreement between either of immunoassay and LC-MS/MS although there was no significant difference in the mean values of 25(OH)D3 among three different methods before treatment and both agreement coefficients and mean values of immunoassays against that of LC-MS/MS are not satisfactory after treatment.
Table 3.
Passing–Bablok regression and concordance correlation analysis for each immunoassay against LC-MS/MS total or 25(OH)D2
| Passing–Bablok regression |
Concordance correlation |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | 95%CI | Slope | 95%CI | Cusum test | CCC | 95%CI | r | Cb | ||
| Before-Centaur | 16.9 | 10.8 to 22.1 | 0.48 | 0.34 to 0.65 | p>0.05 | 0.43 | 0.25 to 0.58 | 0.52 | 0.83 | |
| Before-Elecsys | −5.3 | −19.4 to 3.6 | 1.12 | 0.87 to 1.47 | p>0.05 | 0.69 | 0.52 to 0.80 | 0.69 | 0.99 | |
| After-Centaur | −13.8 | 4.4 to 19.8 | 0.53 | 0.42 to 0.73 | p>0.05 | 0.39 | 0.25 to 0.51 | 0.68 | 0.58 | |
| After-Elecsys | 13.8 | −22.9 to 1.9 | 1 | 0.78 to 1.22 | p>0.05 | 0.61 | 0.45 to 0.73 | 0.75 | 0.81 | |
| Adjusted 25(OH)D2 | ||||||||||
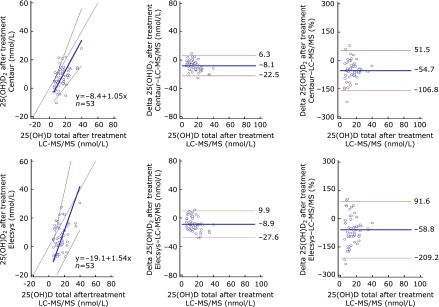
| Centaur | −8.4 | −13.1 to −3.7 | 1.05 | 0.73 to 1.50 | p>0.05 | 0.34 | 0.17 to 0.49 | 0.54 | 0.63 | |
| Elecsys | −19.1 | −30.8 to 10.9 | 1.54 | 1.07 to 2.50 | p>0.05 | 0.26 | 0.09 to 0.42 | 0.41 | 0.64 | |
CCC, concordance correlation coefficient; CI, confidence interval; r, Pearson’s correlation coefficient; Cb, bias correction factor; Before-Centaur, Centaur values before treatment; Before-Elecsys, Elecsys values before treatment; After-Centaur, Centaur values after treatment; After-Elecsys, Elecsys values after treatment.
Fig. 1.
Comparison of 25(OH)D total levels for the two immunoassays against LC-MS/MS total by Passing–Bablok regression analysis (left panels) and Bland–Altman plots [middle (nmol/L) and right panels (%)], for before treatment (A) and after treatment (B). Left panels: thin black lines denote lines of identity; thick blue lines denote regression lines; red lines denote 95% confidence intervals; blue circles denote individual samples. Middle and right panels: thick blue lines denote mean biases; red lines denote 95% confidence intervals; blue circles denote individual samples.
According to the Bland–Altman plots for analyzing assay bias, the mean biases for Elecsys (before/after treatment, −0.8 nmol/L/−9.3 nmol/L) were not different from those for Centaur (before/after treatment, −2.9 nmol/L/−12.1 nmol/L), with striking bias increases after ergocalciferol treatment for both assays. By expressing the bias in percentage, the data showed a similar tendency as described above.
To determine whether there was a difference between Centaur and Elecsys in the recovery of 25(OH)D2, we calculated 25(OH)D2 concentrations of each immunoassay. The values and percentages of 25(OH)D2 for immunoassays are shown in Table 4. The paired t test of the values of 25(OH)D2 showed no significant difference between Centaur and Elecsys (p>0.05). So did the paired t test of percentage 25(OH)D2 for Centaur and Elecsys. The results indicate comparable 25(OH)D2 cross-reactivities between Centaur and Elecsys assays.
Table 4.
Estimated 25(OH)D2 concentrations (nmol/L) and percentage 25(OH)D2 cross-reactivity (%) for each immunoassay compared to LC-MS/MS 25(OH)D2
| Estimated 25(OH)D2 concentration mean ± SD (nmol/L) | Percentage 25(OH)D2 cross-reactivity mean ± SD (%) | Commercial claim for 25(OH)D2 cross-reactivity (%) | |
|---|---|---|---|
| Centaur | 7.5 ± 7.6 | 45.3 ± 54.2 | 105 |
| Elecsys | 6.8 ± 9.8 | 41.2 ± 76.4 | 92 |
SD: standard deviation.
The results of CCC, Passing–Bablok regression analysis and Bland–Altman plots for 25(OH)D2 of immunoassays against LC-MS/MS 25(OH)D2 are shown in Table 2 and Fig. 2. In regard to the 25(OH)D2 recovery, both assays showed poor agreement against LC-MS/MS, with CCC = 0.34 for Centaur and 0.26 for Elecsys. The slope for Centaur, 1.05 (95% CI of 0.73 to 1.50), was not different from 1, but its intercept, −8.4 (95% CI of −13.1 to −3.73) was still evident. The slope for Elecsys, 1.54 (95% CI of 1.07 to 2.50) and its intercept, −19.1 (95% CI of −30.79 to −10.92) were both statistically significant, indicating a positive systemic bias and negative constant bias for this assay against LC-MS/MS. The Bland–Altman plots showed comparable mean bias [−8.1 nmol/L (−54.7%) for Centaur and −8.9 nmol/L (−58.8%) for Elecsys], but Elecsys had a broader bias distribution than Centaur in 25(OH)D2 recovery. The concentration of 25(OH)D2 in serum before vitamin D treatment was too low to detect and not shown in Table 3.
Fig. 2.
Comparison of the adjusted 25(OH)D2 levels for the two immunoassays against LC-MS/MS 25(OH)D2 by Passing–Bablok regression analysis (left panels) and Bland–Altman plots [middle (nmol/L) and right panels (%)]. Left panels: thin black lines denote lines of identity; thick blue lines denote regression lines; red lines denote 95% confidence intervals; blue circles denote individual samples. Middle and right panels: thick blue lines denote mean biases; red lines denote 95% confidence intervals; blue circles denote individual samples.
Discussion
Although there is no doubt that levels of 25(OH)D in serum is a marker of vitamin D nutritional status, the assay of serum 25(OH)D is not standardized. LC-MS/MS is now widely accepted as the gold standard for 25(OH)D analysis, but they have not been offered by most laboratories. Immunoassays is widely used to measure serum 25(OH)D concentrations. This situation raised a concern about the accuracy of test results for 25(OH)D. Although several diagnostic companies recently claimed that the 25(OH)D immunoassays had been largely improved, we found a poor agreement between immunoassays and the LC-MS/MS assay in measuring 25(OH)D concentrations in serum in the present studies. More importantly, the immunoassays markedly underestimate 25(OH)D2 levels in serum when compared with LC-MS/MS.
The obvious disagreement, demonstrated in slope, intercept and CCC, between immunoassays and LC-MS/MS in 25(OH)D3 cross-reactivity has also been reported by several investigators previously.(18–20) Because of the lipophilic nature of 25(OH)D, the immunoassays, without extraction step, are more vulnerable to non-specific matrix effects, which are caused by mainly liquids and lately claimed any substances in plasma or serum samples for direct assays.(13) Matrix effects are unpredictable in individual samples for different assays, therefore contributing to measuring variation.(21) On the other hand, the immunoassays are designed to displace the analyte from its binding protein, which involves disrupting DBP. The percentages of free 25(OH)D based on their own protocols and the interaction with other less known factors such as DBP are other explanations for their variable performances against the gold reference LC-MS/MS.(22) The narrow distribution of values (mostly <50 nmol/L) in our study might also play a role in the unsatisfactory performance for immunoassays.
Another potential problem is the assay sensitivity in detecting 25(OH)D2 for immunoassays. Due to its solvent extraction step to separate 25(OH)D2 and 25(OH)D3, LC-MS/MS is regard as the gold reference for high specificity and accuracy. As for immunoassays, those unable to release 25(OH)D from DBP in the same step as capture of the analyte, tend to show spurious 25(OH)D2 results.(23) With 25(OH)D released in the absence of capture moiety, it is not surprising that Centaur and Elecsys exhibit under-recovery of 25(OH)D2. The analyte captures employed for Centaur and Elecsys are specific antibodies and DBP, respectively. As DBP showing a low affinity for 25(OH)D2, Elecsys and Centaur may need to pay more attention in this respect, which can also be ascertained from our data that the intercept, slope and CCC of Elecsys showed poor performance in 25(OH)D2 cross-reactivity.(24)
The analysis of percentage 25(OH)D2 cross-reactivity demonstrated a comparable performance for Centaur and Elecsys, but was significantly lower than what Le Goff et al.(14) previously reported and what Centaur (105%) and Elecsys (92%) claimed. Le Goff et al.(14) treated subjects with larger dosage of vitamin D2 for longer duration, thus resulting markedly higher 25(OH)D2 levels than ours.(25) Previous studies reported that most immunoassays had difficulties in measuring low concentrations of 25(OH)D total, so our lower 25(OH)D2 concentration range may account for poor performance in detecting 25(OH)D2 by the immunoassays in our study.(26) Therefore, we deduced that low 25(OH)D2 cross-reactivity for the two immunoassays may be related to their difficulties in detecting low 25(OH)D2 concentrations. The differences between our results and commercial claims might be due to the source of 25(OH)D2 used. In studies by Cavalier et al.(15) and studies by Horst et al.,(26) the recovery of 25(OH)D2 for immunoassays kits was performed by adding exogenous 25(OH)D2 to the human serum samples, unlike our present studies using samples containing endogenous 25(OH)D2 after vitamin D2 treatment. Spiking exogenous 25(OH)D metabolites have been well known to affect analytical cross-reactivity with vitamin D compounds when using the immunoassays. One study reported an around 50% of 25(OH)D2 cross-reactivity for Elecsys although the sample size was relatively small and vitamin D was not supplemented to these subjects.(27) In the present study, we used larger sample size and fixed dosage of vitamin D2 and demonstrated 41.2% and 45.3% of 25(OH)D2 cross-reactivity for Elecsys and Centaur.
There are some limitations in our study. One problem was that our samples were collected in the summer. Different amount of sun exposure may result in variability of the 25(OH)D concentrations. During the one month interval between May and June, for sample collection, the sun exposure was gradually increased, which might have resulted in the elevation of 25(OH)D3 concentrations, thus might have affected the accuracy of 25(OH)D2 measuring concentration. Another limitation is that the LC-MS/MS method were arbitrarily regarded as a gold reference with 100% recovery of 25(OH)D2 and 25(OH)D3, which was a theoretical assumption. One limitation for LC-MS/MS is its inability to discriminate between 25(OH)D and its inactive isomer C-3 epimers, thus overestimate 25(OH)D concentrations when the C-3 epimers account for a considerable proportion of the circulating 25(OH)D3 concentration.(28)
Carter reported that the results of the LC-MS/MS assay developed as a candidate reference measurement procedure by NIST were on average 11.2% lower than those given by routine LC-MS/MS methods.(13) These data indicate that most routine LC-MS/MS assays may have overestimated 25(OH)D by failing to resolve a molecule having the same mass as 25(OH)D and a similar fragmentation pattern. However, the LC-MS/MS method employed in the present study uses calibrators traceable to SRM 972 from NIST to ensure its correctness. Its correctness was also qualified by Endocrinology Proficiency Testing Program of New York State Department of Health. Given the discrepancies found among different sorts of assays, it is important to standardize all assays, including LC-MS/MS methods, to achieve comparable accuracy.
Taken together, Centaur and Elecsys lack agreement with LC-MS/MS both in 25(OH)D3 and 25(OH)D2 measurement. Moreover, both immunoassays has cross-reactivity lower than 50% for 25(OH)D2, which is much lower than what has been previously reported and commercially claimed. Thus, clinicians should carefully interpret the results when using Centaur or Elecsys immunoassay to measure serum 25(OH)D concentrations in individuals who have received vitamin D2 supplements to avoid overestimating vitamin D deficiency.
Acknowledgments
This work was supported by the grants 81072219, 81272973 and 81471055 from the National Natural Science Foundation of China.
The authors are grateful to KingMed Diagnostics for the provision of LC-MS/MS measurements and the Roche Elecsys vitamin D total measurements in their division corporations, and are also to the laboratory of Institute of Metabolism and Endocrinology, the Second Xiangya Hospital of Central South University for the provision of Siemens ADVIA Centaur vitamin D total measurements.
Abbreviations
- CDC
the Centers for Disease Control and Prevention
- CI
confidence interval
- DBP
vitamin D binding protein
- HPLC
high performance liquid chromatography
- IS
internal standards
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- NIST
the National Institute for Standards and Technology
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- 25(OH)D2
25-hydroxyvitamin D2
- 25(OH)D3
25-hydroxyvitamin D3
- SD
standard deviation
- SRM
standard reference materials
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goltzman D, Hendy GN, White JH. Vitamin D and its receptor during late development. Biochim Biophys Acta. 2014;1849:171–180. doi: 10.1016/j.bbagrm.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 4.Visweswaran RK, Lekha H. Extraskeletal effects and manifestations of Vitamin D deficiency. Indian J Endocrinol Metab. 2013;17:602–610. doi: 10.4103/2230-8210.113750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siasos G, Tousoulis D, Oikonomou E, et al. Vitamin D serum levels are associated with cardiovascular outcome in coronary artery disease. Int J Cardiol. 2013;168:4445–4447. doi: 10.1016/j.ijcard.2013.06.151. [DOI] [PubMed] [Google Scholar]
- 6.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 7.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99:1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 8.Ajuria-Morentin I, Mar-Medina C, Bereciartua-Urbieta E, et al. Lack of transferability between different immunoassays and LC-MS/MS for total 25-hydroxyvitamin D measurement and disagreement defining deficiency. Scand J Clin Lab Inv. 2013;73:82–86. doi: 10.3109/00365513.2012.743165. [DOI] [PubMed] [Google Scholar]
- 9.Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144 Pt A:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Stoecklin E, Eggersdorfer M. A glimpse of vitamin D status in Mainland China. Nutrition. 2013;29:953–957. doi: 10.1016/j.nut.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 11.Yu S, Fang H, Han J, et al. The high prevalence of hypovitaminosis D in China: a multicenter vitamin D status survey. Medicine (Baltimore) 2015;94:e585. doi: 10.1097/MD.0000000000000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Binkley N, Krueger DC, Morgan S, Wiebe D. Current status of clinical 25-hydroxyvitamin D measurement: an assessment of between-laboratory agreement. Clin Chim Acta. 2010;411:1976–1982. doi: 10.1016/j.cca.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12:19–28. doi: 10.2174/138945011793591608. [DOI] [PubMed] [Google Scholar]
- 14.Le Goff C, Peeters S, Crine Y, Lukas P, Souberbielle JC, Cavalier E. Evaluation of the cross-reactivity of 25-hydroxyvitamin D2 on seven commercial immunoassays on native samples. Clin Chem Lab Med. 2012;50:2031–2032. doi: 10.1515/cclm-2012-0164. [DOI] [PubMed] [Google Scholar]
- 15.Cavalier E, Wallace AM, Carlisi A, Chapelle JP, Delanaye P, Souberbielle JC. Cross-reactivity of 25-hydroxy vitamin D2 from different commercial immunoassays for 25-hydroxy vitamin D: an evaluation without spiked samples. Clin Chem Lab Med. 2011;49:555–558. doi: 10.1515/CCLM.2011.072. [DOI] [PubMed] [Google Scholar]
- 16.Glendenning P, Taranto M, Noble JM, Musk AA. Current assays overestimate 25-hydroxyvitamin D3 and underestimate 25-hydroxyvitamin D2 compared with HPLC: need for assay-specific decision limits and metabolite-specific assays. Ann Clin Biochem. 2006;43:23–30. doi: 10.1258/000456306775141650. [DOI] [PubMed] [Google Scholar]
- 17.Janssen MJ, Wielders JP, Bekker CC, et al. Multicenter comparison study of current methods to measure 25-hydroxyvitamin D in serum. Steroids. 2012;77:1366–1372. doi: 10.1016/j.steroids.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Denimal D, Ducros V, Dupré T, et al. Agreement of seven 25-hydroxy vitamin D3 immunoassays and three high performance liquid chromatography methods with liquid chromatography tandem mass spectrometry. Clin Chem Lab Med. 2014;52:511–520. doi: 10.1515/cclm-2013-0434. [DOI] [PubMed] [Google Scholar]
- 19.Górriz Pintado S, Estela Burriel PL. Influence of the immunoassay used in measurement of serum vitamin D levels. Endocrinol Nutr. 2014;61:123–129. doi: 10.1016/j.endonu.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–159. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 21.Heijboer AC, Blankenstein MA, Kema IP, Buijs MM. Accuracy of 6 routine 25-hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem. 2012;58:543–548. doi: 10.1373/clinchem.2011.176545. [DOI] [PubMed] [Google Scholar]
- 22.Farrell CJ, Soldo J, McWhinney B, Bandodkar S, Herrmann M. Impact of assay design on test performance: lessons learned from 25-hydroxyvitamin D. Clin Chem Lab Med. 2014;52:1579–1587. doi: 10.1515/cclm-2014-0111. [DOI] [PubMed] [Google Scholar]
- 23.Hollis BW. Comparison of equilibrium and disequilibrium assay conditions for ergocalciferol, cholecalciferol and their major metabolites. J Steroid Biochem. 1984;21:81–86. doi: 10.1016/0022-4731(84)90063-3. [DOI] [PubMed] [Google Scholar]
- 24.Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93:3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 25.Carter GD. 25-hydroxyvitamin D: a difficult analyte. Clin Chem. 2012;58:486–488. doi: 10.1373/clinchem.2011.180562. [DOI] [PubMed] [Google Scholar]
- 26.Horst RL. Exogenous versus endogenous recovery of 25-hydroxyvitamins D2 and D3 in human samples using high-performance liquid chromatography and the DiaSorin LIAISON Total-D Assay. J Steroid Biochem Mol Biol. 2010;121:180–182. doi: 10.1016/j.jsbmb.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Knudsen CS, Nexo E, Hojskov CS, Heickendorff L. Analytical validation of the Roche 25-OH Vitamin D Total assay. Clin Chem Lab Med. 2012;50:1965–1968. doi: 10.1515/cclm-2011-0964. [DOI] [PubMed] [Google Scholar]
- 28.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.