Abstract
Postoperative analgesia in laboratory rats is complicated by the frequent handling associated with common analgesic dosing requirements. Here, we evaluated sustained-release buprenorphine (Bup-SR), sustained-release meloxicam (Melox-SR), and carprofen gel (CG) as refinements for postoperative analgesia. The aim of this study was to investigate whether postoperative administration of Bup-SR, Melox-SR, or CG effectively controls behavioral mechanical and thermal hypersensitivity in a rat model of incisional pain. Rats were randomly assigned to 1 of 5 treatment groups: saline, 1 mL/kg SC BID; buprenorphine HCl (Bup HCl), 0.05 mg/kg SC BID; Bup-SR, 1.2 mg/kg SC once; Melox-SR, 4 mg/kg SC once; and CG, 2 oz PO daily. Mechanical and thermal hypersensitivity were tested daily from day–1 through 4. Bup HCl and Bup-SR attenuated mechanical and thermal hypersensitivity on days 1 through 4. Melox-SR and CG attenuated mechanical hypersensitivity–but not thermal hypersensitivity–on days 1 through 4. Plasma concentrations, measured by using UPLC with mass spectrometry, were consistent between both buprenorphine formulations. Gross pathologic examination revealed no signs of toxicity in any group. These findings suggest that postoperative administration of Bup HCl and Bup-SR—but not Melox-SR or CG—effectively attenuates mechanical and thermal hypersensitivity in a rat model of incisional pain.
Abbreviations: Bup HCl, buprenorphine HCl; Bup-SR, sustained-release buprenorphine; CG, carprofen gel; Melox-SR, sustained-release meloxicam
Postoperative analgesia is a vital aspect of laboratory animal medicine. Investigators have a responsibility to follow an effective and safe pain management protocol for research animals that have undergone surgical procedures. Pain and distress are serious animal welfare concerns that directly affect animal physiology and can result in altered research data.1,17,30 Continued refinement of pre-, intra-, and postoperative pain management in rodents is necessary to improve animal wellbeing, obtain high-quality research data, and ensure compliance with standards set forth by the Guide for the Care and Use of Laboratory Animals.21
Many classes of analgesics are available to veterinary practitioners, but in the laboratory setting, the options tend to be simpler and typically involve 1 of 2 drug classes, opioids and NSAID. Buprenorphine HCl (Bup HCl), a partial μ-opioid receptor agonist, has long been the ‘gold standard’ for postoperative analgesia in laboratory animals due to the drug's prolonged plasma half-life and effective analgesic properties.15,28 Buprenorphine effectively controls mild to moderate postoperative pain in rodents for 6 to 12 h.16 Because many rodent surgical procedures might cause pain for at least 48 h, researchers must handle these animals at least twice daily during this time period to readminister buprenorphine. Repeated dosing requires frequent handling of surgically manipulated animals, resulting in handling-associated stress.1 In addition, handling an animal frequently likely is disruptive to its cagemates and potentially to animals in the same room. Because of their analgesic and antiinflammatory properties, NSAID are often used either in conjunction with or as an alternative to opioids to control pain in laboratory animals.11,33 Meloxicam and carprofen are 2 NSAID that preferentially inhibit cyclooxygenase 2 and thus prostaglandin synthesis.10,11 Although generally considered safe, reported side effects of NSAID include gastrointestinal ulceration, altered platelet function, and renal dysfunction.11
Novel formulations of opioid and NSAID analgesics have recently been introduced to the veterinary market and include sustained-release injectables,2,5,14,22 gel-based oral compounds,6,19 and transdermal patches.13,18,25,37 Our group previously demonstrated the effectiveness of sustained-release buprenorphine (Bup-SR) in controlling mild to moderate incisional pain in rats.7 Another study found that Bup-SR successfully controlled orthopedic surgical pain in rats.14 These alternative formulations show great potential in decreasing the stress associated with frequent handling and dosing requirements. Many of these products are still considered new in the veterinary market, and few evidence-based recommendations for their use in laboratory animal species are available. The main goal of the current study was to refine postoperative analgesia by using longer-lasting or gel-formulation products. To this end, we investigated whether Bup-SR, sustained-release meloxicam (Melox-SR), or carprofen gel (CG) provided postoperative analgesia in the rat plantar incisional model according to results of behavioral testing. We hypothesized that Bup-SR, Melox-SR, and CG would provide effective postoperative analgesia as evidenced by reduced pain responses in this model.
Materials and Methods
Animals.
Adult (n = 84; weight, 372 ± 2.04 g) male Sprague–Dawley rats (Rattus norvegicus; Charles River, Wilmington, MA) were used in this study. Rats were singly housed in static microisolation cages with ALPHA-dri paper bedding (Shepherd Specialty Papers, Milford, NJ) on a 12:12-h dark:light cycle, at 70 to 74 °F (21 to 23 °C), and 30% to 70% relative humidity. They had unlimited access to commercial food (Teklad Global 18% Protein Rodent Diet 2018, Harlan Laboratories, Madison, WI) and water purified by reverse osmosis. Sentinel rats were free of rat Theiler virus, rat coronavirus, Kilham rat virus, rat parvovirus, rat minute virus, Toolan H1 virus, lymphocytic choriomeningitis virus, murine adenovirus types 1 and 2, reovirus type 3, Sendai virus, pneumonia virus of mice, Mycoplasma pulmonis, and endo- and ectoparasites. Experiments were conducted with approval by the Administrative Panel for Laboratory Animal Care at Stanford University. All animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals.21 Rats were weighed daily after completion of behavioral testing (at 1200) from day –1 through 4. At the conclusion of the study, rats were euthanized by carbon dioxide asphyxiation followed by a secondary physical method.
Study design.
Two experiments were conducted: 1) the incisional pain model and behavioral testing (n = 33) and 2) a plasma concentration study (n = 51). In the first experiment, rats were randomly assigned to receive 1 of 5 treatments: saline (n = 8; 1 mL/kg SC; 0.9% NaCl, Hospira, Lake Forest, IL) at 5 min prior to skin incision followed by twice-daily dosing for 3 d; Bup HCl (n = 5; 0.05 mg/kg SC; Buprenorphine hydrochloride, 0.3 mg/mL, Hospira) at 10 min prior to skin incision followed by twice-daily dosing for 3 d; Bup-SR (n = 6; 1.2 mg/kg SC; Buprenorphine SR-LAB, 1 mg/mL, Zoopharm, Fort Collins, CO) at 10 min prior to skin incision; Melox-SR (n = 7; 4.0 mg/kg SC; Meloxicam SR, 2 mg/mL, Zoopharm) at 10 min prior to skin incision; or CG (n = 7; 2 oz QD for 2 d before and 2 d after surgery; MediGel CPF, 2-oz cup, Clear H2O, Portland, ME). The group sizes varied due to the removal of data outliers from the study. During the treatment period, CG was the only food source for this group (standard rodent diet was removed from each cage), and CG cups were weighed before placement and after removal to monitor daily gel consumption. Administered in this fashion, each rat was estimated to receive 5 mg/kg carprofen daily, as recommended by the manufacturer.
In the second experiment (plasma concentration), rats were randomly assigned to receive 1 of 4 treatments (n = 12 per treatment): Bup HCl (0.05 mg/kg SC twice-daily for 3 d); Bup-SR (1.2 mg/kg SC once); Melox-SR (4.0 mg/kg SC once); and CG (2 oz QD for 4 d). Three rats per treatment group underwent blood collection by terminal cardiocentesis at each time point (1, 2, 3, and 4 d after initial treatment). Rats were necropsied by 2 blinded, experienced clinicians (TLS and SCA) in consultation with a board-certified veterinary pathologist.
Surgery.
Anesthesia was induced in rats by using 3% to 5% isoflurane inside an induction chamber followed by maintenance by using mask delivery of 1% to 2% isoflurane and 100% oxygen. Sterile eye lubrication was applied, and rats were placed on a circulating warm water blanket for the duration of the surgery. Cefazolin (20 mg/kg SC; GlaxoSmithKline, Research Triangle Park, NC) and warm 0.9% NaCl (10 mL/kg SC) were administered prior to incision. The surgical procedure was performed as described previously.4 Each rat was placed in dorsal recumbency, and the plantar surface of the left (ipsilateral) hindpaw was aseptically prepared and draped. Once the animal reached a surgical plane of anesthesia (as indicated by a lack of withdrawal response to toe pinch), a 1-cm longitudinal skin incision was made in the left hindpaw, beginning 0.5 cm from the tibiotarsus and extending 1 cm distally. The flexor digitorum brevis muscle was identified, elevated, and incised longitudinally without disrupting the muscle attachments. Hemostasis was achieved with gentle pressure when necessary. The muscle was released and the skin incision closed with 2 interrupted horizontal mattress sutures (5-0 polyglactin 910, Ethicon, Somerville, NJ). Triple-antibiotic ointment (Taro Pharmaceuticals, Hawthorne, NY) was applied to the incision site. Rats were placed in a heated recovery cage and monitored continuously before being returned to their home cage.
Behavioral testing.
Rats were acclimated to the behavioral testing environment daily for 3 d prior to surgery, from 0900 to 1200 each day. Rats underwent behavioral testing of mechanical and thermal hypersensitivity 1 d prior to surgery to acquire baseline data and then daily beginning 1 d after surgery for 4 consecutive d, between 0900 and 1200 each day. Prior to each behavioral testing session, rats were given 15 min to acclimate in their home cage after being transported to the behavioral testing laboratory.
Response to mechanical stimuli.
Rats were placed individually in a plastic chamber (20 × 12 × 8 cm) on an elevated wire-grid platform with 1 cm2 perforations and given 15 min to acclimate within the apparatus. Von Frey monofilaments with calibrated bending forces (10 g, Aesthesio, DanMic Global, San Jose, CA) were applied perpendicularly to the plantar surface of each hindpaw for 10 trials. Each stimulus was administered for 1 s on a different location on the plantar surface. The pads, toes, and heels were excluded from stimulation. Withdrawal responses were measured as the number of times a rat lifted the paw completely off the grid due to stimulation during the 10 trials. Mechanical hypersensitivity was defined as a significant increase in paw withdrawal frequency caused by application of focal mechanical stimuli. Each rat's right (contralateral) hindpaw served as its control.
Response to thermal stimuli.
Rats were placed individually in a plastic chamber (20 × 12 × 13 cm) on an elevated glass platform preheated to 28 °C and given 15 min to acclimate within the testing apparatus. Radiant heat from a 50-W light bulb (Plantar Analgesia Meter, IITC Life Science, Woodland Hills, CA) was focused on the middle of the plantar surface of each hindpaw. A 20-s cutoff was used to prevent tissue injury. Each hindpaw was tested 4 times, with at least 1 min between trials. Withdrawal latency was measured as the mean of the last 3 trials. Thermal hypersensitivity was defined as a significant decrease in paw withdrawal latency caused by application of focal thermal stimuli. Each rat's right (contralateral) hindpaw served as its control.
Plasma concentration of analgesics.
Blood samples were collected by terminal cardiocentesis into heparinized tubes at 1, 2, 3, and 4 d after the initial administration of each analgesic (at 0900). Three rats from each treatment group were assigned to each of the 4 time points. In addition, blood was collected from 3 naïve rats for negative control samples. Blood was collected at 1200. All samples were centrifuged, and plasma was collected and stored at –80 °C. Buprenorphine concentration was analyzed by UPLC with mass spectrometry, at a lower limit of detection of 0.1 ng/mL. Meloxicam and carprofen concentrations were analyzed by liquid chromatography with mass spectrometry detection, at a lower limit of detection of 1.0 µg/mL and 0.05 µg/mL, respectively (Pharmacology Analytical Laboratory, College of Veterinary Medicine, NC State University).
Statistical analyses.
Data were analyzed by using repeated-measures ANOVA with Bonferroni correction for multiple comparisons (R Development Core Team, 2015) to examine differences in withdrawal responses within groups over time. Data were expressed as mean ± SEM. A P value of less than 0.05 was considered significant.
Results
Body weight.
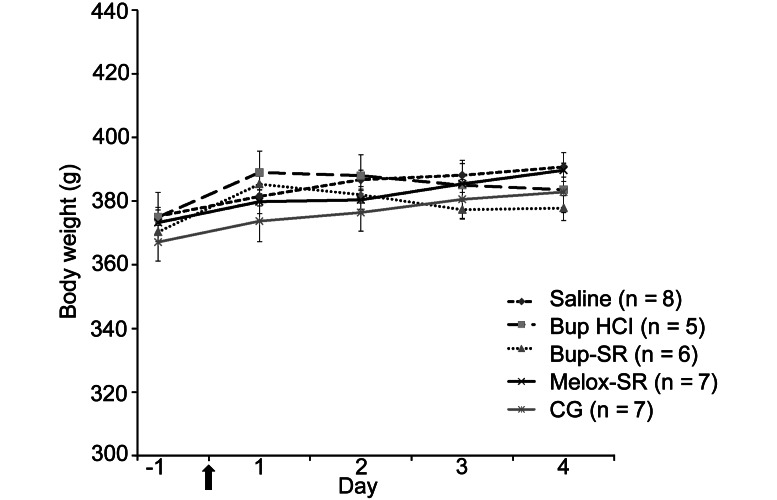
Body weight did not differ between treatment groups throughout the course of the study (Figure 1).
Figure 1.
Daily body weight (mean ± SEM) of rats in saline, Bup HCl, Bup-SR, Melox-SR, and CG treatment groups. Arrow indicates day of surgery.
Responses to mechanical hypersensitivity.
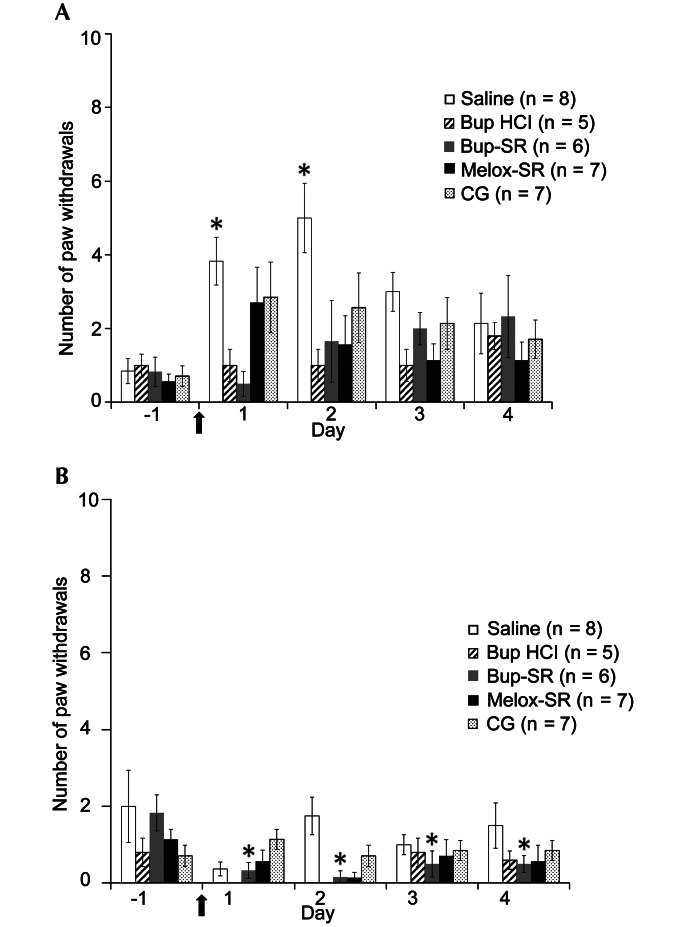
In the ipsilateral hindpaws of the saline group, mechanical hypersensitivity increased significantly (P < 0.05) from day –1 (0.9 ± 0.3 paw withdrawals) to days 1 (3.8 ± 0.7) and 2 (5.0 ± 0.9). Mechanical hypersensitivity in the ipsilateral hindpaws of the Bup HCl and Bup-SR groups did not differ between days –1 (1.0 ± 0.3 and 0.8 ± 0.4, respectively), 1 (1.0 ± 0.4 and 0.5 ± 0.3, respectively), and 2 (1.0 ± 0.4 and 1.7 ± 1.1, respectively). Mechanical hypersensitivity in the contralateral hindpaw of the Bup-SR group but not the Bup HCl rats decreased significantly (P < 0.05) between day –1 (1.8 ± 0.5 and 0.8 ± 0.4, respectively) and days 1 (0.3 ± 0.2 and 0.0 ± 0.0, respectively), 2 (0.2 ± 0.2 and 0.0 ± 0.0, respectively), 3 (0.5 ± 0.3 and 0.8 ± 0.4, respectively), and 4 (0.5 ± 0.2 and 0.6 ± 0.2, respectively). The Bup HCl group showed no withdrawal of the contralateral hindpaw on days 1 and 2. In the Melox-SR and CG groups, mechanical hypersensitivity in the ipsilateral hindpaw did not differ between days –1 (0.6 ± 0.2 and 0.7 ± 0.3, respectively), 1 (2.7 ± 1.0 and 2.9 ± 1.0, respectively), and 2 (1.6 ± 0.8 and 2.6 ± 0.9, respectively). Measurements from the contralateral hindpaw in the Melox-SR and CG groups did not differ at any time point (Figure 2).
Figure 2.
Effects of saline, Bup HCl, Bup-SR, Melox-SR, and CG on mechanical hypersensitivity (mean ± SEM) of the (A) ipsilateral and (B) contralateral hindpaws. In the Bup HCl group, 0 withdrawals of the contralateral hindpaw were recorded on days 1 and 2. Arrow indicates day of surgery. *, Value significantly (P < 0.05) different from that on day –1 in the same treatment group.
Responses to thermal hypersensitivity.
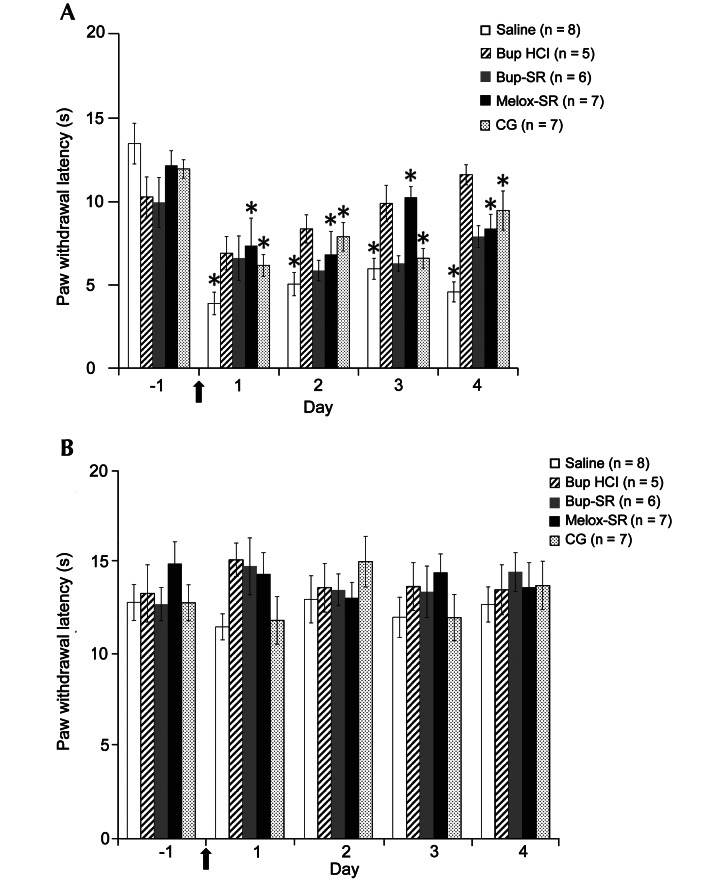
Thermal hypersensitivity in the ipsilateral hindpaw of the saline group increased significantly (P < 0.05) from day –1 (13.5 ± 1.2 s) to days 1 (3.9 ± 0.7 s), 2 (5.0 ± 0.7 s), 3 (6.0 ± 0.6 s), and 4 (4.6 ± 0.6 s). There were no differences in thermal hypersensitivity in the Bup HCl and Bup-SR groups’ ipsilateral hindpaw measurements. Thermal hypersensitivity in the ipsilateral hindpaw of rats that received Melox-SR increased significantly (P < 0.05) from day –1 (12.2 ± 0.9 s) to days 1 (7.3 ± 1.7 s), 2 (6.8 ± 1.4 s), 3 (10.2 ± 0.6 s), and 4 (8.4 ± 0.9 s). Similarly, there were significant increases in the thermal hypersensitivity in the ipsilateral hindpaw of the CG rats from day –1 (11.9 ± 0.6 s) to days 1 (6.2 ± 0.7 s), 2 (7.9 ± 0.9 s), 3 (6.6 ± 0.6 s), and 4 (9.5 ± 1.2 s). Contralateral hindpaw measurements did not differ at any time point in any group (Figure 3).
Figure 3.
Effects of saline, Bup HCl, Bup-SR, Melox-SR, and CG on paw withdrawal latency (s, mean ± SEM) of (A) ipsilateral and (B) contralateral hindpaws. Arrow indicates day of surgery. *, Value significantly (P < 0.05) different from that on day –1 in the same treatment group.
Plasma concentrations of analgesics.
The plasma concentration of buprenorphine was highest at the day-2 time point for both Bup HCl (1.0 ± 0.1 ng/mL) and Bup-SR (1.2 ± 0.3 ng/mL), although these values were not significantly different from those on days 1, 3, or 4. Plasma concentrations were consistent across time points, and there was no statistical difference between treatment groups at any time point (Figure 4). The plasma concentration of meloxicam in the Melox-SR group peaked at day 1 (18.5 ± 2.5 µg/mL); this value differed significantly from the concentrations on days 2 (5.9 ± 1.0 µg/mL), 3 (2.9 ± 1.0 µg/mL), and 4 (2.4 ± 1.9 µg/mL). The plasma concentration of carprofen in the CG group was similar on days 1 (16.5 ± 1.9 µg/mL), 2 (21.4 ± 1.2 µg/mL), and 3 (22.4 ± 3.5 µg/mL) but decreased significantly (P < 0.05) on day 4 (1.3 ± 0.1 µg/mL; Figure 5).
Figure 4.
Plasma concentration of buprenorphine (ng/mL, mean ± SEM) in rats treated with either Bup HCl or Bup-SR (n = 3 at each time point). Plasma samples from naïve rats (n = 3) were used as negative controls for the assay.
Figure 5.
Plasma concentrations (μg/mL, mean ± SEM) of meloxicam and carprofen in rats treated with either Melox-SR or CG (n = 3 at each time point). Plasma samples from naïve rats (n = 3) were used as negative controls for the assays. *, Value significantly (P < 0.05) different from that on day –1 in the same treatment group.
CG consumption.
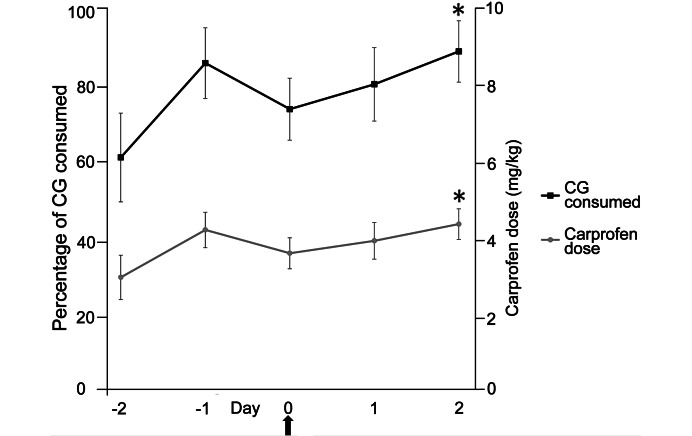
The percentage of CG consumed was lowest (but not statistically significantly) on the first day it was offered (day –2; 62% ± 11%). The percentage consumed on day 2 (89% ± 8%) was significantly different from that on day –2. Consumption tended to decrease on day 0, the day of surgery. According to the manufacturer's estimated carprofen dose of 5 mg/kg in 2 oz of product, rats received 3.1 ± 0.6 mg/kg carprofen on day –2, with a significant increase in dose by day 2 (4.5 ± 0.4 mg/kg; Figure 6).
Figure 6.
CG consumption (%, left) and estimated carprofen dose (mg/kg, right). Arrow indicates day of surgery. Data are presented as mean ± SEM. *, Value significantly (P < 0.05) different from that on day –2.
Pathology.
Gross pathologic examination revealed no abnormalities in any of the rats in this study. All anatomic characteristics were within normal limits.
Discussion
The current study shows that, in a rat incisional pain model, Bup HCl (0.05 mg/kg SC BID for 2 d) or Bup-SR (1.2 mg/kg SC once) attenuated both mechanical and thermal hypersensitivity and maintained a consistent plasma concentration. Melox-SR (4.0 mg/kg SC once) or CG (2 oz QD) attenuated mechanical—but not thermal—hypersensitivity. In all treatment groups, rats maintained their body weight throughout the study period and no gross pathology was seen at necropsy. These data do not support our hypothesis that all drugs evaluated would attenuate mechanical and thermal hypersensitivity in this pain model.
The main goal of this study was to refine postoperative analgesia in rats by using an incisional model of acute minor pain. Our group has used this model extensively and found it to be a reliable procedure to replicate and assess pain behavior due to minor incisional pain. Currently, Bup HCl is the standard of care for postoperative analgesia in laboratory rodents, but it requires administration multiple times daily, resulting in frequent handling and increased stress of the animals. Because of the longer duration of action of the sustained-release formulation (Bup-SR), animal handling and consequently stress were minimized in the current study; therefore we consider that the use of Bup-SR is a refinement of postoperative analgesia. Furthermore, the results of the present study were consistent with those in another study, in which we used the rat incisional pain model and showed that mechanical hypersensitivity lasted 1 to 2 d whereas thermal hypersensitivity persisted as long as 4 d.23 In addition, 0.3 or 1.2 mg/kg Bup-SR attenuated postoperative mechanical and thermal hypersensitivity for at least 48 h in this model.7 The present study, through the use of 1.2 mg/kg Bup-SR, confirms previous findings and further demonstrates the consistent plasma concentrations of buprenorphine. Only the plasma concentrations of Bup HCl and Bup-SR on days 1 and 2 were maintained close to the reported effective plasma concentration threshold (1 ng/mL) of buprenorphine in mice37 and rats.14 Although the plasma concentration of Bup HCl and Bup-SR seemed to be increased on day 4, these concentrations were not statistically higher than those of days 1, 2, and 3; these results may reflect variability due to mass spectrometry as well as the small sample size (n = 3/group/day). Given that 1.2 mg/kg Bup-SR has been reported to cause mild sedation,7 this effect cannot be ruled out as a cause of the changes in mechanical hypersensitivity that we noted in the contralateral hindpaws of the Bup-SR group. We consider that sedation was mild at most, because it did not affect the animals’ body weights throughout the study; in fact, the body weights of animals in every treatment group were consistent over the 4-d postoperative period. This observation supports the conclusion that the rat incisional pain model causes acute minor pain compared with a more severely painful procedure, such as laparotomy, after which the weights of untreated rats decreased.12 In the current study, we also examined all rats grossly at necropsy and found no pathologic changes in any rat in the opioid treatment groups.
Meloxicam and carprofen are NSAID that preferentially inhibit cyclooxygenase 2.10,26 Both drugs are commonly used in the research environment due to their long durations of action and multiple routes of administration. In addition to evaluating Bup-SR, we sought to further refine postoperative analgesia by evaluating the use of Melox-SR and CG. We found that Melox-SR and CG attenuated mechanical hypersensitivity for at least 48 h in this model. These findings are similar to previous studies in which several NSAID (for example, indomethacin,35,36 flunixin,32 celecoxib,35 etoricoxib,35 naproxen35) reduced mechanical hyperalgesia and tactile allodynia in the rat incisional pain model. In addition, carprofen at 5 mg/kg SC reportedly achieved effective postoperative analgesia in a laparotomy model in rats.29,38 In the current study, neither Melox-SR nor CG was able to attenuate thermal hypersensitivity during the postoperative period. Perhaps the complex mechanisms of thermal nociception differ from those of mechanical nociception and that the doses required to attenuate thermal hypersensitivity are higher than those required to attenuate mechanical hypersensitivity. Due to the wide range of analgesic and antiinflammatory effects of NSAID, there is no definitive plasma concentration level that correlates with analgesia across species.2,22 Therefore, our group was interested in evaluating plasma concentrations of NSAID that provide effective postoperative analgesia in this model.
In the present study, the plasma concentration of Melox-SR was highest on day 1 (18 µg/mL) and significantly lower on day 2 (5 µg/mL) and thereafter; in comparison, the plasma concentration of CG remained higher than 16 µg/mL as long as the product was provided. After CG was discontinued on d 2, the plasma concentration was maintained for an additional 24 h (day 3) and then dropped at 48 h after CG removal (that is, day 4). Plasma concentrations of meloxicam (20 mg/kg PO) and carprofen (10 mg/kg PO) in C57BL/6 mice were reported to be 16.7 and 20.3 µg/mL, respectively.20 In humans, the therapeutic plasma concentration of carprofen is 10 µg/mL in patients with rheumatoid arthritis.9 Because of reported taste neophobia in rats,3,24,31 we decided to introduce CG (2 oz QD) 2 d prior to the surgical procedure. We found that rats consumed less CG on the first day of exposure and on the day of the surgical procedure and anesthesia recovery; however CG consumption steadily increased on subsequent days. According to the amount of CG consumed, our rats received carprofen in the recommended dose range (1 to 5 mg/kg/d)34 throughout the study. Because oral carprofen (5 mg/kg) in flavored gelatin has been reported to reduce food (17%) and water consumption (13%) in rats,12 it is important to monitor body weights and hydration status. We found that all rats receiving Melox-SR or CG maintained their body weights, with a positive trend, throughout the study period. A similar increase in body weight occurred in a study in which rats received injectable carprofen.12 The analgesic effect provided by 5 mg/kg carprofen may last 5 h after a single injection.27 Given these encouraging findings, CG is a promising analgesic formulation that appears to maintain a sufficient plasma concentration to achieve extended or more consistent analgesia because of the ability to self-administrate as needed; however, further studies are required. In light of the decreased consumption on the first day of exposure and on the day of surgery, other supportive measures (for example, subcutaneous fluid administration, close monitoring after surgery), should be implemented if needed. Going by our experience, rats found CG palatable, and they readily consumed it once they became acclimated to its taste and presence in the cage. Similar to our results from the rats that received the opioid formulations, there was no gross pathologic evidence of toxicity in any of the animals in the NSAID groups. These formulations of meloxicam and carprofen provide alternatives to opioids for postoperative analgesia that minimizes animal handling and supports a refined research technique. Although we did not investigate multimodal analgesia in the present study, because it would confound the analgesic effect of each individual drug, we strongly believe that a multimodal analgesic approach should be adopted when considering the use of NSAID (that is, the combination of NSAID with local anesthetics or other adjunctive drugs) to provide effective postoperative analgesia.
Several considerations warrant attention regarding the use of these analgesic options. Of note, the formulation of Bup-SR that we used does not need to be refrigerated but Melox-SR does. Therefore, we allowed Melox-SR to warm to room temperature for at least 30 min before use. One disadvantage we encountered was that both Bup-SR and Melox-SR were highly viscous, making drug preparation and injection somewhat difficult, but neither group of rats demonstrated any skin irritation at the injection site, contrary to previous reports.2,5,14 Pica behavior, which occurred in rats treated with buprenorphine,8 was not observed in the current study. A financial analysis of the drugs (assuming a 350-g rat) revealed minor differences in cost. The standard-of-care cost of Bup HCl (0.05 mg/kg BID for 6 doses) costs approximately $5.10; Bup-SR (1.2 mg/kg, 1 dose) is $7.14, Melox-SR (4.0 mg/kg, 1 dose) is $10.50; and CG (2 oz, SID, 4 doses) costs $7.00. These differences in costs do not account for labor charges, which increase the cost of daily or twice-daily drugs (Bup HCl and CG). We only investigated sustained-release and gel formulations in the current study, but other types of long-lasting analgesics in the veterinary market should be further investigated by using the same model or other pain models (for example, laparotomy, thoracotomy, neuropathic pain, cancer pain, chemotherapeutic-induced peripheral neuropathy). Different classes of analgesics (for example, opioids, NSAID, local anesthetics, α2 agonists, N-methyl-D-aspartate receptor antagonists, adjunctive drugs, other novel analgesics) should be considered depending on the type of research performed. Further research on the various analgesics available is critical to better inform investigators, IACUC members, and animal care programs regarding the best options for postoperative pain management that have the least adverse effect on research objectives. According to the findings from the current study, we recommend the use of either Bup HCl (0.05 mg/kg SC BID) or Bup-SR (1.2 mg/kg SC, 1 dose) as single-agent options for postoperative analgesia in an incisional pain model in rats. We suggest that further investigation be performed on the use of Melox-SR and CG in a multimodal analgesic plan, given that our results do not support their use as single agents in the control of incisional pain in this model.
Acknowledgments
We thank Elias Godoy and Sandy Tainter for their technical assistance and Drs Mark Papich and Kristen Messenger for drug plasma concentration analyses. In addition, we thank Janis Atuk-Jones for her assistance in formatting and editing the manuscript. This work was supported in part by the NIH Research Education Program for Laboratory Animal Veterinarians Training Grant (R25OD010452).
References
- 1.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 2.Bauer C, Frost P, Kirschner S. 2014. Pharmacokinetics of 3 formulations of meloxicam in cynomolgus macaques (macaca fascicularis). J Am Assoc Lab Anim Sci 53:502–511. [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer DJ, Christenson TJ, Clark KR, Powell SK, Swain RA. 2003. Acetaminophen as a postsurgical analgesic in rats: a practical solution to neophobia. Contemp Top Lab Anim Sci 42:20–25. [PubMed] [Google Scholar]
- 4.Brennan TJ, Vandermeulen EP, Gebhart GF. 1996. Characterization of a rat model of incisional pain. Pain 64:493–501. [DOI] [PubMed] [Google Scholar]
- 5.Carbone ET, Lindstrom KE, Diep S, Carbone L. 2012. Duration of action of sustained-release buprenorphine in 2 strains of mice. J Am Assoc Lab Anim Sci 51:815–819. [PMC free article] [PubMed] [Google Scholar]
- 6.Christy AC, Byrnes KR, Settle TL. 2014. Evaluation of medicated gel as a supplement to providing acetaminophen in the drinking water of C57BL/6 mice after surgery. J Am Assoc Lab Anim Sci 53:180–184. [PMC free article] [PubMed] [Google Scholar]
- 7.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (rattus norvegicus). J Am Assoc Lab Anim Sci 53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 8.Clark JA, Jr, Myers PH, Goelz MF, Thigpen JE, Forsythe DB. 1997. Pica behavior associated with buprenorphine administration in the rat. Lab Anim Sci 47:300–303. [PubMed] [Google Scholar]
- 9.Derendorf H, Hochhaus G, 1995. Handbook of pharmacokinetic/pharmacodynamic correlation. Boca Raton (FL): CRC Press. [Google Scholar]
- 10.Engelhardt G. 1996. Pharmacology of meloxicam, a new non-steroidal anti-inflammatory drug with an improved safety profile through preferential inhibition of COX-2. Br J Rheumatol 35 Suppl 1:4–12. [DOI] [PubMed] [Google Scholar]
- 11.Fish RE, Brown MJ, Danneman PJ, Karas AZ, 2008. Anesthesia and analgesia in laboratory animals. 2nd ed. London: Academic Press. [Google Scholar]
- 12.Flecknell PA, Orr HE, Roughan JV, Stewart R. 1999. Comparison of the effects of oral or subcutaneous carprofen or ketoprofen in rats undergoing laparotomy. Vet Rec 144:65–67. [DOI] [PubMed] [Google Scholar]
- 13.Foley PL, Henderson AL, Bissonette EA, Wimer GR, Feldman SH. 2001. Evaluation of fentanyl transdermal patches in rabbits: blood concentrations and physiologic response. Comp Med 51:239–244. [PubMed] [Google Scholar]
- 14.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 15.Fox JG, Anderson LC, Loew FM, Quimby FW. 2002. Laboratory animal medicine. 2nd ed. New York (NY): Academic Press. [Google Scholar]
- 16.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13. [PubMed] [Google Scholar]
- 17.Goldkuhl R, Klockars A, Carlsson HE, Hau J, Abelson KS. 2010. Impact of surgical severity and analgesic treatment on plasma corticosterone in rats during surgery. Eur Surg Res 44:117–123. [DOI] [PubMed] [Google Scholar]
- 18.Hofmeister EH, Egger CM. 2004. Transdermal fentanyl patches in small animals. J Am Anim Hosp Assoc 40:468–478. [DOI] [PubMed] [Google Scholar]
- 19.Hovard A, Teilmann A, Hau J, Abelson K. 2014. The applicability of a gel delivery system for self-administration of buprenorphine to laboratory mice. Lab Anim 49:40–45. [DOI] [PubMed] [Google Scholar]
- 20.Ingrao JC, Johnson R, Tor E, Gu Y, Litman M, Turner PV. 2013. Aqueous stability and oral pharmacokinetics of meloxicam and carprofen in male C57BL/6 mice. J Am Assoc Lab Anim Sci 52:553–559. [PMC free article] [PubMed] [Google Scholar]
- 21.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): The National Academies Press. [Google Scholar]
- 22.Kendall LV, Hansen RJ, Dorsey K, Kang S, Lunghofer PJ, Gustafson DL. 2014. Pharmacokinetics of sustained-release analgesics in mice. J Am Assoc Lab Anim Sci 53:478–484. [PMC free article] [PubMed] [Google Scholar]
- 23.McKeon GP, Pacharinsak C, Long CT, Howard AM, Jampachaisri K, Yeomans DC, Felt SA. 2011. Analgesic effects of tramadol, tramadol-gabapentin, and buprenorphine in an incisional model of pain in rats (rattus norvegicus). J Am Assoc Lab Anim Sci 50:192–197. [PMC free article] [PubMed] [Google Scholar]
- 24.Mickley AG, Hoxha Z, Biada JM, Kenmuir CL, Bacik SE. 2006. Acetaminophen self-administered in the drinking water increases the pain threshold of rats (rattus norvegicus). J Am Assoc Lab Anim Sci 45:48–54. [PubMed] [Google Scholar]
- 25.Park I, Kim D, Song J, In CH, Jeong SW, Lee SH, Min B, Lee D, Kim SO. 2008. Buprederm, a new transdermal delivery system of buprenorphine: pharmacokinetic, efficacy and skin irritancy studies. Pharm Res 25:1052–1062. [DOI] [PubMed] [Google Scholar]
- 26.Ricketts AP, Lundy KM, Seibel SB. 1998. Evaluation of selective inhibition of canine cyclooxygenase 1 and 2 by carprofen and other nonsteroidal anti-inflammatory drugs. Am J Vet Res 59:1441–1446. [PubMed] [Google Scholar]
- 27.Roughan JV, Flecknell PA. 2001. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 90:65–74. [DOI] [PubMed] [Google Scholar]
- 28.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals. Lab Anim 36:322–343. [DOI] [PubMed] [Google Scholar]
- 29.Roughan JV, Flecknell PA. 2004. Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav Pharmacol 15:461–472. [DOI] [PubMed] [Google Scholar]
- 30.Sneddon LU, Elwood RW, Adamo SA, Leach MC. 2014. Defining and assessing animal pain. Anim Behav 97:201–212. [Google Scholar]
- 31.Speth RC, Smith MS, Brogan RS. 2001. Regarding the inadvisability of administering postoperative analgesics in the drinking water of rats (rattus norvegicus). Contemp Top Lab Anim Sci 40:15–17. [PubMed] [Google Scholar]
- 32.St A Stewart L, Martin WJ. 2003. Evaluation of postoperative analgesia in a rat model of incisional pain. Contemp Top Lab Anim Sci 42:28–34. [PubMed] [Google Scholar]
- 33.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154. [DOI] [PubMed] [Google Scholar]
- 34.Wenger S. 2012. Anesthesia and analgesia in rabbits and rodents. J Exot Pet Med 21:7–16. [Google Scholar]
- 35.Whiteside GT, Harrison J, Boulet J, Mark L, Pearson M, Gottshall S, Walker K. 2004. Pharmacological characterisation of a rat model of incisional pain. Br J Pharmacol 141:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto T, Sakashita Y, Nozaki-Taguchi N. 2000. Anti-allodynic effects of oral COX-2 selective inhibitor on postoperative pain in the rat. Can J Anaesth 47:354–360. [DOI] [PubMed] [Google Scholar]
- 37.Yun M, Jeong S, Pai C, Kim S. 2010. Pharmacokinetic- pharmacodynamic modeling of the analgesic effect of bupredermTM, in mice. Health 2:824–831. [Google Scholar]
- 38.Zegre Cannon C, Kissling GE, Goulding DR, King-Herbert AP, Blankenship-Paris T. 2011. Analgesic effects of tramadol, carprofen or multimodal analgesia in rats undergoing ventral laparotomy. Lab Anim (NY) 40:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]