Abstract
Mouse cage and bedding changes are potentially stressful to mice and are also labor- and resource-intensive. These changes are often performed on a calendar-based schedule to maintain a clean microenvironment and limit the concentrations of ammonia to which mice and workers are exposed. The current study sought to establish a performance-based approach to mouse cage-changing that uses urine spot characteristics as visual indicators of intracage ammonia levels. Colorimetric ammonia indicators were used to measure ammonia levels in individually-ventilated cages (IVC) housing male or female mice (n =5 per cage) of various strains at 1 to 16 d after cage change. Urine spot characteristics were correlated with ammonia levels to create a visual indicator of the cage-change criterion of 25 ppm ammonia. Results demonstrated a consistent increase in ammonia levels with days since cage change, with cages reaching the cage-change criterion at approximately 10 d for IVC containing male mice and 16 d for those with female mice. Ammonia levels were higher for male than female mice but were not correlated with mouse age. However, urine spot diameter, color, and edge characteristics were strongly correlated with ammonia levels. Husbandry practices based on using urine spot characteristics as indicators of ammonia levels led to fewer weekly cage changes and concomitant savings in labor and resources. Therefore, urine spot characteristics can be used as visual indicators of intracage ammonia levels for use of a performance (urine spot)-based approach to cage-changing frequency that maintains animal health and wellbeing.
Changing mouse cages and bedding is an integral husbandry activity in most laboratory animal facilities. The resources associated with providing fresh bedding and cleaning and autoclaving cages and the time spent by animal care staff to accomplish these tasks are considerable. Cage changing is often performed according to a calendar-based schedule to maintain a clean microenvironment according to human standards, although animal health and wellbeing are considered also.13 The current study explores the validity of using a performance (urine)-based approach to reduce cage-changing frequency in a barrier rodent housing facility.
An advantage of frequent mouse cage and bedding changes is the reduced accumulation of wastes within the animals’ microenvironment. Ammonia and other wastes accumulate in rodent cages at concentrations that vary according to the sex, strain, and health status of the animals housed; bedding; environment; time between cage changes; and number of animals per cage.6,10,12,14-17,20
Studies suggest that high ammonia concentration contributes to health effects in mice, including lesions in nasal and olfactory mucosa.4,20 There is no established guideline for exposure limits for ammonia in mice, largely because there is insufficient literature to conclude what levels cause health effects in this species.16 Ammonia is irritating to human skin, eyes, and lungs, and the current exposure limit set by the Occupational Health and Safety Administration and the American Conference of Governmental Industrial Hygienists is 50 ppm maximal exposure or 25 ppm exposure averaged over an 8-h work day.1,9 In the absence of clear exposure limits in rodents, 50 ppm is often used as a guideline for maximal ammonia exposure for rodents as well.15
Frequent cage changing may have unintended negative consequences. Rodents are highly sensitive to the odors and pheromones in their microenvironment, making the changing of the cage and bedding a stressful event.2 Frequent cage changing has been associated with increased stereotypical and aggressive behaviors in mice18,19 as well as decreased body mass3 and increased pup mortality.13 Cage changing is a human occupational health concern as well, because it exposes laboratory animal technicians and others present in the animal facility to allergens, dust, ammonia, and potential infectious agents. These risks are mitigated in most facilities by the use of face masks and laminar-flow change stations or biosafety cabinets during cage changing.
The schedule established for cage and bedding changes in a rodent facility should balance the positive and negative aspects of changing the animals’ microenvironment from the regulatory and animal health and wellbeing perspectives with the occupational health and labor concerns from a facility-management perspective. The laboratory animal regulations allow flexibility by recommending a performance-based approach for cage-changing frequency, leaving the decision to be made according to professional judgment and communication between researchers and animal care staff as well as verification of microenvironmental conditions.7
Converting from a calendar-based cage-changing schedule to a performance (urine spot)-based approach has the potential to save time and resources as it maintains animal health and welfare. To accomplish this conversion, easily recognized criteria are needed to guide animal care staff to change mouse cages at appropriate intervals. The current study was undertaken to evaluate ammonia levels and correlate these with urine-spot characteristics in high-density mouse IVC over time to test the hypothesis that these parameters can be used to guide the cage-changing frequency for mice. The overall goal was to establish a performance-based approach for mouse cage changing that uses easily recognized visual indicators of ammonia levels in mouse cages, thus allowing animal care staff to convert from a calendar-based to a performance (urine spot)-based cage-changing schedule while maintaining animal health and wellbeing.
Materials and Methods
Animals.
Healthy adult mice (Mus musculus) of both sexes with a variety of altered genes (uMT, A20, AhR, ApoE, Baff, Bim, Blimp1, Btk, C3, CARD11, CD19, CD40L, CD40R, CD45, Cg1, EuMyc, FcRg, H2, HS1, IFIH1, Ifnar1, IFNg, IL6, IL17, IL21, IL23R, IL25, IlRg, IRF4, Itch, JIP3, JIP4, LDLR, Ly5.1, M167, Mb1, mHEL, MyD88, Nurr77, OTI, PKCβ, Ptpn22, R4A, Rag2, RIP-mOva, RORgT, ROSA, SH2B3, sHel, STS1, TAB1, Taci, TCR, and Vβ5) on background strains Balb/c, C57Bl/6, 129, and NSG, primarily produced by inhouse breeding, were used in this study. The average age of mice at the time of evaluation was 154 d (range, 29 to 598 d), and all mice in each cage tested were the same age. Mice were obtained from either approved vendors or inhouse breeding. All mice used in this study were on an IACUC-approved protocol. All mice were treated humanely in accordance with standards described in the Guide for the Care and Use of Laboratory Animals.7 Mice were observed daily by the animal care staff for overall health and wellbeing, as determined by lack of disease and behavioral distress. Animal care staff was blinded to the test conditions under study when making health and welfare assessments. A veterinary assessment of all mice at the time of the ammonia sensor test revealed no visible clinical or behavioral abnormalities.
Housing and equipment.
All mice used in this study were housed as single-sex groups of 5 adults in polysulfone IVC (small mouse cages, Thoren Caging Systems, Hazelton, PA) with a floor space of approximately 67 in.2 (7.7 in. × 12.2 in. × 5.3 in.). Cages were contained in racks holding a maximum of 120 cages and were supplied by an automatic watering system (Edstrom Industries, Waterford, WI) with water purified by reverse osmosis and treated with 1 to 2 ppm chlorine. HEPA-filtered air was circulated actively through IVC at 65 to 70 changes hourly. As measured by using digital indicators in a subset of test cages (n = 13), the intracage temperature was 78.4 ± 0.3 °F (25.8 ± 0.1 °C) and relative humidity was 32% ± 2%. Corncob bedding (diameter, 1/4 in.; Bed-O'cobs, The Andersons, Maumee OH) was added at the same volume (approximate depth, 1/2 in.) to all clean cages by using an automatic bedding dispenser (Lynx Product Group, Wilson, NY), and bedded cages were autoclaved prior to use. All mice had unrestricted access to irradiated rodent chow (Purina LabDiet 5053, PharmaServ, Framingham, MA).
Cages used in this study were located in 2 rooms within a barrier-maintained SPF facility from which sentinel mouse serum was tested quarterly (Clinical Standard Panel, IDEXX Laboratories, Columbia, MO) and annually (Basic Standard Panel, IDEXX Laboratories) and confirmed free of mouse hepatitis virus, minute virus of mice, generic parvovirus (NS1), mouse parvovirus (types 1 through 5), mouse norovirus, Theiler murine encephalomyelitis virus, epizootic diarrhea of infant mice, Sendai virus, Mycoplasma pulmonis, pneumonia virus of mice, reovirus 3, lymphocytic choriomeningitis virus, and ectromelia virus. Feces and tape tests of sentinel mice were confirmed negative for pinworms and fur mites through inhouse testing. Cages were manipulated only in vertical laminar-flow change stations (MaxiMiser, Thoren Caging Systems) located within these rooms. Environmental parameters of the housing rooms were maintained at temperatures of 73 ±2 °F, relative humidity of 30% to 70%, 12:12-h light:dark cycles, and 12 to 15 air changes hourly.
Prior to this study, animal care staff changed mouse cages at a frequency of once weekly for cages housing 3 to 5 mice and once every 2 wk for cages housing 1 or 2 mice, with approximately 50% of mouse cages in the facility changed weekly.
Ammonia measurements.
A colorimetric ammonia indicator (Small Animal Ammonia Sensor, Pacific Sentry, Redmond, WA) was suspended from the wire top into the front center of each cage (Figure 1) for 2 h during the light cycle at 1 (n = 20), 2 (n = 20), 3 (n = 24), 4 (n = 28), 5 (n = 28), 6 (n = 28), 7 (n = 28), 8 (n = 28), 9 (n = 28), 10 (n = 12), 11 (n = 28), 12 (n = 24), 14 (n = 28), and 16 (n = 17) days after the previous cage change. Ages and strains of mice were distributed randomly among test groups. Intracage ammonia data were recorded according to the color of the central disk on the low-humidity (less than 50%) side of the sensor, with yellow indicating less than 1 ppm ammonia (designated by the number 1); light green indicating 1 to 25 ppm ammonia (2); medium green indicating 25 to 50 ppm ammonia (3); dark blue indicating more than 50 ppm ammonia (4); and intermediate colors designated by the numbers 1.5, 2.5, and 3.5. Ammonia sensors were assigned randomly to cages on each day of testing and replaced according to manufacturer recommendation. A sufficient supply of sensors was available to avoid using sensors on consecutive days and to allow random assignment among cages. Any cage with a score of 3 or higher (≥25 ppm ammonia; n = 108) was changed at the end of the 2-h exposure period and excluded from future time point analyses. Each indicator was photographed at the end of the 2-h exposure period for confirmation of the assigned number.
Figure 1.
Ammonia sensor suspended from wire top within a mouse cage. The central disk is a light-green color, designated by the number 2, indicating an intermediate (1–25 ppm) ammonia level.
Urine-spot assessment.
The urine-spot characteristics of diameter (in inches), color (light, medium, or dark brown), edge (diffuse or distinct), and location (front or back of cage), as seen from the bottom of each cage, were noted at the end of the 2-h exposure period. The colorimetric sensors were then observed and coded (1 to 4) for ammonia levels. The same person did all assessments, but spot characteristics were evaluated in all test cages prior to and independent of ammonia level assessments, in an attempt to reduce potential bias. Each cage bottom was photographed to confirm urine-spot characteristics and correlate these with ammonia levels.
Statistical analysis.
Means, standard deviations, linear trends across time, scatter plots, and correlations were calculated from numerical data by using Excel functions (Microsoft, Redmond, WA). SAS version 9.4 (SAS Institute, Cary, NC) was used for other statistical analyses. Sex-associated differences for ammonia levels and urine-spot diameter, color, and edge characteristics were evaluated by using t tests. Homogeneity of variance between sexes was checked by using a folded F test; if significant, a Satterthwaite approximation was used to adjust the degrees of freedom. The relationships between ammonia and urine-spot diameter, color, and edge characteristics were analyzed by using a γ statistic (for comparing ordinal-by-ordinal relationships) applied to a 2-way contingency table. P < 0.05 was used throughout to indicate statistical significance.
Results
Ammonia measurements.
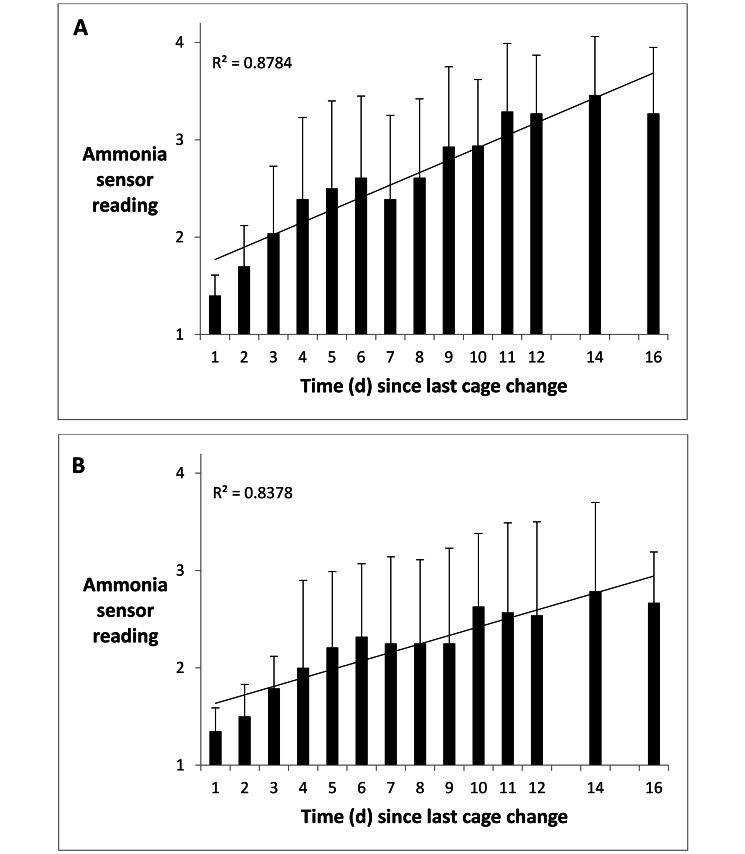
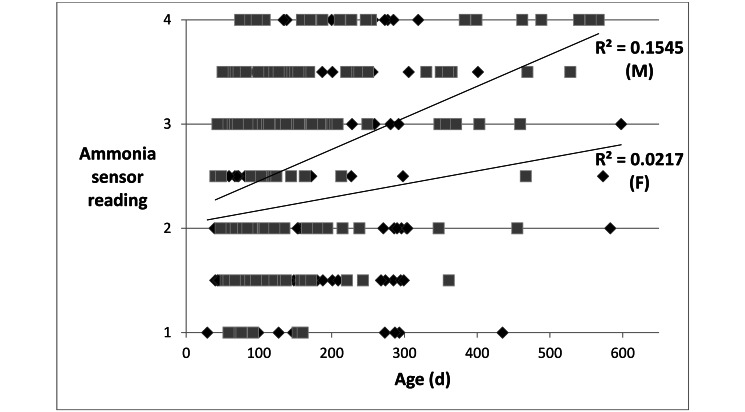
Intracage ammonia levels, as indicated by the central disk color of ammonia sensors at the end of the 2-h exposure period, generally increased with increasing time (days) since last cage change (Table 1, Figure 2). In cages housing 5 adult male mice, the data points fit a linear model, with a coefficient of determination (R2) of 0.8784. Ammonia levels reached the cage-change criterion (25 ppm ammonia or more) at approximately 10 d after last cage change (Figure 2 A). In cages housing 5 adult female mice, the data points fit a linear model, with a coefficient of determination (R2) of 0.8378. Ammonia levels reached the cage-change criterion (25 ppm or more) at approximately 16 d after last cage change (Figure 2 B). Therefore, ammonia levels increased more rapidly, and reached the cage-change criterion approximately 6 d earlier, in IVC housing 5 male mice compared with IVC housing 5 female mice. Age of mice at the time of the 2-h evaluation was not strongly correlated with ammonia levels, with R2 values of 0.1545 for male mice and 0.0217 for female mice (Figure 3). In general, ammonia levels were significantly (P < 0.01) higher in IVC housing male mice than female mice (Table 2).
Table 1.
Ammonia level (mean ± 1 SD) in cages holding 5 male or 5 female mice at 1 to 16 d after cage change
| Time (d) after cage change | Ammonia level in cages holding 5 mice (no. of cages evaluated) |
|
| Male | Female | |
| 1 | 1.4 ± 0.2 (10) | 14. ± 0.2 (10) |
| 2 | 1.7 ± 0.4 (10) | 1.5 ± 0.3 (10 |
| 3 | 2.0 ± 0.7 (12) | 1.8 ± 0.3 (12) |
| 4 | 2.4 ± 0.8 (14) | 2.0 ± 0.9 (14) |
| 5 | 2.5 ± 0.9 (14) | 2.2 ± 0.8 (14) |
| 6 | 2.6 ± 0.8 (14) | 2.3 ± 0.8 (14) |
| 7 | 2.4 ± 0.9 (14) | 2.3 ± 0.9 (14) |
| 8 | 2.6 ± 0.8 (14) | 2.3 ± 0.9 (14) |
| 9 | 2.9 ± 0.8 (14) | 2.3 ± 1.0 (14) |
| 10 | 2.9 ± 0.7 (8) | 2.6 ± 0.8 (4) |
| 11 | 3.3 ± 0.7 (14) | 2.6 ± 0.9 (14) |
| 12 | 3.3 ± 0.6 (12) | 2.5 ± 1.0 (12) |
| 14 | 3.5 ± 0.6 (14) | 2.8 ± 0.9 (14) |
| 16 | 3.3 ± 0.7 (11) | 2.7 ± 0.5 (6) |
Ammonia level codes: 1, less than 1 ppm ammonia (color of indicator disk, yellow); 2, 1–25 ppm (light green); 3, 25–50 ppm (medium green); and 4, more than 50 ppm (dark blue).
Figure 2.
Ammonia sensor readings (mean ± 1 SD) at 1 to 16 d after cage change. (A) Cages housing 5 adult male mice. The criterion ammonia level for cage change (sensor reading 3, that is, 25 ppm or greater) first occurred at approximately 10 d after last cage change. (B) Cages housing 5 adult female mice. The criterion ammonia level for cage change (sensor reading 3, that is, 25 ppm or greater) first occurred at approximately 16 d after last cage change.
Figure 3.
Ammonia sensor readings by mouse age in cages housing 5 adult male (black diamond) or 5 adult female (gray square) mice. Ammonia levels are coded as 1 (0–1 ppm), 2 (1–25 ppm), 3 (25–50 ppm), and 4 (more than 50 ppm). No significant correlation between age and ammonia level is seen in cages housing male mice (R2 = 0.1545) or female mice (R2 = 0.0217).
Table 2.
Ammonia levelaand urine spot diameter, color,band edgeccharacteristics (mean ± 1 SD) on day 1-16 after cage change in cages housing 5 male or 5 female mice
| Male Cages (n = 135) | Female Cages (n = 123) | P | |
| Ammonia | 2.7 ± 0.9 | 2.2 ± 0.9 | <0.0001d |
| Diameter (in.) | 3.0 ± 1.2 | 2.3 ± 1.1 | <0.0001d |
| Color3 | 1.9 ± 0.7 | 1.6 ± 0.7 | <0.0026d |
| Edge4 | 1.5 ± 0.5 | 1.3 ± 0.5 | <0.0058d |
Ammonia level codes: 1, less than 1 ppm ammonia (color of indicator disk, yellow); 2, 1–25 ppm (light green); 3, 25–50 ppm (medium green); and 4, more than 50 ppm (dark blue).
Color codes: 1, light brown; 2, medium brown; and 3, dark brown.
Edge codes: 1, diffuse; and 2, distinct.
Value significantly (P < 0.01) different between male and female mice.
Urine-spot assessment.
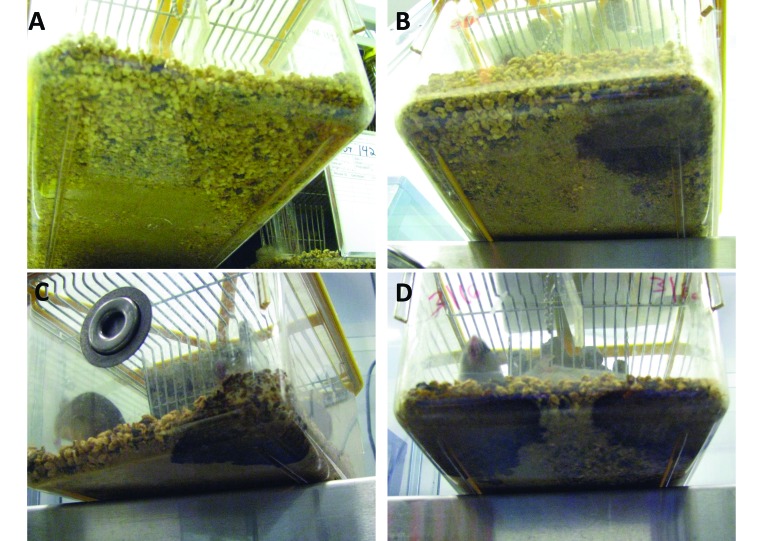
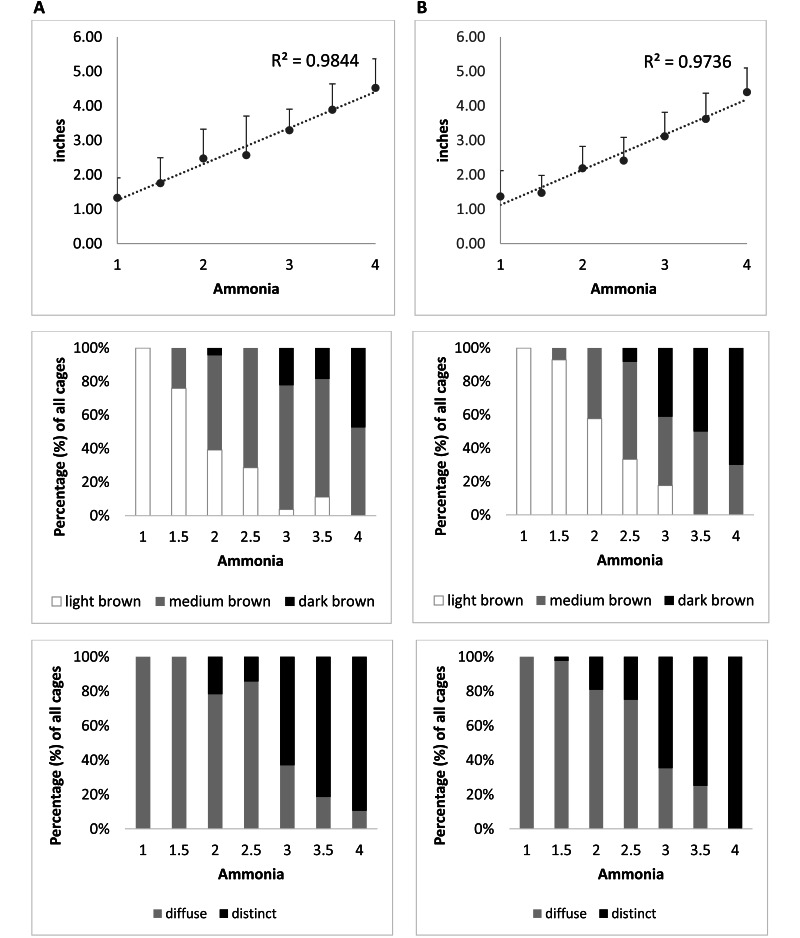
The urine spots observed in this study varied from indistinct small patches to large areas of the cage bottom, and they varied in color from light brown with diffuse borders to dark brown with distinct edges (Figure 4). Urine spot characteristics were strongly associated with numerical scores assigned for ammonia levels, with scores of 1 (less than 1 ppm ammonia) represented by small (diameter, 1 to 2 in.), light-brown urine spots with diffuse boundaries (Figure 4 A); scores of 2 (1 to 25 ppm ammonia) represented by moderate-sized (diameter, 2 to 3 in.), medium-brown urine spots with diffuse boundaries (Figure 4 B); scores of 3 (25 to 50 ppm ammonia, the cage-change criterion) represented by moderate- to large-sized (diameter, 3 to 4 in.), dark-brown urine spots with distinct edges (Figure 4 C); and scores of 4 (more than 50 ppm ammonia) represented by large (diameter, 5 to 6 in.), dark-brown urine spots with distinct edges (Figure 4 D). The pattern was so consistent that the numerical ammonia level, and thus the cage-change criterion, could be readily predicted from the characteristics of the urine spot as seen from the bottom of the cage. The urine spots in cages housing male mice had significantly (P < 0.01) larger diameters, darker colors, and more distinct edges than did those in cages housing female mice (Figure 5, Table 2). Statistical analysis also demonstrated strong correlations between ammonia levels and urine spot diameter, color, and edge characteristics (Table 3). Approximately 80% of urine spots were located at the front of the cage (data not shown), which was opposite from the area typically used for nesting and the automatic watering system.
Figure 4.
Representative urine spots in cage bottoms corresponding to ammonia sensor readings of (A) 1 (0–1 ppm), (B) 2 (1–25 ppm), (C) 3 (25–50 ppm), and (D) 4 (more than 50 ppm).
Figure 5.
Urine spot characteristics at different ammonia levels in cages housing (A) 5 male or (B) 5 female mice. Spot diameter (inches; upper panels) shows high correlation with ammonia levels in cages housing both male mice (R2 = 0.9844) and female mice (R2 = 0.9736). Spot color (light brown [white], medium brown [gray], or dark brown [black]; middle panels) is illustrated as a percentage of all urine spots at each ammonia level in cages housing male or female mice. Spot edge (diffuse [gray] or distinct [black]; lower panels) is illustrated as a percentage of all urine spots at each ammonia level in cages housing male or female mice. In all graphs, ammonia levels are coded as 1 (0–1 ppm), 2 (1–25 ppm), 3 (25–50 ppm), and 4 (more than 50 ppm).
Table 3.
Contingency table for ammonia levelaand urine spot diameter, color,band edgeccharacteristics in all cages housing 5 mice
| Ammoniaa | Diameter (in.) | Color3 | Edge4 | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 1 | 2 | ||
| 1 | 11 | 8 | 2 | 1 | 0 | 0 | 0 | 11 | 0 | 0 | 11 | 0 |
| 1.5 | 71 | 33 | 35 | 2 | 1 | 0 | 0 | 61 | 10 | 0 | 70 | 1 |
| 2 | 49 | 2 | 34 | 9 | 3 | 1 | 0 | 24 | 24 | 1 | 39 | 10 |
| 2.5 | 19 | 0 | 13 | 4 | 1 | 1 | 0 | 6 | 12 | 1 | 15 | 4 |
| 3 | 44 | 0 | 4 | 27 | 12 | 1 | 0 | 4 | 27 | 13 | 16 | 28 |
| 3.5 | 35 | 0 | 0 | 12 | 18 | 4 | 1 | 3 | 23 | 9 | 7 | 28 |
| 4 | 29 | 0 | 0 | 3 | 11 | 13 | 2 | 0 | 13 | 16 | 2 | 27 |
| Statistic | γ | 0.87 | 0.82 | 0.88 | ||||||||
| SE | 0.02 | 0.03 | 0.03 | |||||||||
| P | 0.0001d | 0.0001d | 0.0001d | |||||||||
Data are given as the number of cages with the indicated characteristics.
Ammonia level codes: 1, less than 1 ppm ammonia (color of indicator disk, yellow); 2, 1–25 ppm (light green); 3, 25–50 ppm (medium green); and 4, more than 50 ppm (dark blue).
Color codes: 1, light brown; 2, medium brown; and 3, dark brown.
Edge codes: 1, diffuse; and 2, distinct.
Ammonia levels significantly (P < 0.0001) correlated with urine spot diameter, color, and edge characteristics.
Changes in husbandry practices.
At the completion of this study, the urine-spot characteristics representing various ammonia levels were demonstrated to animal care staff by using cages in the dirty cagewash area. Staff members were trained to use a combination of urine spot diameter, color, and edge characteristics to identify ammonia levels representing the cage-change criterion. As a result, new husbandry practices were established in which mouse cages were changed according to a performance-based approach instead of a calendar-based schedule. The number of mouse cages changed weekly decreased from approximately 50% to approximately 33% of total mouse cages in the facility. Concomitant reductions in bedding costs, cagewash use, and autoclave time and labor were realized as well. The health and wellbeing of the mice, as determined by lack of disease and behavioral distress observed by animal care staff, were not adversely affected by the implementation of these new husbandry practices.
Discussion
The overall goal of this study was to establish a performance (urine spot)-based approach for mouse cage-change frequency by using easily recognized visual indicators of ammonia levels in mouse cages. The colorimetric ammonia sensors used in this study demonstrated that ammonia levels in IVC housing 5 adult mice generally increased with the number of days after cage change, increasing more rapidly in IVC housing male compared with female mice. There was no evidence of a statistical correlation between age of animal and ammonia concentration. Animal care staff was able to use the characteristics of urine spots that were visible from the cage bottom as visual indicators of ammonia levels that reached the cage-change criterion. A performance-based approach to cage changing in this facility, using urine spot characteristics, has decreased the frequency of cage changing, increased cost savings, and maintained animal wellbeing.
Converting from a calendar-based to a performance (urine spot)-based cage-changing schedule is consistent with the intent of the Guide for the Care and Use of Laboratory Animals,7 which encourages the use of professional judgment and communication between researchers and animal care staff in making this type of decision. The Guide states that “The frequency of bedding change depends on multiple factors…” and “There is no absolute frequency of bedding changes.”7 The Guide also recommends verification of microenvironmental conditions to ensure that pollutants, such as ammonia, are kept at nonirritating levels to protect the wellbeing of the animals.7 Several previous studies have examined the effects of cage-change frequency on contaminant levels and animal health, but there is significant variation in the experimental parameters used, as summarized previously.15
The 2 primary contaminants to which a mouse is exposed in a closed housing environment are carbon dioxide and ammonia.10 Previous work demonstrated elevated carbon dioxide concentrations in the area of the cage where animals congregate, with carbon dioxide levels between 1 and 9 d after cage change at less than 3000 ppm,16 which is much lower than the suggested experimental limit of 15,000 ppm.8 In contrast, ammonia increases over time in an unchanged rodent cage because of the accumulation of urine and feces. Ammonia is produced by the breakdown of urea in urine by the urease enzyme in fecal microorganisms or bedding.11 Ammonia concentration in mouse cages can increase to more than 50 ppm, levels which have been reported by some investigators to cause pathology of murine respiratory mucosa.4,20 Ammonia was the parameter of choice for this study because its accumulation is potentially significant to animal and human health and wellbeing. The ammonia sensor used in this study is semiquantitative but produces results that are sufficient for husbandry purposes. The sensors are cost-effective and easily monitored and can be used to substantiate cage-change decisions rapidly or refresh the training of husbandry staff. The intracage ammonia concentration likely is diluted slightly when the cage lid is lifted to suspend the indicator, but it quickly reequilibrates to the test level. Previous work illustrated that mouse cage bedding, as seen from above, is increasingly soiled with urine and feces between 1 and 17 d after cage change.15 The urine spot, which we propose here as an effective visual indicator, is advantageous because it is apparent from the bottom of the IVC and is usually opposite the water supply and thus can be seen without opening the cage and usually without disturbing the mice.
In the absence of specific ammonia exposure limits in rodents, we used 25 ppm ammonia as the cage change criterion in the current study, in light of the human exposure limit of 25 ppm averaged over an 8-h workday, as set by the Occupational Safety and Health Administration and the American Conference of Governmental Industrial Hygienists.1,9 This level is consistent with exposure limits set in previous investigations15 and avoids exposing the mice to 50 ppm ammonia, which is the maximal exposure limit for humans.1,9 The highest housing density permitted at our institution (5 adult mice) was used in the current study to provide the most extreme conditions of ammonia accumulation that would be seen in this facility with normal adult mice of various strains and genetic makeups. According to recommendations in the Guide,7 5 mice (weight, 15 to 25 g each) require 12 in.2 floor space per mouse, or 60 in.2 total, and 5 mice each weighing more than 25 g require 15 in.2 floor space per mouse, or 75 in.2 total. The IVC used in the current study provide 67 in.2, which is insufficient for most adult mice according to the Guide standards. An IACUC-approved exception to the Guide at our institution allows 5 adult mice to be housed in these IVC, as long as the mice remain healthy according to the attending veterinarian.
The results of this study showed that IVC housing 5 adult male mice reached the cage-change criterion of 25 ppm ammonia (that is, a code of 3 or greater, or medium green or darker on the indicator) at approximately 10 d after last cage change, which is approximately 6 d earlier than the corresponding point in IVC housing 5 adult female mice (reached at 16 d). We found that, in general, IVC housing male mice had significantly higher ammonia levels than did those housing female mice. These results are consistent with previous findings of sex-associated differences in cage microenvironments12 and the excretion of major urinary proteins.5 A previous study using a calendar-based approach with similar caging, bedding, and ventilation rates found that cage changes every 14 d represented the optimal husbandry practice in that facility.13 According to the ammonia data presented here, the previous calendar-based standard in our facility—that is, changing the cages and bedding every 7 d for cages housing 3 to 5 adult mice—was at least 3 d earlier than needed for most male mice and at least 9 d earlier than needed for most female mice. This discrepancy is likely even more pronounced for cages housing 3 or 4 adult mice. Considering the results of the current study, the previous practice of changing the cages and bedding every 14 d for cages housing 1 or 2 adult mice was likely excessive in most cases, as well.
We had expected that ammonia levels would increase with the increasing age of mice, but this hypothesis was not supported for either male or female mice in this study. From a management perspective, this finding makes husbandry decisions easier and more consistent, because urine spots and the number of mice can be assessed independently of mouse age. In addition, the propensity of mice to congregate at the inner, darker end of the cage and excrete urine at the opposite (outer) end of the cage made urine-spot assessments easier and less disruptive to the mice. Urine spots located at the inner end of the cage are readily seen by briefly removing the cage from the rack to observe the underside of the cage, with minimal disturbance to the mice.
The animal care staff was easily convinced of the rationale for changing husbandry practices when presented with the consistent correlation between ammonia levels and urine-spot characteristics. The staff readily became accustomed to identifying the cage-change criterion by using a combination of urine spot diameter, color, and edge characteristics. Color and edge characteristics are inherently subjective, but practice by staff and oversight by the attending veterinarian ensured consistency. In addition, experienced staff members and investigators were familiar with the visible agitation in mice exposed to newly changed cages and bedding, and their experiences helped to bolster the argument to decrease the frequency of cage changes. Considerable evidence indicates that changing the cage and bedding is a significant stressor to rodents that carries potential health effects2,3,13,18,19 as well as being an occupational health concern for humans. Members of the animal care staff in our facility wear face masks to protect themselves from dust and particulate matter, despite the fact that all mouse cages in the barrier facility are changed in laminar-flow changing stations or biosafety cabinets.
This study was limited to cages housing 5 healthy adult mice and did not differentiate between strains; this design was intentional so that results could be applied to any mouse strain in the facility. We presume that IVC housing fewer than 5 healthy adult mice reach the cage-change criterion at even later time points, but this study did not specifically address this assumption. In addition, disease states causing polyuria and combinations of adult mice and neonates were not addressed. Previous studies6,10,13-17 have compared a variety of bedding and caging types, whereas our current results are based on a single type of bedding and a single type of mouse caging. The focus of our study was to identify readily used visual criteria that the facility's animal care staff could use to identify cages that had reached ammonia levels requiring a bedding change. Therefore, the normal population of mice in the facility was the most appropriate population to study. In the future, it would be useful to expand the current work to assess the influences of type of bedding and caging, breeding status, mouse strain, health status, and number of mice per cage, to produce a complete picture of appropriate cage-change frequency that could be applied universally. The specific urine-spot characteristics that correlate to the cage-change criterion need to be verified in different facilities and with different caging, bedding, and mouse strains.
In summary, ammonia levels in cages housing 5 healthy adult mice increased at 1 to 16 d after last cage change, reaching the cage-change criterion of 25 ppm at approximately 10 d for male mice and 16 d for female mice. These findings, along with significant differences in ammonia levels and urine spot characteristics in IVC housing male compared with female mice, bolster the argument for changing cages on a performance-based rather than a calendar-based schedule. This report presents a reliable method to determine cage-changing schedule by using the intracage ammonia levels indicated by urine-spot characteristics. The visual appearance of the urine spot, including its size, color density, and edge definition as seen from the bottom of the cage, varies significantly with the intracage ammonia level and can be used to indicate the cage-change criterion. This performance-based method is currently in use at our institutional vivarium, resulting in less frequent cage changes, considerable savings in staff time and facility resources, and the maintenance of animal health and wellbeing.
Acknowledgments
The authors thank the animal care staff of the Office of Animal Care at Seattle Children's Research Institute for their assistance. In addition, Dr James Bassuk, from the Department of Continuous Performance Improvement at Seattle Children's Research Institute, provided valuable advice.
References
- 1.American Conference of Governmental Industrial Hygienists. 2007. Threshold limit values (TLVs) and biological exposure indices (BEI). Cincinnati (OH): ACGIH. [Google Scholar]
- 2.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 3.Beynen AC, van Tintelen G. 1990. Daily change of cage depresses mass gain in mice. Z Versuchstierkd 33:106–107. [PubMed] [Google Scholar]
- 4.Buckley LA, Jiang XZ, James RA, Morgan KT, Barrow CS. 1984. Respiratory tract lesions induced by sensory irritants at the RD50 concentration. Toxicol Appl Pharmacol 74:417–429. [DOI] [PubMed] [Google Scholar]
- 5.Cheetham SA, Smith AL, Armstrong SD, Beynon RJ, Hurst JL. 2009. Limited variation in the major urinary proteins of laboratory mice. Physiol Behav.96: 253–261. [DOI] [PubMed] [Google Scholar]
- 6.Horn MJ, Hudson SV, Bostrom LA, Cooper DM. 2012. Effects of cage density, sanitation frequency, and bedding type on animal wellbeing and health and cage environment in mice and rats. J Am Assoc Lab Anim Sci 51:781–788. [PMC free article] [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academy Press. [Google Scholar]
- 8.Krohn TC, Hansen AK. 2000. The effects and tolerances for carbon dioxide in relation to recent developments in laboratory animal housing. Scand J Lab Anim Sci 27:173–181. [Google Scholar]
- 9.Occupational Safety and Health Administration. [Internet]. 2012. Occupational Safety and Health Standards. Regulations (Standards – 29 CFR), Table Z-1 Limits of Air Contaminants.[Cited 15 April 2014]. Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9992
- 10.Perkins SE, Lipman NS. 1995. Characterization and quantification of microenvironmental contaminants in isolator cages with a variety of contact beddings. Contemp Top Lab Anim Sci 34:93–98. [PubMed] [Google Scholar]
- 11.Rahija RJ. 2007. Gnotobiotics. p 217–233. In: Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL, The mouse in biomedical research,, 2nd ed., Boston (MA): Academic Press. [Google Scholar]
- 12.Reeb C, Jones R, Bearg D, Bedigian H, Myers DD, Paigen B. 1998. Microenvironment in ventilated animal cages with differing ventilation rates, mice populations, and frequency of bedding changes. Contemp Top Lab Anim Sci 37:43–49. [PubMed] [Google Scholar]
- 13.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73. [DOI] [PubMed] [Google Scholar]
- 14.Riskowski GL, Harrison PC, Memarzadeh F. 2006. Mass generation rates of ammonia, moisture, and heat production in mouse cages with two bedding types, two mouse strains, and two room relative humidities. ASHRAE Trans 112:134–144. [Google Scholar]
- 15.Rosenbaum MD, VandeWoude S, Johnson TE. 2009. Effects of cage-change frequency and bedding volume on mice and their microenvironment. J Am Assoc Lab Anim Sci 48:763–773. [PMC free article] [PubMed] [Google Scholar]
- 16.Silverman J, Bays DW, Cooper SF, Baker SP. 2008. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci 47:57–62. [PMC free article] [PubMed] [Google Scholar]
- 17.Smith E, Stockwell JD, Schweitzer I, Langley SH, Smith AI. 2004. Evaluation of cage micro-environment of mice housed on various types of bedding materials. Contemp Top Lab Anim Sci 43:12–17. [PubMed] [Google Scholar]
- 18.Van Loo PL, Van der Meer E, Kruitwagen CL, Koolhaas JM, Van Zutphen LF, Baumans V. 2004. Long-term effects of husbandry procedures on stress-related parameters in male mice of two strains. Lab Anim 38:169–177. [DOI] [PubMed] [Google Scholar]
- 19.Van Loo PL, Van Zutphen LF, Baumans V. 2003. Male management: coping with aggression problems in male laboratory mice. Lab Anim 37:300–313. [DOI] [PubMed] [Google Scholar]
- 20.Vogelweid CM, Zapein KA, Honigford MJ, Li L, Li H, Marshall H. 2011. Effects of a 28-day cage-change interval on intracage ammonia levels, nasal histology, and perceived welfare of CD1 mice. J Am Assoc Lab Anim Sci 50:868–878. [PMC free article] [PubMed] [Google Scholar]