Abstract
Behavior and health, including the incidence of chronic idiopathic diarrhea, can vary widely among NHP reared indoors. We hypothesized that factors during gestation account for some of the variability in chronic diarrhea risk that cannot be explained by postnatal environment, genes, or known physiologic deficits. We hypothesized that, among macaques reared indoors postnatally, outdoor housing during gestation (when the dam engaged with a large, species-typical social group) would be protective against diarrhea as compared with gestation experienced in an indoor setting. We also hypothesized that exposure to routine husbandry and veterinary care in utero would increase diarrhea rates in offspring. We built models to test the influence of specific events during pregnancy as well as their interactions with anxiety-related genotype as a way of understanding gene×environment interaction on the development of diarrhea in indoor-reared rhesus macaques. Although previous reports have suggested that rearing by the mother in an indoor environment is preferable to nursery rearing, we found that whether gestation occurred indoors (in single or pair housing) or outdoors (in a large social group) better explained the variability in diarrhea rate in our study population of indoor-reared macaques. Furthermore, the diarrhea incidence was associated with nervous temperament and serotonin transporter promoter genotype. Several significant interactions indicated that some of these effects were specific to subsets of animals. Our results demonstrate that the prenatal environment can have unexpected lasting health consequences.
Abbreviations: 5HTT, serotonin transporter (gene); ACTH, adrenocorticotropic hormone; AIC, Akaike information criterion; MAOA, monoamine oxidase A (gene)
Even before becoming pregnant, women often begin taking dietary supplements and initiate exercise regimens to ensure the best possible environment for fertilization, attachment, and embryo growth. This behavior reflects the recognition that even the very earliest stages of fetal development are affected by the environment. The fetal environment can have lifelong consequences for health and behavior through mechanisms collectively referred to as ‘biological sensitivity to context,’ which prepares the individual for challenges it is likely to experience throughout its life.31
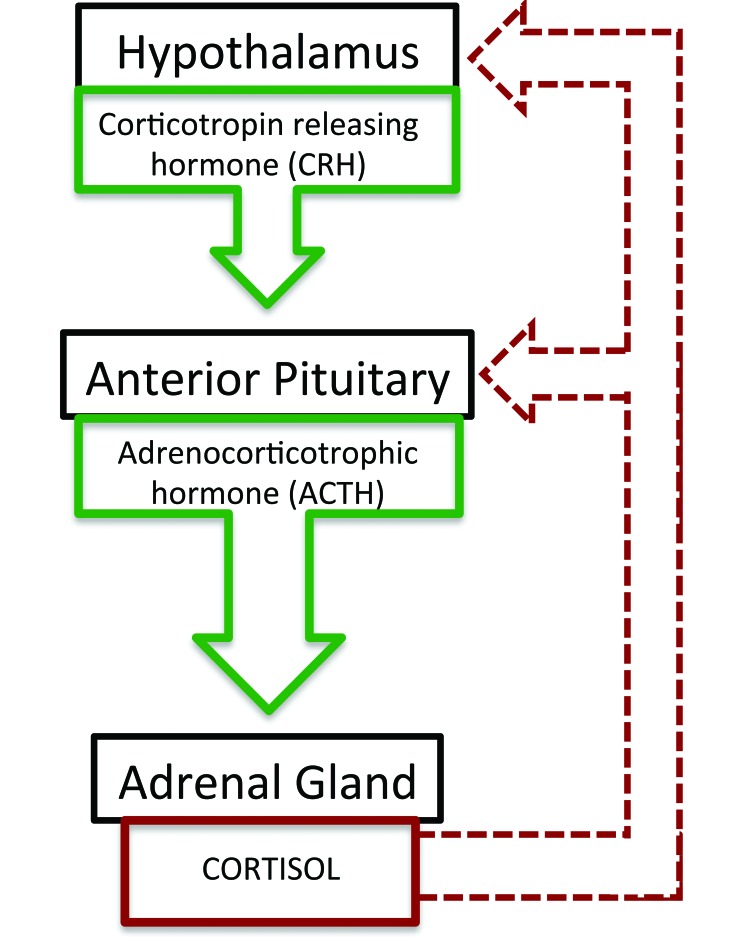
Research in mice, for example, has shown an effect of prenatal stress on the development of anxiety-like behaviors and deficits in social behavior.27,78 This is described as an effect of priming of the hypothalamic–pituitary–adrenal axis, leading to alterations in set-point or impaired negative feedback control owing to epigenetic modification (Figure 1).11,28 Prenatally stressed rodents have shown both increased magnitude and duration of the hypothalamic–pituitary–adrenal stress response.11 However, prenatally stressed animals that were reared postnatally by a dam that was not experimentally exposed to stress during pregnancy showed reduced behavioral effects of their gestational exposure, suggesting that although prenatal stress may increase the likelihood of anxiety-like behaviors, its effect is at least partially reversible by experiencing a particular postnatal environment.27 Similar correlations exist in humans, with prenatally stressed persons exhibiting impairments in social behavior and cognitive function.11 In addition, in a rhesus macaque model, prenatal stress can lead to changes in the gastrointestinal microflora for at least the first 6 mo postnatally.6 Although it is easy to posit a mechanism between nutritional stress during gestation and the gastrointestinal development of the offspring, dams in the cited studies6 were exposed to only a mild psychologic stressor, revealing complex effects of psychosocial stress on fetal development. Therefore, expecting that multiple types of gestational stress might affect an animal's overall health during early life is reasonable (Figure 1).
Figure 1.
The hypothalamic–pituitary–adrenal (HPA) axis is an important regulatory system (green solid arrows) for cortisol, a hormone involved in the stress response as well as metabolism, inflammation, and other important homeostatic functions. The HPA axis operates through negative feedback (red dashed arrows), which may be blunted or sensitized due to chronic stress during development.
In addition to influencing later health, the gestational environment may influence an offspring's general behavioral and physiologic approach to its postnatal environment, including to postnatal stressors. Interindividual differences in how animals consistently behave in their environments are often described in terms of personality,14 a term that encompasses both traits (dispositions in response), and motives (incentives for response). Personality describes the consistent patterns in behavior and affect seen within a subject across time and situations and includes the type, magnitude, and duration of responses.11,28,57 Personality can be seen both as a cause and consequence of behavior: personality is a cause of the way an animal interacts with its environment and other animals, and personality is a consequence of genetics, life history (including the prenatal environment), and health status and is constantly updated through the subject's experiences with its environment and other animals.40,64,84,87 Therefore, in this view, personality, which can be influenced by prenatal experience and which can be associated with appraisal of and response to stressors,3,29,49,52,55 might mediate the effect of prenatal experience on health. Studies in both humans and NHP have shown personality to be a risk factor for diseases, including nonpathogenic diarrhea, as well as deficits in immunity that are associated with disease.10,15,57,85
Therefore, a potentially useful indicator of comprehensive health in rhesus macaques in biomedical research facilities is diarrhea incidence, defined as the number of cases in the population in a given time period. Diarrhea is the leading cause of morbidity, especially for prepubescent NHP and accounts for more than 50% of animal hospitalizations annually in primate research centers.32,39 Diarrhea is a common clinical symptom associated with many diseases and syndromes and is therefore useful as a general indicator of stress on the system.56 Sometimes diarrhea is due to a pathogen (that is, Shigella, norovirus), but often times it is not.60,82,86 Nursery-reared NHP and those reared indoors by their mothers are known to have especially high incidence of diarrhea, but significant and unexplained variability within those groups mimics the high variability found in other measures of stress response.19,59
Multiple causes and effects of stress on the animal, and the presence of interindividual differences in response, complicate the relationship between stress and the development of diarrhea.48,55,64 Differences in perception of stress, and variation in the magnitude of the stress response, may increase susceptibility to recurrent diarrhea in some individuals, leading to increased variation.10,37,85 Moreover, interactions between body systems are evident such as evidence that the composition of the microbiome feeds back on behavior: Bravo and colleagues found a decrease in anxiety-related behaviors in mice inoculated with the probiotic Lactobacillus revealing a direct relationship between the microflora community and behavior.9 Stress, including social stress, has been shown to augment inflammatory conditions through dysregulation of the gut microbiota and inhibition of probiotic or anti-inflammatory strains of bacteria.4,5,26,62 Dysbiosis, if not outright diarrhea, following life stresses is commonly cited in reports of chronic or recurrent diarrhea in humans and other animals.33 Animals may be especially prone to postinfection diarrhea when the glucocorticoid (that is, the hypothalamic–pituitary–adrenal) stress system is dysregulated, as in prenatally stressed progeny.24,69
Given that the gestational environment has the potential to generate system set points (the values that the homeostatic system works to maintain in domains, such as temperature and plasma cortisol concentration) and prepare the animal for its postnatal environment, we evaluated the relationship between potential gestational stress of the dam and diarrhea risk of offspring. Dams that rear their offspring indoors are heterogeneous in terms of the gestational experiences of their fetuses, and their offspring are more variable in tests of behavior and personality at 3 mo of age.61,63 Some dams are relocated to indoor housing during their pregnancy, other indoor-housed females may experience social disruption in the form of change or loss of their social partner during pregnancy, and each may have other experiences during their pregnancy including illness, medication, or sedation. Previous literature suggests each of these experiences is a potential stressor associated with rises in circulating cortisol, compromised immune function, or the development of anxiety-like behaviors.6,18,53,65,68,79 Similarly, infants might be selected for nursery rearing due to difficult birth, dam or infant health, colony management, or research purposes. In short, animals that are born and reared in an indoor setting at primate facilities often constitute a group whose prenatal experiences are quite variable. We sought to better understand the role of prenatal factors on animal health among NHP born and reared indoors.
Over the years, poor outcomes have been associated with indoor or nursery rearing without the consideration of the prenatal environment.75,77,82 Behaviorists and colony management experts consider mother rearing, even if indoors, preferable to nursery rearing unless necessitated by a specific research protocol and approved through IACUC review.26,27,47,62,72,75 Mother rearing, even when indoors, offers the infant the opportunity to form an important bond with its mother and to learn appropriate social behaviors typical to macaque society. In addition, mother-reared infants receive breast milk, known to have significant benefits on physical and physiologic development.4 However, the indoor mother-reared infants are rarely outside of their mother's arm reach and do not have the opportunity to learn about rhesus macaque social structure through interaction with multiple members of each age–sex class. Nursery-reared infants have only peer contact during the early rearing period. The consequences of each rearing environment may produce juveniles that are more or less sensitive to particular types of stressors they are likely to encounter.
Materials and Methods
Data were collected from all infants in the BioBehavioral Assessment program at the California National Primate Research Center, whose rearing type was coded as ‘indoor mother-reared’ or ‘nursery-reared,’ and who were born between 1 January 2001 and 31 December 2007. Indoor mother-reared infants were housed in single-adult cages and may have had access to their mother's pairmate and her infant (if she had one) in an adjacent cage for 8 to 24 h daily. Cages included perch bars and at least one toy or other enrichment item. The mother and infant had visual and auditory access to as many as 50 other animals in the room. All animals had unlimited access to water and were fed a prescribed number of standard monkey chow biscuits (LabDiet, Purina Mills International, St Louis, MO) twice daily. Nursery-reared infants are raised in incubators in isolation until approximately 30 d of age, during which time they received formula (Enfamil) every 2 h. At approximately 30 d of age, they were introduced to a social pairmate of similar age. After a few days of habituation, the 2 infants are allowed continuous contact in ‘quad cages,’ which were smaller versions of the standard indoor housing system containing perch bars and wire mesh. These cages also had plush toys, pacifiers, and towels for the infants. Infants were provided formula and gradually were transitioned onto soaked monkey chow and finally standard chow. During the years evaluated in this study, selected infants were nursery-reared primarily to derive a SPF colony.7 All animals were cared for in compliance with protocols approved by the IACUC at the University of California–Davis and adhered to the requirements of the Animal Welfare Act, USDA regulations, and the Guide for the Care and Use of Laboratory Animals.1,41 The University of California–Davis (including the California National Primate Research Center) is fully AAALAC-accredited.
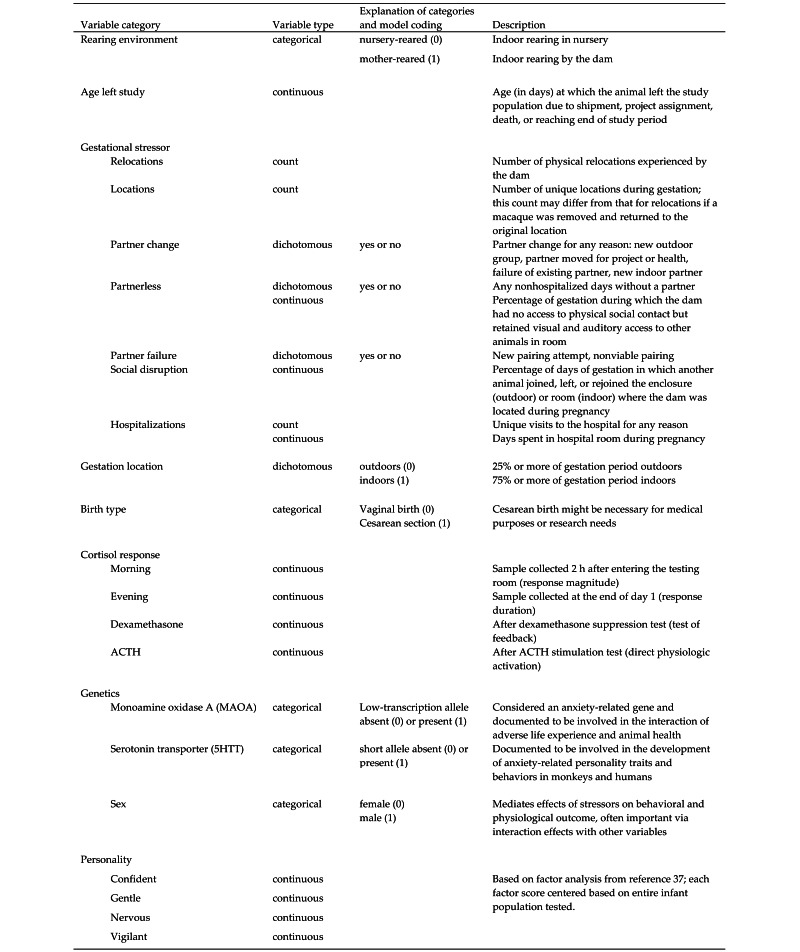
Description of variables.
For each infant, data regarding gestational variables were determined by using the digital colony management system at the center. By using the estimated conception date as the start date (estimated by veterinarians according to fetal size at palpation or ultrasonography), all dam movements, cagemate changes, veterinary treatments, and research involvement were recorded. Descriptions of each variable are found in Figure 2. Postnatal health records were reviewed for each animal from birth until August 1 of the animal's third year of life or its removal from the colony due to research assignment, sale, or death. All incidents requiring veterinary care were categorized as either related to gastrointestinal ailments (diarrhea, colitis) or nongastrointestinal (trauma, obstetrical, dental care, other illness). Gestation location was coded as a dichotomous variable, whereby animals spending at least 25% of their gestation outdoors were classified as ‘outdoor-gestated,’ whereas those that spend 25% or less of their gestation outdoors (that is, 75% or more of their gestation indoors) were classified as ‘indoor-gestated.’ To determine this value, the gestation length for each animal was the birth date minus the conception date.
Figure 2.
Description of variables.
At the center, animals routinely are moved from one location to another for a variety of reasons: research needs, social concerns, health concerns, and other colony management needs (for example, room reorganizations). Both the number of times an animal was moved (that is, no. of relocations) and the number of new locations (that is, no. of locations) were determined for each animal during gestation. For indoor animals, cage number changes within the same room were not considered because, broadly, the social environment remained intact. Although these 2 variables of the number of relocations and physical relocations are related, there are important differences when, for example, an animal is returned to its original location after one or more stays in the veterinary ward for treatments.
Relocation into the hospital required special consideration, because it was highly likely that, during the time that the subject and dam were cohoused, hospitalization might be due to the dam's illness, and both dam and infant might consequently experience significant physiologic stress: the hospital ward is a unique environment where the make-up of the room changes daily and caretakers enter frequently to assess or treat one or more animals. In addition, during their hospital stay, animals are frequently supplemented with orange-flavored drink (Tang, Mondelez International, Deerfield, IL), rice cereal, or additional produce. Therefore, number of hospital relocations (that is, hospitalizations) was considered a distinct variable such that count and total length of hospitalization(s) during pregnancy (continuous measure) were evaluated as variables of interest.
Significant effort is made to provide all indoor-housed animals with suitable partners for social contact either intermittently (approximately 0730 to 1530) or continuously (24 h daily). Sometimes one partner may be relocated for project, management, mating, or social reasons, or, rarely, the euthanasia of one partner. Because we suspect that these may be important social events, we documented all dams’ partner changes during gestation (social disruption, partner failure, partner change, and proportion of time animals were partnerless; Figure 2).
We queried the center's internal medical database for all cases of gastrointestinal health events (diarrhea) for those animals evaluated in the BioBehavioral Assessment program from 2001 through 2007. Data were coded from day of birth until the animal was assigned to a research protocol, the animal was shipped to another facility, the animal died or was euthanized, or until 1 August of the animal's third year of life (which we refer to as the animal ‘aging out’ of the study). Most infants are born in March through July; this cut-off was selected to reflect the start of the first active mating season for young females as well as to facilitate health coding. The incidence of diarrhea described here is used as the number of cases in the population per day in analyses, owing to variation in the duration of assessment between animals. Digital medical records were confirmed by inspection of written hospitalization records. When discrepancies were found, the information from the written medical record was used. A case of diarrhea was defined as rectal culture followed by treatment (antibiotics or other) or a hospitalization record with the admission note containing the term ‘diarrhea.’ Separate diarrhea events were distinguished by notation of a clearly dated discharge diagnosis and relocation out of the hospital ward to either the home cage or a new nonhospital location. Results of pathogen culture were not considered in the analyses that follow in light of the absence of statistical correlation between pathogen infection and diarrhea symptoms in the published literature as well as veterinary clinical experience.8,30,39,82
Biobehavioral assessment.
Between 90 and 120 d of age, infants were brought to a testing room at approximately 0900 for a 25-h long biobehavioral assessment,35,61,63 which involves a series of behavioral and physiologic assessments administered in a fixed order throughout the day, either in the animal's temporary holding cage or in a test cage in the next room. At the end of the 25-h period (approximately 1000 on day 2), infants were returned to their mothers or the nursery from which they came.
The first assessment began approximately 15 min after arrival, and other assessments were conducted until nearly 1600 of day 1; observations resumed the next morning. At the end of the 25-h period, the observer rated each animal according to a set of 16 trait adjectives to describe overall temperament. Exploratory and confirmatory factor analyses revealed 4 temperament dimensions: Gentle (gentle, calm, flexible, curious), Nervous (nervous, fearful, timid, not calm, not confident), Confident (confident, bold, active, curious, playful), and Vigilant (vigilant, not depressed, not tense, not timid).35 Blood samples were collected 4 times: 1100 (morning) and 1600 (evening) of day 1 and 0830 (dexamethasone) and 0900 (ACTH) on day 2. After the second sample, animals received dexamethasone (500 μg/kg IM), a synthetic exogenous steroid; consequently, the third sample evaluated the magnitude of endogenous cortisol suppression. After this sample, animals received ACTH (2.5 IU), and 30 min later, the final blood sample was collected to test the responsiveness of the adrenal gland to ACTH stimulation.
Blood samples were used to determine monoamine oxidase A and serotonin transporter promoter genotypes by using published procedures.44 For this analysis, animals were dichotomized as possessing (or not) 1 or 2 low-activity alleles for monoamine oxidase promoter (MAOA) or the short version of the serotonin transporter promoter allele (5HTT). Alleles for these genes vary in their effect on transcriptional activity due to minor variations in gene nucleotide sequences. For MAOA, the low-expression allele is commonly associated with increased vulnerability to adversity and the short 5HTT allele with increased anxiety-related behaviors.17,43,46
Data analysis.
Data were imported into R for analysis.75 Poisson regressions were built with the count of gastrointestinal health events as the outcome variable. All models included the age at which the animal left the study either due to sale, research assignment, death, or aging out at the conclusion of the study period. Models were built in a stepwise fashion by using a priori hypotheses beginning with the earliest time point in the animal's life and progressing with age of influence. Therefore, models started with genotype followed by gestation location and gestational stress exposure and ended with rearing location and personality and cortisol response during the biobehavioral assessment testing paradigm. In this way, our model-building methods were theory-driven based on life-history chronology. Interaction effects were included only if sufficient theory or evidence was available in the literature, and terms within those interactions were hypothesized as main effects. In addition, all variables contained in the model as part of an interaction effect were included as main effects.
Models were ranked by using the Akaike Information Criterion (AIC), a descriptor of how well models within a set fit the existing data. Models are penalized for increasing number of predictors and rewarded for goodness of fit, with the best model having the lowest AIC score.74 All models adding up to 95% of AIC weighting were carried through to the next stepwise addition of predictors. Models are assigned weighting based on their AIC score and their relative likelihood as representative of the data compared with other models in the set. The 95% weighting is considered analogous to a 95% confidence interval that the best model is included within the models selected.13 For the final step of our model building, we applied the principle of parsimony: if a more complicated model differed by less than 7 units from its less complicated counterpart, those additional variables were excluded from the parsimonious model.38 This method has been suggested as maintaining only variables with biologic relevance and reducing the tendency to overfit models. To this end, models were maintained in the form(s) with the highest likelihood(s) (lowest AIC score) until all step-wise additions were completed, and then a parsimonious model was derived to include fewest variables present in the models of the last step of the analysis that still maintained goodness of fit. We present β coefficients in all model tables (effect size equals e^β), but we only interpret results in detail for the parsimonious model.
We evaluated the fit of the entire model and not the statistical significance of individual variables within the model. As such, some models contain main and interaction effects that themselves are not statistically significant but that, when included, the model as a whole is better at explaining the data than when the nonsignificant variables were removed. Although we report statistical values of individual variables, we did not make model-building decisions based on P values of individual variables and therefore did not correct for multiple comparisons.12
Results
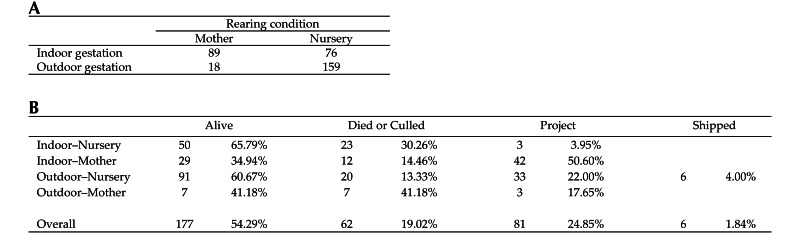
The numbers of indoor and outdoor gestation locations were approximately evenly split in our cohort (177 outdoor, 165 indoor; Figure 3 A). The majority of dams (274) spent either all or none of their gestation indoors, whereas the remaining 68 animals moved locations and were classified according to the described scheme. Postnatally, two thirds of animals were nursery reared (235 macaques), and the remainder (107) were indoor mother-reared. Animals aging out of the study on August 1st of their third year of life accounted for 54.3% of the study population. Other animals died or were culled over health concerns (19.0%), were shipped to another facility (<1%), or were placed on an active project (24.9%) before they reached the designated study end. Range of time spent in the study cohort was 150 to 1272 d (mean ± 1 SD, 935.0 ± 332.5 d). The age at which the macaque left the study pool and the reason for leaving were not equally balanced across groups. (Figure 3 B). Overall, animals experience 1.35 ± 0.86 diarrhea events each during the study period. Nearly half (46.9%) of animals did not experience any clinically relevant diarrhea events while under observation for this study.
Figure 3.
Breakdown of subject pool. (A) Rearing type and gestation location. To be classified as gestated indoors, at least 75% of the duration of the pregnancy was spent indoors; otherwise the offspring was classified as outdoor gestated. Postnatally, all infants in this study were reared indoors, either by their mother or in the nursery. (B) Reasons for animals exiting the study. Alive, alive at end of study period (that is, ‘aged out’ of cohort); died or culled, macaque died or was culled for medical reasons; project, macaque was assigned to an active project and thus removed from the cohort; shipped, macaque was sent to another facility and thus lost to follow-up. A macaque's stint in the study population varied between animals and was included in all models.
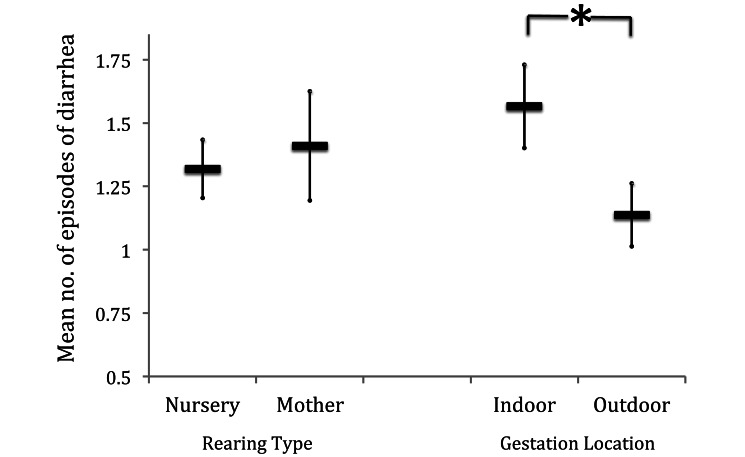
Age at which the animal left the study was included in all models because of the large variability across subjects and the known effect of age on diarrhea risk.30,42 Given that the primary purpose of this study was to evaluate the influence of gestational exposures on early-life diarrhea risk, we first tested the effects of gestation location compared with rearing location in isolation. The β coefficient for gestation location was –0.387 (SE, 0.099), whereas on its own, gestation location better explained the variation in diarrhea incidence than did postnatal rearing location: animals gestated outdoors had significantly (β = –0.387, P < 0.01) lower diarrhea risk than did those gestated indoors (that is, this model had a lower AIC and a significant β coefficient; Figure 4). With this confirmation, we continued our analysis and began building our multivariate models of cumulative risk according to the age at which each variable first had the potential to affect the offspring.
Figure 4.
Mean number of episodes of diarrhea according to rearing type (mother, reared indoors by mother; nursery, reared indoors in nursery) or gestation location. *, P < 0.01; bar, SE.
Our models were built according to our a priori hypotheses in a stepwise fashion by increasing age at first influence on the animal. Step 1 tested anxiety-related genes and genetic sex and the additive and interaction effects between them. The 4 best-fit models which were carried through to step 2 included the main effects of serotonin transporter and monoamine oxidase genotype, and 2 of the models included sex. Models also included interaction effects between the 2 anxiety-related genes or between each gene of interest and sex (Table 1).
Table 1.
Best-fit models for diarrhea risk that include only genetic variables
| gene1 | gene2 | gene3 | gene4 | |
| Intercept | −0.277 (0.931) | −0.576 (0.973) | −0.243 (0.932) | −0.358 (0.966) |
| Age | 0.080 (0.136) | 0.123 (0.141) | 0.072 (0.137) | 0.085 (0.140) |
| 5HTT | −0.370b (0.128) | −0.560b (0.170) | −0.290b (0.146) | −0.293a (0.147) |
| MAOA | 0.083 (0.137) | 0.239 (0.171) | 0.178 (0.161) | 0.173 (0.161) |
| Sex | — | 0.002 (0.182) | — | 0.057 (0.128) |
| 5HTT × MAOA | — | — | −0.337 (0.313) | −0.331 (0.313) |
| 5HTT × sex | — | 0.449 (0.262) | — | — |
5HTT, presence of serotonin transporter gene short allele; MAOA, presence of monoamine oxidase A low-expression gene; sex, male (referent) or female
Data are presented as β coefficients (SE) such that 0.0 indicates no difference associated with that variable. The age that a macaque left the study was used as a standardization variable in all models as a measure of accumulated risk, because all animals entered the study at birth and because the duration in the population varied. Because the models presented are all within 2 AIC units of each other, each has a high likelihood of being the best-fit model for these data. The models differ according to which main and interaction effects are included from the set of genetic influences tested.
P < 0.05
P < 0.01
In step 2 of model building, we added variables that took place during gestation: gestation location, number of locations, time (days) in hospital, number of relocations, and number of days of gestation in which there was some type of social disruption as defined by an animal (re)joining or leaving the enclosure or room (Table 2). We also tested models with interaction effects between gestation location and each of the other variables. The set of best-fit models from this step includes 8 unique but similar sets of these variables of interest.
Table 2.
Best-fit models that include genetic and prenatal environmental variables
| gest1 | gest2 | gest3 | gest4 | gest5 | gest6 | gest7 | gest8 | |
| Intercept | −1.374 | −0.194 | −1.527 | −0.771 | −1.418 | −0.218 | −1.129 | −0.838 |
| (1.02) | (1.07) | (1.03) | (1.04) | (1.02) | (1.07) | (1.01) | (1.04) | |
| log(age at end) | 0.318a | 0.218 | 0.327a | 0.232 | 0.318a | 0.219 | 0.269 | 0.233 |
| (0.15) | (0.15) | (0.15) | (0.15) | (0.15) | (0.15) | (0.15) | (0.15) | |
| 5HTT | −0.724c | −0.649c | −0.703c | −0.657c | −0.695c | −0.645c | −0.44c | −0.649c |
| (0.17) | (0.17) | (0.17) | (0.17) | (0.17) | (0.17) | (0.13) | (0.17) | |
| MAOA | −0.032 | 0.144 | 0.362a | 0.128 | 0.042 | 0.461b | −0.221 | 0.449a |
| (0.25) | (0.25) | (0.17) | (0.25) | (0.24) | (0.17) | (0.22) | (0.18) | |
| Sex | −0.412 | −0.433a | −0.377 | −0.437a | −0.344 | −0.425 | −0.248 | −0.426 |
| (0.21) | (0.22) | (0.22) | (0.22) | (0.21) | (0.22) | (0.17) | (0.22) | |
| Gestation location | −0.924c | −1.198c | −0.713c | −0.946c | −0.809c | 0.564a | −0.805c | −0.797c |
| (0.19) | (0.32) | (0.17) | (0.19) | (0.22) | (0.27) | (0.18) | (0.18) | |
| No. of locations | — | −0.484b | — | — | — | −0.518c | — | — |
| (0.15) | (0.15) | |||||||
| No. of relocations | — | — | — | −0.413b | — | — | — | −0.444c |
| (0.13) | (0.13) | |||||||
| Social disruption | — | — | — | — | −0.135 | −1.103c | — | — |
| (0.08) | (0.31) | |||||||
| Time (d) in hospital | −0.687 | — | −0.6 | — | — | — | −0.584 | — |
| (0.45) | (0.44) | (0.43) | ||||||
| 5HTT × sex | 0.651a | 0.588a | 0.595a | 0.595a | 0.576a | −0.596a | — | 0.565a |
| (0.27) | (0.27) | (0.27) | (0.27) | (0.26) | (0.29) | (0.26) | ||
| MAOA × sex | −0.477 | −0.585a | −0.511 | −0.564 | −0.528 | — | — | −0.583a |
| (0.3) | (0.29) | (0.29) | (0.3) | (0.3) | (0.29) | |||
| Gestation location × sex | 0.825b | 0.876c | 0.774b | 0.838b | 0.751b | 0.846b | 0.677b | 0.807b |
| (0.26) | (0.26) | (0.25) | (0.26) | (0.26) | (0.26) | (0.25) | (0.26) | |
| Gestation location × MAOA | 0.717a | 0.572a | — | 0.581a | 0.632a | — | 0.686* | — |
| (0.29) | (0.29) | (0.29) | (0.29) | (0.29) | ||||
| Gestation location × no. of locations | 0.29 | — | — | — | 0.346 | — | — | |
| (0.2) | (0.2) | |||||||
| Gestation location × no. of relocations | — | — | — | 0.359a | — | — | — | 0.409b |
| (0.15) | — | — | — | (0.14) | ||||
| Gestation location × social disruption | — | — | — | — | 0.131 | — | — | — |
| (0.08) | ||||||||
| Gestation location × time (d) in hospital | 0.665 | — | 0.582 | — | — | — | 0.558 | — |
| (0.45) | (0.44) | (0.43) | ||||||
| AIC value and weight | 770.06 | 773.12 | 774.33 | 774.61 | 774.74 | 775.03 | 775.58 | 776.58 |
| 0.55 | 0.12 | 0.06 | 0.06 | 0.05 | 0.05 | 0.03 | 0.02 |
5HTT, presence of serotonin transporter gene short allele; MAOA, presence of monoamine oxidase A low-expression gene
Data are presented as β coefficients (SE) such that 0.0 indicates no difference associated with that variable. See Figure 2 for complete description of variables.
P < 0.05
P < 0.01
P < 0.001
Next, in step 3, we added the variables of birth type, rearing location, and interactions between birth or rearing type and sex, anxiety-related genes (5HTT and MAOA), and gestation location. None of these variables improved the models relative to their fit without these additional variables; therefore, given the rule of parsimony none of these were retained for the next step.
In step 4, we evaluated models including test scores from the BioBehavioral Assessment to include the final phase of development considered in our hypotheses, the early postnatal period. Specifically we tested blood cortisol concentration at each of the 4 described test points, personality factors, and their interactions with each other and with the other variables for which we had specific hypotheses (Table 3).
Table 3.
Best-fit models that including genetic and gestational variables, early postnatal exposure, personality, and cortisol response
| post1 | post2 | post3 | post4 | post5 | post6 | |
| Intercept | −0.950 | −1.824 | −1.599 | −0.897 | −0.664 | −1.552 |
| (1.165) | (1.126) | (1.144) | (1.181) | (1.17) | (1.162) | |
| log(age at end) | 0.298 | 0.335a | 0.307 | 0.295 | 0.278 | 0.306 |
| (0.164) | (0.162) | (0.164) | (0.166) | (0.164) | (0.166) | |
| 5HTT | −0.53b | −0.59b | −0.523b | −0.525b | −0.542b | −0.517b |
| (0.185) | (0.186) | (0.185) | (0.185) | (0.184) | (0.185) | |
| MAOA | 0.080 | −0.059 | 0.091 | 0.076 | 0.124 | 0.087 |
| (0.254) | (0.257) | (0.255) | (0.254) | (0.254) | (0.255) | |
| Sex | −0.284 | −0.272 | −0.273 | −0.284 | −0.267 | −0.272 |
| (0.236) | (0.232) | (0.236) | (0.236) | (0.235) | (0.236) | |
| Gestation location | −1.323c | −0.933c | −0.981c | −1.321c | −1.333c | −0.984c |
| (0.367) | (0.224) | (0.226) | (0.367) | (0.366) | (0.227) | |
| No. of locations | −0.564c | — | — | −0.560c | −0.560c | — |
| (0.159) | (0.159) | (0.159) | ||||
| No. of relocations | — | — | −0.479 | — | — | −0.476c |
| (0.141) | (0.141) | |||||
| Time (d) in hospital | — | −0.713 | — | — | — | — |
| — | (0.467) | — | — | — | — | |
| Rearing location | 0.407a | 0.316 | 0.401a | 0.398a | 0.498a | 0.394a |
| (0.192) | (0.192) | (0.191) | (0.198) | (0.202) | (0.197) | |
| Nervous | 0.345b | 0.350b | 0.340b | 0.361c | 0.327a | 0.361c |
| (0.119) | (0.118) | (0.118) | (0.084) | (0.139) | (0.084) | |
| Gentle | — | — | — | −0.037 | — | −0.041 |
| — | — | — | (0.196) | — | (0.195) | |
| ACTH stimulation | −0.001 | 0 | −0.001 | −0.001 | — | −0.001 |
| (0.001) | (0.001) | (0.001) | (0.001) | — | (0.001) | |
| Cortisol sample 2 (evening, day 1) | — | — | — | — | −0.003 | — |
| — | — | — | — | (0.002) | — | |
| 5HTT × sex | 0.639a | 0.694a | 0.620a | 0.635a | 0.632a | 0.615a |
| (0.278) | (0.28) | (0.278) | (0.279) | (0.279) | (0.279) | |
| MAOA × sex | −0.675a | −0.570a | −0.693a | −0.671a | −0.717a | −0.688a |
| (0.310) | (0.313) | (0.313) | (0.31) | (0.316) | (0.313) | |
| Gestation location × sex | 0.722b | 0.691a | 0.689a | 0.731b | 0.710a | 0.693a |
| (0.278) | (0.275) | (0.276) | (0.282) | (0.283) | (0.28) | |
| Gestation location × MAOA | 0.749a | 0.869b | 0.744a | 0.750a | 0.709a | 0.744a |
| (0.298) | (0.300) | (0.300) | (0.298) | (0.298) | (0.300) | |
| Gestation location × no. of locations | 0.383 | — | — | 0.379 | 0.383 | — |
| (0.218) | (0.218) | (0.217) | ||||
| Gestation location × no. of relocations | — | — | 0.443b | — | — | 0.441b |
| (0.157) | (0.157) | |||||
| Gestation location × time (d) in hospital | — | 0.689 | — | — | — | — |
| (0.467) | ||||||
| Rearing location × nervous | −0.738c | −0.679c | −0.749c | −0.719c | −0.716c | −0.725c |
| (0.162) | (0.154) | (0.162) | (0.152) | (0.166) | (0.152) | |
| Nervous × ACTH stimulation | 0 | 0 | 0 | — | — | — |
| (0.001) | (0.001) | (0.001) | ||||
| Nervous × cortisol sample 2 | — | — | — | — | 0.001 | — |
| (0.002) | ||||||
| Gentle × ACTH stimulation | — | — | — | 0 | — | 0 |
| (0.002) | (0.002) | |||||
| AIC value and weight | 712.19 | 713.7 | 713.75 | 714.42 | 715.54 | 716.03 |
| 0.37 | 0.17 | 0.17 | 0.12 | 0.07 | 0.05 |
5HTT, presence of serotonin transporter gene short allele; MAOA, presence of monoamine oxidase A low-expression gene
Data are presented as β coefficients (SE) such that 0.0 indicates no difference associated with that variable. See Figure 2 for complete description of variables.
P < 0.05
P < 0.01
P < 0.001
After this final building step, we pared down the model to include only those variables present in each of the best performing models from step 4, to identify the most parsimonious model—that with the fewest variables that retained its goodness of fit and reduced the likelihood of overfitting.41 Table 4 summarizes the best, most parsimonious model.
Table 4.
Best-fit parsimonious model
| β | SE | |
| Main effects | ||
| Intercept | −1.329 | 1.183 |
| Age | 0.325a | 0.166 |
| Sex | −0.303 | 0.239 |
| 5HTT | −0.569b | 0.187 |
| MAOA | 0.046 | 0.259 |
| Gestation location | −1.263c | 0.383 |
| No. of locations | −0.350a | 0.172 |
| Nervous | 0.344b | 0.118 |
| Rearing location | 0.355 | 0.195 |
| Time (d) in hospital | −0.445 | 0.429 |
| ACTH stimulation | 0 | 0.001 |
| Interaction effects | ||
| 5HTT × sex | 0.684 | 0.282 |
| MAOA × sex | −0.616 | 0.315 |
| Gestation location × sex | 0.748b | 0.279 |
| Gestation location × no. of locations | 0.278 | 0.243 |
| Rearing location × nervous | −0.722c | 0.161 |
| Gestation location × time (d) in hospital | 0.426 | 0.429 |
| Gestation location × MAOA | 0.786b | 0.301 |
| Nervous × ACTH stimulation | 0 | 0.001 |
5HTT, presence of serotonin transporter gene short allele; MAOA, presence of monoamine oxidase A low-expression gene
Data are presented as β coefficients such that 0.0 indicates no difference associated with that variable. See Figure 2 for complete description of variables.
P < 0.05
P < 0.01
P < 0.001
In the best-fit model (Table 4), the presence of the short form of the serotonin transporter gene (5HTT) had a protective effect, with macaques at reduced risk for diarrhea relative to those with the long form (β = –0.569, P < 0.01; Figure 5). The low-expression gene for MAOA was included as well, although the presence of the low-expression form was associated with a nonstatistically significant increase in diarrhea incidence. Although the main effect of MAOA was not statistically significant in the best-fit model, because removing this variable greatly reduced the fit of the model and increased its AIC value, MAOA was preserved in the parsimonious model. The interaction effects involving MAOA added in later steps of model building suggests that the gene is important in understanding diarrhea risk in some groups of animals but not others.
Figure 5.
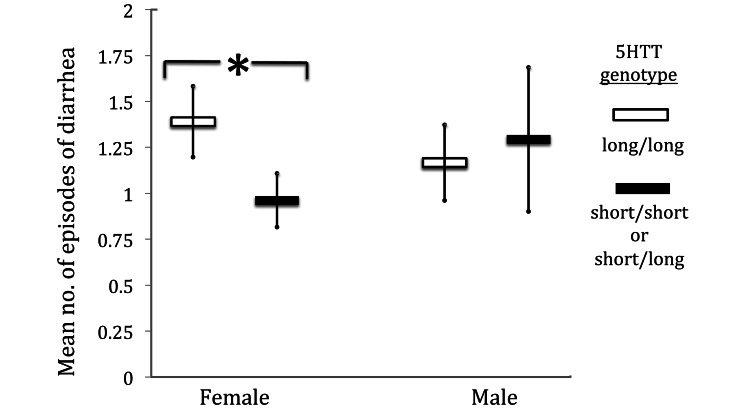
Number of diarrhea episodes in male and female macaques according to the presence (filled symbols) or absence (open symbols) of the serotonin transporter short allele. *, Interaction effect (P < 0.05); bar, SE.
Animal sex is also included, although not statistically significant on its own, because removing sex decreased the model fit. Both anxiety-related genes had interactions with sex (sex × 5HTT: β = 0.684, P > 0.05; sex ×MAOA: β = –0.616, P > 0.05), such that the short allele was protective for female macaques, whereas there was no effect of 5HTT genotype for male macaques.
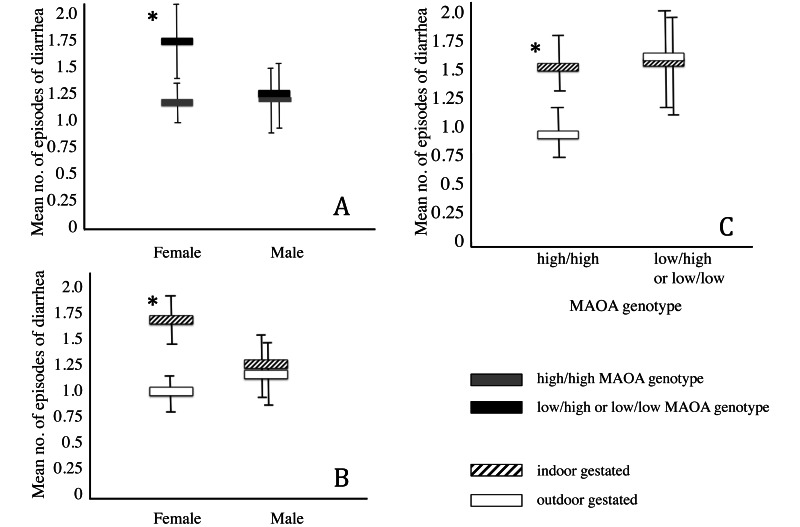
Outdoor gestation location had a strong protective effect (β = –1.263, P < 0.001), which we expected from its strong predictive value in the univariate models first tested (Figure 3). Gestation location also interacted with many of the other variables considered in this study, including sex (β = 0.748, P < 0.01; outdoor gestation was particularly protective for female macaques) and MAOA genotype (β = 0.786, P < 0.01; the high activity allele was protective for outdoor-gestated animals), as well as nonsignificant interactions that were important to model fit, including number of unique locations and number of days spent in the hospital. A difference in diarrhea risk based on both gestation location and MAOA genotype was only found for female macaques (Figure 6).
Figure 6.
Mean number of diarrhea episodes according to (A) the sex of the macaque and presence (black) or absence (gray) of the low-expression MAOA allele, (B) the gestation location (indoor, hashed; outdoor, open) and MAOA genotype, and (C) gestation location and sex of animal. Because the gene is sex-linked, male macaques have only a single allele (either high or low expression). Bar, SE; interaction effect, P < 0.05.
Animals with a nervous temperament had a significantly greater risk of diarrhea (β = 0.334, P < 0.01), but this effect was moderated by rearing location (which by itself was not a significant predictor): those macaques scoring high on the nervous personality scale had higher diarrhea incidence when they were nursery-reared than when they were mother-reared (interaction β = –0.722, P < 0.001). Also present as an interaction effect with nervous temperament score was response to ACTH stimulation. The coefficient for this interaction, as well as for the main effect of ACTH stimulation, was 0; however, removing these terms decreased the overall model fit. None of the other cortisol measurements (immediately after transfer to testing location, evening of day 1, and after dexamethasone suppression) were included in any of the stepwise models or in the final model. Finally, contrary to expectation, animals that experienced more unique locations in the move histories had a reduced risk for diarrhea (β = –0.350, P < 0.05).
Some variables, such as social disruption, appear in best-fit models during the stepwise model-building process but were not included in the final model. This situation might occur when the explanatory power of one variable is masked by similar but stronger correlations with other variables or by high level of correlation between 2 variables such that only one can be included in the model at a time. In the case of the social disruption variable, number of locations or number of relocations might have played that role.
Discussion
Nursery rearing has repeatedly been found to have deleterious effects on health outcomes in rhesus macaques.59,67,81 Other studies have shown that indoor-reared animals have significantly higher lifetime diarrhea incidence than their outdoor-reared peers.32,59,67 The present results, however, suggest that important influences begin even before birth: within the indoor-reared cohort, factors experienced in utero contribute to diarrhea incidence during the first 3 y of life; in fact, the location of gestation (indoors compared with outdoors) is a more important predictor of diarrhea incidence than is the type of postnatal indoor-rearing experience. Assuming that indoor gestation is stressful, our results are consistent with other research showing that exposure of a pregnant female macaque to stressors is associated with offspring birth weight, immune development and function, and gastrointestinal function.6,21-23
Outdoor gestation not only had a strong protective effect, reducing the diarrhea incidence on its own, but it also interacted with several variables (Table 4). That gestation location is so integral to the model demonstrates its important role in supporting the development of healthy macaques. In the final parsimonious model, gestation location had interactions with sex, MAOA genotype, number of locations, and days in hospital. Both gestation location and MAOA genotype influenced diarrhea incidence in female but not male macaques (Figure 6).
Indoor rearing type, whether mother- or nursery- reared, was not significant in the model as a main effect but did improve the model when included as an interaction effect with the animal's nervous personality rating. Although more nervous animals overall showed greater diarrhea incidence, this increased risk was especially pronounced when they were also nursery-reared. Personality influences an animal's response to its environment as well as its perception of that environment.2,14,48 Nervous animals, therefore, may perceive nursery rearing as more stressful than do their peers that score lower regarding nervous temperament. Because these animals are bred for use in biomedical research, this interindividual difference in response to a particular environment may have consequences for researchers if not weighed when considering an animal's response to experimental designs.25,34,52,88
The putative stressful experiences of pregnant female macaques in the model were number of locations, number of days hospitalized, number of relocations, and the percentage of time spent indoors. Although these experiences appear to be primarily behavioral stressors for the dam (that is, an animal is relocated to another cage), many of these experiences have physiologic aspects. For example, for many relocations and physical examinations, animals are sedated with ketamine, a dissociative anesthetic that, after prenatal exposure, affects offspring behavior through interaction with the MAOA genotype.17 Although we found no interaction effect between MAOA genotype and physical relocations on diarrhea occurrence in the current study, our measure of relocations was neither specific to ketamine exposure nor necessarily inclusive of all ketamine exposures. The interaction effect between MAOA and gestation location is large (effect size, 2.19)—animals gestated outdoors that carry the MAOA low-expression allele are less likely to respond effectively to the environmental change of relocation indoors and have a greater diarrhea risk compared with macaques that do not carry that allele.
The genes we examined in this study are specifically involved in anxiety-related behavior and sensitivity to the environment.16,43 The low-expression allele of the MAOA gene is generally associated with increased vulnerability to adverse environments, although it is important to consider that such a gene may also increase vulnerability to positive environmental influences, as described in the biologic sensitivity-to-context models.31 That the presence of the short allele of the serotonin transporter (5HTT) had a protective effect for female macaques but not male macaques may reflect social factors that differ between the sexes (Figure 5). This sex-associated difference was unexpected, given the consistent evidence in the NHP literature of association between the short allele and anxiety-related behavior.45,49,78 However, some evidence suggests that expression of anxiety-related behaviors may be an effective mechanism for coping with stressors. The expression of stereotypic behaviors (often considered a sign of poor animal wellbeing) is a demonstrated, effective means of coping with perceived stressors for some animals.54 This idea of multiple, different coping strategies, some of which are more or less effective in particular environments, was first described as a dichotomous ‘reactive–proactive’ coping strategy.47,48 This theory postulates that, given different environments, an animal might adopt one of multiple coping strategies; that the particular strategy adopted is often heavily influenced its genes, personality, and life experience; and that the strategy adopted might be more or less effective at enabling the animal to overcome the stressor.47,48 Therefore, in the population studied here, female macaques possessing the short version of the 5HTT gene might have a more effective coping strategy than do those with the long allele, given a particular developmental environment. Similarly, for the MAOA gene, absence of the low-expression (risk susceptibility) gene is only protective for animals gestated outdoors, supporting the importance of the relationship between genes and environment in health.
Whether the adverse effect of the low-activity MAOA allele is due to the prenatal experience of the relocation to indoor housing or to the other features of indoor living that followed the relocation is unclear. All of the animals in our study were reared indoors and therefore experienced similar husbandry and environmental features, highlighting the interactions between genes and the environment.25,66,71,76,77,81 A comparison of animals gestated indoors and reared outdoors would be valuable but is only feasible for fostered infants, given that it would be dangerous to the infants to include them in a social group formation with their mothers immediately after birth.
Surprisingly, the variables proposed as prenatal stressors had protective effects, reducing the diarrhea incidence with increasing exposure. As the number of unique locations experienced during gestation increased, diarrhea incidence decreased. During postnatal life, relocations are associated with increases in diarrhea risk.65 While the animal is in utero, these exposures may serve as ‘minor stressors,’ which are suggested by the Adaptive Calibration Model to reduce lifelong sensitivity to stressors when experienced during development.28,29 The Adaptive Calibration Model suggests that very low-risk developmental environments do not sufficiently prepare an animal for the diversity of situations he or she is likely to encounter during a lifetime. That there was an interaction between gestation location and the number of locations experienced during gestation such that the protective effect of increased locations was seen only for indoor-gestated animals supports the Adaptive Calibration Model. In this sense, a pregnant dam in indoor housing may perceive her environment as nonthreatening, and she need not physiologically adapt to the range of minor stressors experienced by animals living outdoors, such as temperature fluctuations and other weather changes, environmental pathogen exposure, and routine social stressors. Alternatively, pregnant dams in indoor housing may perceive their environment as especially threatening; therefore the fetus develops either a blunted or hyperactive stress response, neither of which is particularly adaptive in the captive setting. Outdoor-housed dams are exposed to these potential stressors, all of which are expected to occur in her natural environment; thus she—and her developing fetus—may be evolutionarily adapted to readily cope with these stressors.
The variables of number of locations and number of relocations both had similar magnitudes and direction of effect as well as statistical strength in models where they were present. This pattern suggests something about the common aspects of these 2 variables. In the study population, a location move, such as to the hospital, followed by return to the original social group evoked a similar protective effect as did relocation to new group for a pregnant dam—a surprising result. Our hypotheses regarding these variables were that they would increase diarrhea risk in the offspring. However, these types of minor stressors may prepare the offspring for a varied postnatal environment and prime the hypothalamic–pituitary–adrenal axis to produce an effective response.28,29 The specific aspects and consequences of animal handling and movement warrant future study. Our result showing an apparent protective effect of more location movements during gestation goes against our hypothesis that such movement would increase diarrhea incidence in affected offspring. Future work should divide these variables into their component parts (that is, separation from social partners, transportation, reason for movement, duration of separation, known peers in the new location if relocated) to assess which features are most important.
Variables that are important in the model but that were not statistically significant on their own may explain some of the residual variance remaining in diarrhea risk once the other variables are accounted for. Alternatively, perhaps component features of that variable (but not the variable as a whole or as measured) are contributing explanatory power. These variables therefore warrant further investigation to better understand, for example, the features of ‘maleness’ that put an animal at increased risk of diarrhea. In addition, variation in diarrhea incidence could be due to subcategories of that particular variable; a larger dataset might help to explain a significant amount of the variation associated that variable.
The cortisol response to ACTH stimulation and the interaction between this measure and the personality trait nervous were included in the parsimonious model despite not being statistically significant. In other studies involving these correlates of behavior and physiologic stress response, these 2 variables have been the most highly correlated with other measures of stress, for example, hair cortisol (a long-term cortisol measurement) and the expression of stereotypic behaviors.36,80 We believe that these 2 variables explain an important component of the variability between an animal's diarrhea risk during the first few years of life, especially when viewed as ‘set-points’ affecting magnitude and direction of response to stimuli.
Diarrhea is a clinically nonspecific symptom that can result from the dysfunction or dysregulation of numerous body systems and thus is a sensitive measure for overall health.70,73 During development, body systems including the immune system and gastrointestinal tract and cognitive function are vulnerable to effects of the environment.4,20,21,76,77,83 The mechanism by which prenatal effects influence health outcomes may involve epigenetics—the regulation of gene expression by modulation of the chromosome environment in the absence of alteration of the actual genetic code.83 However, this mechanism has not yet been studied in the outbred colony of macaques at our center, due to cost and technical limitations. Maternal circulating cortisol concentrations may drive dysregulation of several downstream systems and determine offspring stress system set points.58 Genetic and environmental exposures influence these values, which in turn influence the magnitude and direction of response to stimuli.
The variables we tested here were chosen on the basis of existing reports in the literature, many of which were experimental case-control studies, which often resulted in increased control over the operationalization of each variable. Although some variables, such as Cesarean section birth, were not well represented in our study population (and therefore we found no association with diarrhea risk), we do not suggest that they are unimportant factors regarding health and development; the contrary has been well-documented in the literature (see reference 51 for review). Very few animals in the outdoor-gestated group were also indoor mother-reared; therefore although we found no interaction effect between gestation and rearing location, a larger study population with a more balanced cohort might reveal an important relationship between these variables. In addition, our distinction between indoor- and outdoor-gestated groups as less than or at least 25% of gestation outdoors, respectively, was based on limited data for animals being moved from one location to the other during gestation, and the movement typically was from outdoors to indoors. Less than 20% of our sample spent between 0% and 100% of gestation time in a single location—meaning that more than 80% spent either all of gestation either indoors or outdoors. It would be valuable to study a larger cohort that experienced a mixed gestation location to evaluate more closely how much time outdoors is recommended.
The groups we studied here, for example with regard to gestational location or rearing type, are not fundamentally equal due to research and colony management concerns involved in animal placement. Because this study resulted from our hypotheses regarding the effect of gestational stress, animals reared outdoors were not included because no animals were gestated indoors and then moved outdoors for early rearing in the available population.
The finding that gestational environment influences diarrhea incidence in early life more than does the specific indoor postnatal environment shows the importance of prenatal experience in important health outcomes. Macaques that develop chronic or recurrent diarrhea often must be euthanized as symptoms progress and recur.3,40,42 Rearing history is a known risk factor for this disease progression, but in light of our current results, the gestational environment for indoor-reared macaques is another important component of that risk. Assuming that our results are confirmed with causal studies, they suggest that pregnant female rhesus macaques should be housed outdoors, in large complex social groups whenever possible for at least 25% of their pregnancy. We recognize that not all facilities are able to maintain outdoor colonies; in those situations, exploring indoor group-housing options and providing sufficient environmental stimulation to pregnant dams may achieve a comparable protective effect.
The current work establishes a connection between typical breeding facility gestational environments and an actual health outcome early in life. Previous work found a relationship between a specific gestational stressor and gut microflora.6,22,23 The current study expands on that information to describe how the standard environments found in research-associated breeding facilities interact with inherent animal traits to influence diarrhea risk, a veterinary and husbandry outcome of concern.
Animal health is a complex amalgamation of genetic, developmental, and environmental influences. Obtaining the healthiest animals with highest possible wellbeing requires efforts to provide the most species-typical environment possible at all phases, from the environment of breeding-age female NHP to the care of pregnant dams, through the early postnatal period, and into puberty. This retrospective analysis suggests the need for a prospective experimental study that can isolate factors associated with gestational stressors and produce clinically useful data that identify the most important influences on early-life diarrhea in NHP.
Acknowledgments
We thank the veterinary staff at the California National Primate Research Center for their participation and assistance. Funding was provided by R24OD010962 (JPC), P51OD011107 (CNPRC base grant), and the Veterinary Scientist Training Program at the University of California School of Veterinary Medicine (Elfenbein).
References
- 1.Animal Welfare Act as Amended. 20087 USC 2131–2156.
- 2.Allport GW. 1937. Personality: a psychological interpretation. Oxford (United Kingdom): Holt. [Google Scholar]
- 3.Ardeshir A, Oslund KL, Ventimiglia F, Yee J, Lerche NW, Hyde DM. 2013. Idiopathic microscopic colitis of rhesus macaques: quantitative assessment of colonic mucosa. Anat Rec (Hoboken) 296:1169–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey MT, Coe CL. 1999. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus macaques. Dev Psychobiol 35:146–155. [PubMed] [Google Scholar]
- 5.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. 2011. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun 25:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey MT, Lubach GR, Coe CL. 2004. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr 38:414–421. [DOI] [PubMed] [Google Scholar]
- 7.Barry PA, Strelow L. 2008. Development of breeding populations of rhesus macaques (Macaca mulatta) that are specific pathogen-free for rhesus cytomegalovirus. Comp Med 58:43–46. [PMC free article] [PubMed] [Google Scholar]
- 8.Blackwood RS, Tarara RP, Christe KL, Spinner A, Lerche NW. 2008. Effects of the macrolide drug tylosin on chronic diarrhea in rhesus macaques (Macaca mulatta). Comp Med 58:81–87. [PMC free article] [PubMed] [Google Scholar]
- 9.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. 2011. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 108:16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breivik T, Sluyter F, Hof M, Cools A. 2000. Differential susceptibility to periodontitis in genetically selected Wistar rat lines that differ in their behavioral and endocrinological response to stressors. Behav Genet 30:123–130. [DOI] [PubMed] [Google Scholar]
- 11.Brunton PJ. 2013. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction 146:R175–R189. [DOI] [PubMed] [Google Scholar]
- 12.Burnham KP, Anderson DR. 2004. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res 33:261–304. [Google Scholar]
- 13.Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. [Google Scholar]
- 14.Capitanio JP. 2008. Personality and disease. Brain Behav Immun 22:647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capitanio JP. 2011. Nonhuman primate personality and immunity: mechanisms of health and disease, p. 233–256. In: Weiss A, King JE, Murray L, Personality and temperament in nonhuman primates. New York (NY): Springer Science. [Google Scholar]
- 16.Capitanio JP, Abel K, Mendoza SP, Blozis SA, McChesney MB, Cole SW, Mason WA. 2008. Personality and serotonin transporter genotype interact with social context to affect immunity and viral set-point in simian immunodeficiency virus disease. Brain Behav Immun 22:676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capitanio JP, Del Rosso LA, Calonder LA, Blozis SA, Penedo MC. 2012. Behavioral effects of prenatal ketamine exposure in rhesus macaques are dependent on MAOA genotype. Exp Clin Psychopharmacol 20:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capitanio JP, Lerche NW. 1998. Social separation, housing relocation, and survival in simian AIDS: a retrospective analysis. Psychosom Med 60:235–244. [DOI] [PubMed] [Google Scholar]
- 19.Capitanio JP, Mason WA, Mendoza SP, Del Rosso LA, Roberts JA. 2006. Nursery rearing and biobehavioral organization. p 191–214. In: Sackett GP, Ruppentahal GC, Elias, Nursery rearing of nonhuman primates in the 21st century. New York (NY): Springer. [Google Scholar]
- 20.Coe CL. 1993. Pyschosocial factors and immunity in nonhuman primates: a review. Psychosom Med 55:298–308. [DOI] [PubMed] [Google Scholar]
- 21.Coe CL, Lubach GR. 2003. Critical periods of special health relevance for psychoneuroimmunology. Brain Behav Immun 17:3–12. [DOI] [PubMed] [Google Scholar]
- 22.Coe CL, Lubach GR. 2005. Prenatal origins of individual variation in behavior and immunity. Neurosci Biobehav Rev 29:39–49. [DOI] [PubMed] [Google Scholar]
- 23.Coe CL, Lubach GR, Schneider ML, Dierschke DJ, Ershler WB. 1992. Early rearing conditions alter immune responses in the developing infant primate. Pediatrics 90:505–509. [PubMed] [Google Scholar]
- 24.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. 2012. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA 109:5995–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coleman K. 2012. Individual differences in temperament and behavioral management practices for nonhuman primates. Appl Anim Behav Sci 137:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins SM. 2001. Stress and the gastrointestinal tract, IV. Modulation of intestinal inflammation by stress: basic mechanisms and clinical relevance. Am J Physiol Gastrointest Liver Physiol 280:G315–G318. [DOI] [PubMed] [Google Scholar]
- 27.de Souza MA, Centenaro LA, Menegotto PR, Henriques TP, Bonini J, Achaval M, Lucion AB. 2013. Prenatal stress produces social behavior deficits and alters the number of oxytocin and vasopressin neurons in adult rats. Neurochem Res 38:1479–1489. [DOI] [PubMed] [Google Scholar]
- 28.Del Giudice M, Ellis BJ, Shirtcliff EA. 2011. The adaptive calibration model of stress responsivity. Neuroscience Biobehav Rev 35:1562–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Giudice M, Hinnant JB, Ellis BJ, El-Sheikh M. 2012. Adaptive patterns of stress responsivity: a preliminary investigation. Dev Psychol 48:775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elfenbein HA, McCowan B. 2012. The epidemiology of diarrhea in a colony of captive rhesus macaques. Program of the 34th meeting of the American Society of Primatologists 20–23 June 2012. Am J Primatol 74:1–82. 10.1002/ajp.2206422038875 [Google Scholar]
- 31.Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. 2011. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Dev Psychopathol 23:7–28. [DOI] [PubMed] [Google Scholar]
- 32.Elmore DB, Anderson JH, Hird DW, Sanders KD, Lerche NW. 1992. Diarrhea rates and risk factors for developing chronic diarrhea in infant and juvenile rhesus monkeys. Lab Anim Sci 42:356–359. [PubMed] [Google Scholar]
- 33.Folks DG, Kinney FC. 1992. The role of psychological factors in gastrointestinal conditions: a review pertinent to DSM-IV. Psychosomatics 33:257–270. [DOI] [PubMed] [Google Scholar]
- 34.Garner JP. 2005. Stereotypies and other abnormal repetitive behaviors: potential impact on validity, reliability, and replicability of scientific outcomes. ILAR J 46:106–117. [DOI] [PubMed] [Google Scholar]
- 35.Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. 2009. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol 51:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottlieb DH, McCowan B, Capitanio JP. 2013. Risk factors for stereotypic behaviors in rhesus macaques (Macaca mulatta): animal's history, current environment, and personality. Am J Primatol 75:995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gwee KA, Graham JC, McKendrick MW, Collins SM, Marshall JS, Walters SJ, Read NW. 1996. Psychometric scores and persistence of irritable bowel after infectious diarrhoea. Lancet 347:150–153. [DOI] [PubMed] [Google Scholar]
- 38.Hegyi G, Garamszegi LZ. 2010. Using information theory as a substitute for stepwise regression in ecology and behavior. Behav Ecol Sociobiol 65:69–76. [Google Scholar]
- 39.Hird DW, Anderson JH, Bielitzki JT. 1984. Diarrhea in nonhuman primates: a survey of primate colonies for incidence rates and clinical opinion. Lab Anim Sci 34:465–470. [PubMed] [Google Scholar]
- 40.Howell S, White D, Ingram S, Jackson R, Larin J, Morales P, Garcia AP, Hicks C, Hopper K, Wagner J. 2012. A bio-behavioral study of chronic idiopathic colitis in the rhesus macaque (Macaca mulatta). Appl Anim Behav Sci 137:208–220. [Google Scholar]
- 41.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 42.Kanthaswamy S, Elfenbein HA, Ardeshir A, Ng J, Smith DG, Hyde DM, Lerche NW. 2013. Familial aggregation of chronic diarrhea disease (CDD) in rhesus macaques (Macaca mulatta). Am J Primatol 76:262–270. [DOI] [PubMed] [Google Scholar]
- 43.Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. 2009. What is an ‘adverse’ environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol Psychiatry 65:770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karere GM, Sullivan E, Kinnally EL, Capitanio JP, Lyons LA. 2012. Enhancing genotyping of MAOA-LPR and 5-HTT-LPR in rhesus macaques (Macaca mulatta). J Med Primatol 41:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinnally EL, Karere GM, Lyons LA, Mendoza SP, Mason WA, Capitanio JP. 2010. Serotonin pathway gene–gene and gene–environment interactions influence behavioral stress response in infant rhesus macaques. Dev Psychopathol 22:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinnally EL, Lyons LA, Abel K, Mendoza S, Capitanio JP. 2008. Effects of early experience and genotype on serotonin transporter regulation in infant rhesus macaques. Genes Brain Behav 7:481–486. [DOI] [PubMed] [Google Scholar]
- 47.Koolhaas J, Korte S, De Boer S, Van Der Vegt B, Van Reenen C, Hopster H, De Jong I, Ruis M, Blokhuis H. 1999. Coping styles in animals: current status in behavior and stress physiology. Neurosci Biobehav Rev 23:925–935. [DOI] [PubMed] [Google Scholar]
- 48.Koolhaas JM. 2008. Coping style and immunity in animals: making sense of individual variation. Brain Behav Immun 22:662–667. [DOI] [PubMed] [Google Scholar]
- 49.Koolhaas JM, de Boer SF, Coppens CM, Buwalda B. 2010. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front Neuroendocrinol 31:307–321. [DOI] [PubMed] [Google Scholar]
- 50.Korte SM, Olivier B, Koolhaas JM. 2007. A new animal welfare concept based on allostasis. Physiol Behav 92:422–428. [DOI] [PubMed] [Google Scholar]
- 51.Kranich J, Maslowski KM, Mackay CR. 2011. Commensal flora and the regulation of inflammatory and autoimmune responses. Semin Immunol 23:139–145. [DOI] [PubMed] [Google Scholar]
- 52.Lewejohann L, Zipser B, Sachser N. 2011. ‘Personality’ in laboratory mice used for biomedical research: a way of understanding variability? Dev Psychobiol 53:624–630. [DOI] [PubMed] [Google Scholar]
- 53.Line SW, Markowitz H, Morgan KN, Strong S. 1991. Effects of cage size and environmental enrichment on behavioral and physiological responses of rhesus macaques to the stress of daily events. p 160–179. In: Novak MA, Petto AJ, Through the looking glass: issues of psychological wellbeing in captive nonhuman primates. Washington (DC): American Psychological Association. [Google Scholar]
- 54.Mason GJ, Latham NR. 2004. Can't stop, won't stop: is stereotypy a reliable animal welfare indicator? Anim Welf 13:S57–S69. [Google Scholar]
- 55.McEwen BS. 1998. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 840:33–44. [DOI] [PubMed] [Google Scholar]
- 56.McEwen BS, Wingfield JC. 2003. The concept of allostasis in biology and biomedicine. Horm Behav 43:2–15. [DOI] [PubMed] [Google Scholar]
- 57.Mehta PH, Gosling SD. 2008. Bridging human and animal research: a comparative approach to studies of personality and health. Brain Behav Immun 22:651–661. [DOI] [PubMed] [Google Scholar]
- 58.Mina TH, Reynolds RM. 2014. Mechanisms linking in utero stress to altered offspring behaviour. Curr Top Behav Neurosci 18: 93–122. [DOI] [PubMed] [Google Scholar]
- 59.Muñoz-Zanzi CA, Thurmond MC, Hird DW, Lerche NW. 1999. Effect of weaning time and associated management practices on postweaning chronic diarrhea in captive rhesus monkeys (Macaca mulatta). Lab Anim Sci 49:617–621. [PubMed] [Google Scholar]
- 60.Nielsen NM, Hansen AV, Simonsen J, Hviid A. 2011. Prenatal stress and risk of infectious diseases in offspring. Am J Epidemiol 173:990–997. [DOI] [PubMed] [Google Scholar]
- 61.Nowak MA, Sackett GP. 2006. The effects of rearing experiences: the early years. p 1–16. In:Sackett G, Ruppentahal G, Elias K. Nursery rearing of nonhuman primates in the 21st century. New York (NY): Springer. [Google Scholar]
- 62.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. 2006. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118:511–521. [DOI] [PubMed] [Google Scholar]
- 63.Porton I, Niebruegge K. 2006. The changing role of hand rearing in Zoo-based primate breeding programs. p 21–24. In: Sackett G, Ruppentahal G, Elias K. Nursery rearing of nonhuman primates in the 21st century. New York (NY): Springer. [Google Scholar]
- 64.Romero LM, Dickens MJ, Cyr NE. 2009. The reactive scope model: a new model integrating homeostasis, allostasis, and stress. Horm Behav 55:375–389. [DOI] [PubMed] [Google Scholar]
- 65.Rommeck I, Anderson K, Heagerty A, Cameron A, McCowan B. 2008. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. J Appl Anim Welf Sci 12:61–72. [DOI] [PubMed] [Google Scholar]
- 66.Rommeck I, Capitanio JP, Strand SC, McCowan B. 2011. Early social experience affects behavioral and physiological responsiveness to stressful conditions in infant rhesus macaques (Macaca mulatta). Am J Primatol 73:692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell RG, Rosenkranz SL, Lee AL, Howard H, DiGiacomo RF, Bronsdon MA, Blakley GA, Tsai CC, Morton WR. 1987. Epidemiology and etiology of diarrhea in colony-born Macaca nemestrina. Lab Anim Sci 37:309–316. [PubMed] [Google Scholar]
- 68.Sachser N, Hennessy MB, Kaiser S. 2011. Adaptive modulation of behavioural profiles by social stress during early phases of life and adolescence. Neurosci Biobehav Rev 35:1518–1533. [DOI] [PubMed] [Google Scholar]
- 69.Sartor RB. 2001. Intestinal microflora in human and experimental inflammatory bowel disease. Curr Opin Gastroenterol 17:324–330. [DOI] [PubMed] [Google Scholar]
- 70.Sartor RB. 2008. Microbial influences in inflammatory bowel diseases. Gastroenterology 134:577–594. [DOI] [PubMed] [Google Scholar]
- 71.Schapiro SJ, Nehete PN, Perlman JE, Sastry KJ. 2000. A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Appl Anim Behav Sci 68:67–84. [DOI] [PubMed] [Google Scholar]
- 72.Sestak K. 2005. Chronic diarrhea and aids: insights into studies with nonhuman primates. Curr HIV Res 3:199–205. [DOI] [PubMed] [Google Scholar]
- 73.Spiller R, Garsed K. 2009. Infection, inflammation, and the irritable bowel syndrome. Dig Liver Dis 41:844–849. [DOI] [PubMed] [Google Scholar]
- 74.Symonds MRE, Moussalli A. 2010. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behav Ecol Sociobiol 65:13–21. [Google Scholar]
- 75.The R Core Team.[Internet] 2012. R: a language and environment for statistical computing. [01 September 2012]. Available at: https://cran.r-project.org/doc/manuals/fullrefman.pdf.
- 76.Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. 2012. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci U S A 109:6490–6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tung J, Gilad Y. 2013. Social environmental effects on gene regulation. Cell Mol Life Sci 70:4323–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Van den Hove DLA, Leibold NK, Strackx E, Martinez-Claros M, Lesch KP, Steinbusch HWM, Schruers KRJ, Prickaerts J. 2013. Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur Neuropsychopharmacol 24:595–607. [DOI] [PubMed] [Google Scholar]
- 79.Van Scott MR, Reece SP, Olmstead S, Wardle R, Rosenbaum MD. 2013. Effects of acute psychosocial stress in a nonhuman primate model of allergic asthma. J Am Assoc Lab Anim Sci 52:157–164. [PMC free article] [PubMed] [Google Scholar]
- 80.Vandeleest JJ, Hamel AF, Meyer J, Novak MA, Mendoza SP, Capitanio JP. 2011. Hair cortisol is correlated with adrenal capacity and influenced by birth timing in infant rhesus monkeys (Macaca mulatta). Program of the 34th meeting of the American Society of Primatologists, Austin, Texas, 16–19 September 2011. Am J Primatol 73:1–120. 10.1002/ajp.2097821104875 [Google Scholar]
- 81.Vandeleest JJ, McCowan B, Capitanio JP. 2011. Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta). Appl Anim Behav Sci 132:81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Tu X, Humphrey C, McClure H, Jiang X, Qin C, Glass RI, Jiang B. 2007. Detection of viral agents in fecal specimens of monkeys with diarrhea. J Med Primatol 36:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. 2004. Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854. [DOI] [PubMed] [Google Scholar]
- 84.Weinstein TA, Capitanio JP. 2008. Individual differences in infant temperament predict social relationships of yearling rhesus monkeys, Macaca mulatta. Anim Behav 76:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitehead WE, Crowell MD, Robinson JC, Heller BR, Schuster MM. 1992. Effects of stressful life events on bowel symptoms: subjects with irritable bowel syndrome compared with subjects without bowel dysfunction. Gut 33:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilk JL, Maginnis GM, Coleman K, Lewis A, Ogden B. 2008. Evaluation of the use of coconut to treat chronic diarrhea in rhesus macaques (Macaca mulatta). J Med Primatol 37:271–276. [DOI] [PubMed] [Google Scholar]
- 87.Wingfield JC. 2013. The comparative biology of environmental stress: behavioural endocrinology and variation in ability to cope with novel, changing environments. Anim Behav 85:1127–1133. [Google Scholar]
- 88.Wurbel H. 2000. Behaviour and the standardization fallacy. Nat Genet 26:263. [DOI] [PubMed] [Google Scholar]