Abstract
Buprenorphine HCl (BUP) is a μ-opioid agonist used in laboratory rodents. New formulations of buprenorphine (for example, sustained-released buprenorphine [BUP SR], extended-release buprenorphine [BUP ER]) have been developed to extend the analgesic duration. In a crossover design, 8 adult rats were injected subcutaneously with either BUP, BUP SR, BUP ER, or saline, after which voluntary running-wheel activity, arterial blood gases, and thermal withdrawal latency were assessed. Wheel running was decreased at 24 h compared with baseline in all treatment groups but returned to baseline by 48 h. Arterial pH, HCO3–, and CO2 were not changed between groups or over time. However, arterial oxygen was lower than baseline in the BUP (–8 ± 2 mm Hg), BUP SR (–7 ± 1 mm Hg), and BUP ER (–17 ± 2 mm Hg) groups compared with saline controls (3 ± 2 mm Hg); the BUP ER group showed the greatest decrease when all time points were combined. BUP increased the withdrawal latency at 1 h (15% ± 3%), whereas BUP ER increased latencies at 4, 8, 12, and 48 h (35% ± 11%, 21% ± 7%, 26% ± 7%, and 22% ± 9%, respectively) and BUP SR prolonged latencies at 24, 48, and 72 h (15% ± 6%, 18% ± 5%, and 20% ± 8%, respectively). The duration of thermal analgesia varied between buprenorphine formulations, but all 3 formulations reduced voluntary-running activity at 24 h after injection and might cause hypoxemia in normal adult rats.
Abbreviations: BUP, buprenorphine hydrochloride; BUP SR, sustained-release buprenorphine; BUP ER, extended-release buprenorphine
Buprenorphine HCl (BUP), a synthetic opiate first synthesized in the late 1960s, has been used extensively in laboratory rodents for many years.8 The antinociceptive effects of BUP are mediated via actions at the μ-opioid receptor, although the drug has been classified as both a full- and a partial μ-opiate receptor agonist.26 BUP administration is associated with minimal toxicity in rodents because its therapeutic index is at least 3 times greater than that of morphine in animals.7 BUP produces minimal to modest respiratory depression, quantified by using arterial blood gas evaluation, even at excessive intravenous doses (3 to 90 mg/kg).9,16 The analgesic efficacy of BUP has been assessed in several models of acute and chronic pain in rodents.4,15 Although efficacious in some models, BUP's duration of action frequently requires repeated postoperative dosing,24 which can reduce body weight and food consumption and affect ambient locomotor activity, thus complicating the ability to use these parameters as signs of postoperative pain.2 In addition, the repeated postoperative handling of rats necessary to redose BUP itself may increase animal stress and further contribute to decreases in postoperative weight gain and food intake.6,14,28 In addition, repeated postoperative dosing of BUP is associated with hyperalgesia, which may limit its usefulness in chronically painful animals.6
To reduce the negative side effects associated with repeated dosing of BUP, new formulations of BUP (for example, sustained-released buprenorphine [BUP SR], extended-release buprenorphine [BUP ER]) have become available recently with the potential to produce long-lasting analgesia. For example, a single dose of BUP SR has shown analgesic efficacy for as long as 72 h in thermal, incisional, and orthopedic pain models in rats.5,11 In addition, BUP ER can achieve thermal analgesia in rats for 5 d; however, data are limited on this formulation due to its novelty (commercially released for rats in 2014).1 These long-lasting formulations may reduce the amount of postoperative animal handling required for multiple injections as well as the associated negative effects. However, no studies have directly evaluated the consequences of these new formulations on important postoperative factors, such as voluntary activity.2
Respiratory depression, manifest as arterial hypercapnia, is commonly associated with pure mu-opioid agonists in rats.9 However, BUP administration appears to have a “ceiling” effect on ventilation, where a maximum effect is seen despite increases in dose.9 Although respiratory rate has been used to estimate opioid-induced respiratory impairment in some models,10 other studies confirm respiratory rate is not an accurate assessment of ventilation due to subsequent changes in tidal volume and dead space ventilation;29 arterial blood gas analysis is the gold standard to detect hypoventilation using arterial carbon dioxide levels.19 To the author's knowledge, no studies have investigated blood gases following either long-acting buprenorphine preparation.
The objective of the current study was to use a crossover design to evaluate the effects of a clinically applicable, single dose of subcutaneous BUP, BUP SR, or BUP ER on voluntary running-wheel activity, resting arterial blood gases, and antinociception according to a thermal withdrawal model in healthy adult rats. Administration of all formulations was hypothesized to produce quantifiable thermal analgesia, reduce voluntary running activity, and result in mild hypoventilation and arterial hypoxemia after injection in healthy adult rats. In addition, the effects were predicted to be of similar magnitude among BUP, BUP SR, and BUP ER but of shorter duration in the BUP group compared with the BUP SR and BUP ER groups.
Materials and Methods
Animals.
Adult male Sprague–Dawley rats (Rattus norvegicus; n = 8; weight 320 ± 13 g; Harlan Laboratories, Indianapolis, IN) were purchased with a surgically implanted femoral arterial catheter connected to an exteriorized port between the shoulder blades. Rats were single-housed in an AAALAC-accredited facility on a 12:12-h dark:light cycle with free access to voluntary running wheels for the entire study duration. They were bedded on cellulose bedding (7099 Tek-Fresh, Harlan Laboratories) which was changed daily to reduce coprophagy and issues with pica. The rats had free access to water and a commercial diet (7002 Teklad 6% Fat Rat Diet, Harlan Laboratories). All experiments were approved by the University of Wisconsin Animal Care and Use Committee and all rats were treated in compliance with the Guide for the Care and Use of Laboratory Animals.18 The rats were free from ectromelia virus, Hantaan virus, K virus, Kilham rat virus, lactic dehydrogenase elevating virus, lymphocytic virus, minute virus of mice, mouse adenovirus type 1 and 2, mouse cytomegalovirus, mouse hepatitis virus, mouse parvovirus, mouse polyoma virus, mouse rotavirus, mouse thymic virus, murine norovirus, pneumonia virus of mice, rat minute virus, rat parvovirus, rat Theiler virus, respiratory enteric virus III, Sendai virus, sialodacryoadenitis virus, Theiler murine encephalomyelitis, Toolan H1 parvovirus, Bordetella bronchoseptica, Helicobacter spp., Mycoplasma pulmonis, Pasteurella multocida, dermatophytes, ectoparasites, and endoparasites. Rats were weighed prior to each treatment and were euthanized by anesthetic overdose at the end of the study.
Study design.
Rats were randomly assigned (www.randomizer.org) to receive either: 1) BUP 0.05 mg/kg SC (0.3 mg/mL; Hospira, Lake Forest, IL); 2) BUP SR 1.2 mg/kg SC (1.0 mg/mL; Zoopharm, Fort Collins, CO); 3) BUP ER 0.65 mg/kg SC (1.3 mg/mL; Animalgesic Labs, Millersville, MD); or 4) 0.9% NaCl as a negative control (0.17 mL/kg SC; Baxter Healthcare, Deerfield, IL) in a nonblinded, crossover design. Injections were made in the morning (between 0600 and 0700), immediately following the rats’ dark cycle. Doses were chosen based on clinically used, analgesic doses of BUP (0.01 to 0.05 mg/kg)24 and BUP SR5,11 or the manufacturer's recommended dose for BUP ER.1 A washout period of at least 7 d was allowed between treatments.
Voluntary running-wheel activity.
To determine the effects of different buprenorphine preparations on voluntary running-wheel activity, rats were single-housed with continual access to voluntary running wheels with magnetic revolution counters (Rodent Activity Wheel, Harvard Apparatus, Holliston, MA). Rats were allowed to acclimate to their cages for at least 1 wk prior to any experimentation. Wheel revolutions were recorded every 24 h between 0700 and 0900 throughout the entire experiment, before baseline arterial blood gases were taken, and prior to any treatments. Baseline revolutions were averaged over the 2 d immediately prior to injection, and revolutions were analyzed daily for 3 d (72 h) after injection.
Arterial blood gas analyses.
To determine the effects of different buprenorphine formulations on ventilation, oxygenation, and arterial pH and bicarbonate concentration, arterial catheters were surgically placed in each rat's left femoral artery prior to its arrival at our facility, exteriorized, secured behind the shoulders, and capped with a removable stainless steel plug. Anesthetic and analgesic agents used during those surgical procedures included ketamine–xylazine (intramuscular or intraperitoneal), isoflurane maintenance, and ketoprofen (subcutaneous). Catheters were made of polyurethane tubing with a dead-space volume of 0.03 to 0.06 mL. Rats were acclimated for at least 1 wk prior to any blood gas sampling, and the catheters were maintained and flushed every 3 to 5 d as recommended by the manufacturer (Harlan Laboratories). During experimentation, the plug was removed and a sterile extension set (B Braun Medical, Bethlehem, PA) shortened to 20 cm and containing approximately 0.2 mL 0.9% NaCl was connected to the exteriorized tubing by using a blunt 22-gauge needle. After the extension set was attached, the rat was placed into the thermal latency testing chamber with the distal port outside of the chamber. The rats were allowed to relax, and once they became quiet, yet awake, the distal, freely accessible port of the tubing was removed without disturbing the animal, and blood was allowed to freely drip until no evidence of flush solution remained in the sample (approximately 4 or 5 drops of whole blood removed). Blood was not withdrawn actively to prevent disturbing the rats and to avoid changes in ventilation associated with their sensing of the blood withdrawal. Arterial blood was then collected passively into a hematocrit tube specifically designed for this purpose (125 µL; safeCliniTubes, Radiometer, Westlake, OH). The ends were sealed, and the sample immediately analyzed for pH, HCO3–, PaO2, and PaCO2 (ABL 800, Radiometer). All results were corrected to the rat's rectal body temperature, which was measured (model 600-1020, Type T Thermocouple Thermometer, Barnant, Barrington, IL) just prior to its placement in the chamber. Samples were obtained prior to treatment (baseline) and at 1, 4, 8, 24, and 48 h after injection. Due to loss of catheter patency during the weeks of crossover experimentation, sample sizes were as follows: BUP, n = 4 at baseline and 1 h; n = 3 at 4 to 48 h; BUP SR, n = 2 at all time points; BUP ER, n = 3 at all time points; saline, n = 3 at all time points.
Withdrawal responses to thermal stimuli.
Thermal sensitivity as an indicator of pain threshold was measured by using the latency of hindlimb withdrawal from a radiant heat stimulus.17 After at least 1 wk of animal handling and catheter care, thermal withdrawal latencies were evaluated in all rats by using a commercial thermal latency device (Ugo Basile, Varese, Italy). Stimulus intensity and the rate of thermistor heating were kept constant throughout the study to establish an average withdrawal latency of 9 to 10 s in a normal rat during baseline readings. The maximal time of heat exposure for all measurements was limited to 20 s to prevent thermal burns. A focused thermal heat stimulus was applied to the plantar surface of each hindpaw, and the time until the paw was lifted in response to the stimulus was defined as the latency interval. Rats were habituated to the device for at least 10 min prior to experimentation. Each rat was tested 3 times with at least 5 min between trials, and the mean latency response was calculated. This pattern allowed sufficient time between latency measurements to prevent learned responses or the development of hyperalgesia secondary to repeated noxious stimuli in quick succession.22 Only the right hindpaw was ultimately used for analyses, to avoid any variability introduced by the femoral artery catheters present in the left hindlimb. Withdrawal latency was measured just prior to injection (baseline) and at 1, 4, 8, 12, 24, 48, and 72 h after treatment.
Statistical analyses.
Thermal withdrawal latency time and the number of wheel revolutions during 24 h were calculated as percentage change from baseline. Blood gas data are reported as the absolute change from baseline values. All are expressed as mean ± SEM and were analyzed by using 2-way repeated-measures ANOVA (time and treatment as factors) to detect differences between groups (SigmaPlot 12.0, Systat Software, San Jose, CA). When a significant time, treatment, or time×treatment interaction effect was seen, the Fisher Least Significant Difference posthoc test was used. A P value less than 0.050 was considered significant.
Results
Voluntary running-wheel activity.
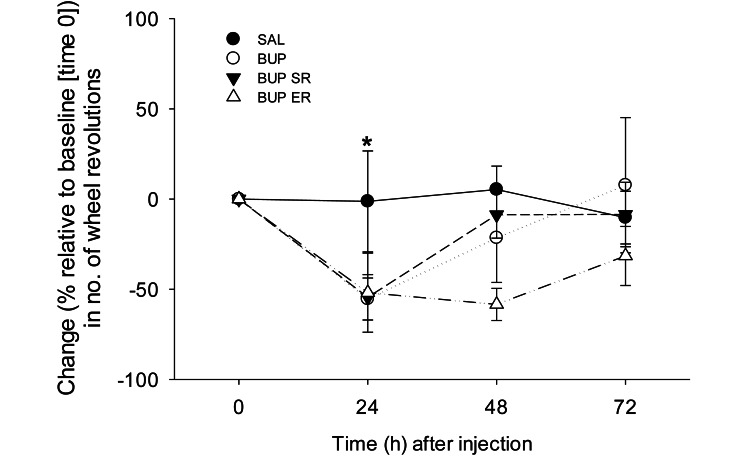
As compared with baseline, the percentage change in the number of running wheel revolutions recorded every 24 h for 72 h after injection showed a significant effect of time (P = 0.011), with no differences between treatments (P = 0.155) or time–treatment interactions (P = 0.271). At 24 h after treatment, the number of wheel revolutions was 41% ± 13% of baseline (time 0) values (P = 0.002), mainly due to the decreases in the BUP (55% ± 11%), BUP SR (54% ± 13%) and BUP ER (52% ± 22%) groups. Wheel revolutions were only minimally changed after injection in the saline group (1% ± 28%, Figure 1). Although the change in revolutions did not differ between the 24- and 48-h time points (P = 0.092), voluntary wheel activity at 24 h was lower than that at 72 h (P = 0.014, Figure 1). The numbers of revolutions at 48 and 72 h after injection did not differ from baseline values (P = 0.079 and 0.354, respectively). Therefore, rats voluntarily ran significantly less at 24 h after injection than at baseline but returned to preinjection activity by 48 h after treatment.
Figure 1.
Effects of saline (control), BUP, BUP SR, and BUP ER on the percentage change from baseline (time 0) in the number of voluntarily run wheel revolutions. There were no significant differences between treatments. However, at 1 d after injection, rats ran significantly less than at baseline; running distance returned to preinjection levels by 48 h after treatment. *, P < 0.05 compared with the values at baseline and 72 h.
Arterial blood gas analyses.
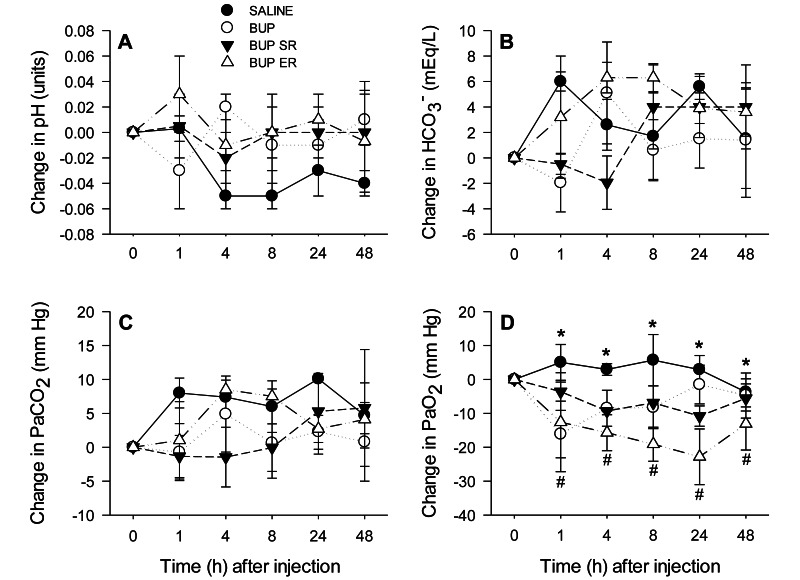
There were no significant time or treatment effects on the change from baseline values of pH, HCO3–, or PaCO2 (all P > 0.050; Figure 2 A through C). In addition, no significant time or interaction effect was found in the change in PaO2 (P = 0.432 and 0.596, respectively). However, treatment had a significant effect on the change in PaO2 from baseline (P < 0.001) between the rats that received BUP (–7.7 ± 2.4 mm Hg), BUP SR (–7.2 ± 1.3 mm Hg) and BUP ER (–16.7 ± 1.9 mm Hg) compared with saline (2.6 ± 1.7 mm Hg; P = 0.012, 0.035, and 0.001, respectively; Figure 2 D). Furthermore, the change in PaO2 in the BUP ER group was significantly different from that in the BUP group (P = 0.030) and BUP SR group (P = 0.042; Figure 2 D).
Figure 2.
Effects of saline (control), BUP, BUP SR, and BUP ER on the absolute change from baseline (time 0) in arterial pH, HCO3–, PaCO2, and PaO2. Neither arterial pH, HCO3–, nor PaCO2 differed over time or between groups. PaO2 values were higher in rats that received saline but lower in those that received BUP ER when compared with all other groups; however, there were no time effects. *, P < 0.05 for saline group compared with BUP, BUP SR, and BUP ER groups; #, P < 0.05 for BUP ER group compared with saline, BUP, and BUP SR groups.
Thermal analgesia.
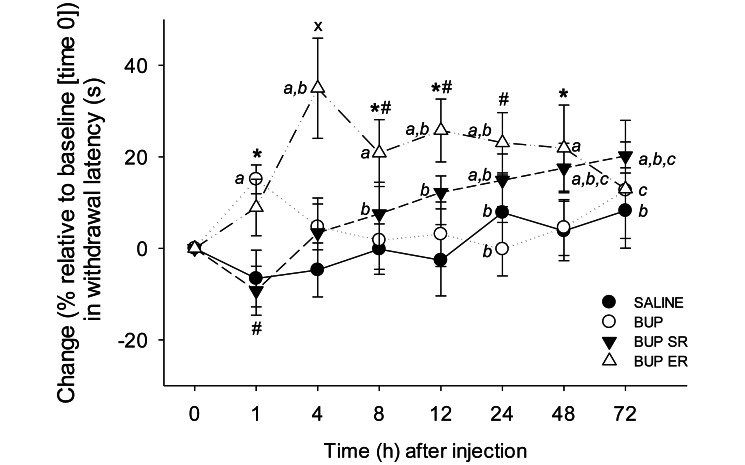
The percentage change in thermal withdrawal latency from baseline values showed significant interaction between time and treatment (P < 0.001, Figure 3). The saline group had no significant changes between baseline and 1-72 h after injection (all P > 0.050), although the values at the 24- and 72-h time points were higher than that at the 1-h time point (P = 0.040 and 0.044, respectively; Figure 3). In the BUP group, withdrawal latency at 1 h was significantly above baseline (15% ± 3%, P = 0.036) and was greater at 1 h in the BUP group compared with the SAL group (P = 0.036, Figure 3). In the BUP SR rats, latencies were significantly above baseline at 24 h (15% ± 6%; P = 0.039), 48 h (18% ± 5%, P = 0.015), and 72 h (20 ± 8%, P = 0.005), whereas in the BUP ER animals, latencies were significantly above baseline at 4 h (35% ± 11%, P < 0.001), 8 h (21% ± 7%, P = 0.004), 12 h (26% ± 7%, P < 0.001), 24 h (23% ± 7%, P = 0.001), and 48 h (22% ± 9%, P = 0.003). However, the withdrawal latencies from 8 to 72 h differed from that at 1 h in the BUP SR rats, and those at 4, 12, and 24 h differed from the 1-h value in the BUP ER group, representing additional analgesia at the later time points (all P < 0.050, Figure 3).
Figure 3.
Effects of saline (control), BUP, BUP SR, and BUP ER on the percentage change from baseline (time 0) in thermal withdrawal latency. In the BUP group, withdrawal latency at 1 h was increased compared with baseline and with that in saline controls at 1 h. Compared with baseline levels, BUP SR increased latencies at 24, 48, and 72 h, but the BUP SR and saline groups did not differ at these time points. Within the BUP SR group, withdrawal latency at 1 h after injection was significantly shorter than those at 8 to 72 h after injection. BUP ER increased withdrawal times from baseline and compared with those of the saline group at 4, 8, 12, 24, and 48 h after treatment. Latencies at 4 to 48 h significantly differed from that at the 1-h time point in the BUP ER group. a, P < 0.050 compared with baseline (time 0) within the same treatment group; b, P < 0.05 compared with 1 h after injection time point within the same treatment group; c, P < 0.05 compared with 4 h after injection within the same treatment group; *, P < 0.05 compared with saline group at the same time point; ×, P < 0.05 compared with all other treatment groups at the same time point; #, P < 0.05 compared with BUP group at the same time point.
Although the withdrawal latencies were prolonged as compared with baseline in the BUP SR group, no significant differences were found between the BUP SR and saline groups at any time point (all P > 0.050, Figure 3). In contrast, the rats given BUP ER had significantly longer latencies than did the saline controls at 4 h (P < 0.001), 8 h (P = 0.021), 12 h (P = 0.002), and 48 h (P = 0.046), but withdrawal latencies at 72 h did not differ between these groups (P = 0.072, Figure 3). These results suggest that BUP ER provides extended thermal analgesia in healthy rats whereas BUP is relatively short-acting.
Discussion
This study confirms the current hypothesis and the results of previous studies in that all buprenorphine formulations produced at least some thermal analgesia in rats. However, the duration of effect differed between compounds, with BUP having the shortest duration and BUP ER lasting at least 48 h. Although BUP SR increased thermal latencies for as long as 72 h, the latencies in this group did not differ from those of saline control rats, making it difficult to draw robust conclusions. In addition, voluntary running-wheel activity decreased for at least 24 h after injection of all 3 formulations but returned to normal levels by 48 h. Preliminary blood gas data suggested that arterial oxygenation was reduced after administration regardless of the formulation, with the greatest decreases in the BUP ER group but that ventilation, quantified by using arterial CO2 levels, was not affected significantly. Further conclusions regarding blood gas data cannot be made due to limited sample sizes. Together, these data suggest that, despite producing thermal antinociception, these formulations of buprenorphine may be associated with clinically important side effects, including reduced voluntary activity and hypoxemia.
Buprenorphine is commonly used as a postoperative analgesic in many laboratory species including rabbits,29,30 mice, and rats.4 Although many studies have verified the analgesic properties of buprenorphine (for review, see reference 3), some did not find a significant analgesic effect in rats12,13 and suggested that other μ-opioid agonists, such as oxymorphone, might be better postoperative choices.13 Discrepancies in results may be attributable to factors such as the route and frequency of administration, drug dose, and choice of analgesic model. For example, although the thermal nociception model tests limb withdrawal in response to a heated, painful stimulus, this assay may not be comparable to soft-tissue or orthopedic postoperative pain.17 In the current study, a single, subcutaneous dose of BUP increased thermal withdrawal latencies, but only briefly (1 h). In comparison, BUP SR achieved thermal analgesia for 24 to 72 h, consistent with previous reports.11 Although latencies did not differ from baseline until 24 h after BUP SR, latencies at 8 h after injection were longer than that at the 1-h time point, indicating at least a modest degree of analgesic associated with BUP SR by 8 h after injection. BUP ER produced thermal analgesia from 4 to 48 h in the current study, consistent with data from the manufacturer.1
Because BUP administration affects ambient locomotor activity in rats, especially during the night period,2,23,27 the effects of all 3 formulations were assessed by using a motor task that is completely voluntary yet requires some self-motivation—running-wheel activity. Voluntary running activity was significantly lower at 24 h after treatment but returned to baseline levels on the second day. However, there were no significant differences between the groups (including the saline-treated group) at either time point. Running-wheel activity is commonly used to measure voluntary rodent activity.20,21,25 Although wheel running and ambient locomotor cage activity frequently correlate, they do not assess the same parameters. For example, voluntary wheel activity amplifies normal cage activity and is a complex act, altering energy balance and neural systems involved in the stress response, mood and reward, rodent food intake, neurotransmitter systems involved in learning and memory, and behavioral processes involving depression and anxiety states.25 Wheel running is not simply a measure of general rat activity, although it is sometimes used as an indicator of general activity, especially when specific guidelines are followed.25 For example, as in the current study, rats should be acclimated to the wheel and 24-h measurements should be used.25 The precise physiologic system affected by the buprenorphine formulations is unclear; the only appropriate conclusion from the current findings is that running-wheel distance is reduced after the administration of BUP, BUP SR, or BUP ER.
Normal PaCO2 and PaO2 values in awake rats are reported as 34.5 ± 3.0 and 90.0 ± 5.5 Torr, respectively.3 Although the limited blood gas data from the current study should be interpreted cautiously, BUP administration in healthy rats breathing room air might not significantly affect PaCO2, as in previous studies of rats and rabbits that showed mild to moderate and ‘ceiling’ effects of BUP on ventilation.9,16,29 In addition, BUP SR and BUP ER administration appeared to have minimal effects on ventilation, arterial pH, and arterial HCO3– levels. However, despite the limitation of small sample sizes, all groups were hypoxemic over the 48 h after treatment in the current study, with decreases of 7 to 17 mm Hg; BUP ER resulted in the most severe hypoxemia, and PaO2 levels after BUP tended to return to baseline faster (by 24 h) than did those for the other groups, possibly due to BUP's shorter pharmacokinetic profile. Because PaCO2 did not change significantly, the contribution of alveolar hypoventilation to the hypoxemia is unclear. However, this pattern is consistent with a report in rabbits, in which BUP administration most likely resulted in ventilation–perfusion abnormalities and concurrent hypoxemia.31 The mechanisms underlying the ventilation–perfusion abnormalities in the current study are unknown; the rats did not exhibit any clinical signs of respiratory disease and were apparently healthy. Perhaps in the current study, the rats developed a degree of pulmonary atelectasis that resulted in hypoxemia, due to reduced running-wheel activity or sedation associated with buprenorphine administration (which was not quantified here). This possibility remains to be tested. In any case, it is unlikely that the hypoxemia was great enough to result in significant tissue hypoxia with subsequent production of lactic acid, given that arterial HCO3– and pH did not change over the course of the study. Furthermore, these data should be considered preliminary because of the small sample sizes, and strong conclusions should not be formulated.
The current study had several limitations in addition to those already discussed above. For example, the investigator performing the withdrawal latency measurements and blood gas analyses was not blinded to the treatments. However, these data are objective, and likely minimal bias was introduced during data acquisition. In addition, 0.9% physiologic saline was used as the negative control solution. More appropriately, control rats should have received the vehicles or carriers for BUP SR and BUP ER, to more accurately test the effects of the buprenorphine itself and not of the additional chemicals involved in the preparation. However, the addition of these control groups would have prolonged the study duration, and ensuring the patency of the arterial catheters would have been increasingly difficult. Skin irritation and inflammatory reactions have previously been associated with subcutaneous long-acting buprenorphine preparations.11 Although all rats appeared healthy throughout our studies and no dermal lesions were observed, inflammatory responses might have contributed to the loss of arterial catheter patency, which resulted in the small sample sizes for blood gas analysis. In addition, the effects of BUP, BUP SR, and BUP ER were investigated for only 48 to 72 h after injection. Given that positive effects (especially thermal analgesia) were still present at 72 h, extending the study duration likely would have provided additional information. A major difference between the current and previous studies is that the current study used only healthy, adult rats. The significant reduction in running-wheel activity and arterial hypoxemia seen in the current animals might be greater in rats that are compromised by sickness, surgery, or other stressors.
In summary, the current findings suggest that the thermal analgesia associated with BUP administration is extended when BUP SR (to 72 h) and BUP ER (to 48 h) are administered. All 3 formulations significantly reduced running-wheel activity for 24 h and may decrease PaO2 for 48 h after injection. Therefore, although these treatments provide analgesia, their administration can also lead to reduced running activity and possible hypoxemia. However, additional blood gas studies need to be performed, and these effects remain to be evaluated in rats with a compromised health status.
Acknowledgments
I thank Allison Clarke, Molly Kelley, and Matteo Nicastri for technical assistance. This study was supported by the Research Animal Wellbeing Fund of the University of Wisconsin Research Animal Resource Center.
References
- 1.Animalgesic Labs. [Internet]. 2015. Studies–Animalgesics. [Cited 06 August 2015] Available at: www.animalgesiclabs.com
- 2.Bourque SL, Adams MA, Nakatsu K, Winterborn A. 2010. Comparison of buprenorphine and meloxicam for postsurgical analgesia in rats: effects on body weight, locomotor activity, and hemodynamic parameters. J Am Assoc Lab Anim Sci 49:617–622. [PMC free article] [PubMed] [Google Scholar]
- 3.Brun-Pascaud M, Gaudebout C, Blayo MC, Pocidalo JJ. 1982. Arterial blood gases and acid-base status in awake rats. Respir Physiol 48:45–57. [DOI] [PubMed] [Google Scholar]
- 4.Christoph T, Kögel B, Schiene K, Méen M, De Vry J, Friderichs E. 2005. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 507:87–98. [DOI] [PubMed] [Google Scholar]
- 5.Chum HH, Jampachairsri K, McKeon GP, Yeomans DC, Pacharinsak C, Felt SA. 2014. Antinociceptive effects of sustained-release buprenorphine in a model of incisional pain in rats (Rattus norvegicus). J Am Assoc Lab Anim Sci 53:193–197. [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper DM, Hoffman W, Wheat N, Lee HY. 2005. Duration of effects on clinical parameters and referred hyperalgesia in rats after abdominal surgery and multiple doses of analgesia. Comp Med 55:344–353. [PubMed] [Google Scholar]
- 7.Cowan A, Doxey JC, Harry EJ. 1977. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol 60:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan A, Lewis JW, Macfarlane IR. 1977. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 60:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahan A, Yassen A, Bijl H, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. 2005. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 94:825–834. [DOI] [PubMed] [Google Scholar]
- 10.Flecknell PA, Liles JH, Wootton R. 1989. Reversal of fentanyl-fluanisone neuroleptanalgesia in the rabbit using mixed agonist–antagonist opioids. Lab Anim 23:147–155. [DOI] [PubMed] [Google Scholar]
- 11.Foley PL, Liang H, Crichlow AR. 2011. Evaluation of a sustained-release formulation of buprenorphine for analgesia in rats. J Am Assoc Lab Anim Sci 50:198–204. [PMC free article] [PubMed] [Google Scholar]
- 12.Gades NM, Wixson SK, Danneman PJ, Tolley EA. 2001. Inefficacy of buprenorphine in rats. Contemp Top Lab Anim Sci 40:7. [PubMed] [Google Scholar]
- 13.Gillingham MB, Clark MD, Dahly EM, Krugner-Higby LA, Ney DM. 2001. A comparison of 2 opioid analgesics for relief of visceral pain induced by intestinal resection in rats. Contemp Top Lab Anim Sci 40:21–26. [PubMed] [Google Scholar]
- 14.Gopal S, Tzeng TB, Cowan A. 2002. Characterization of the pharmacokinetics of buprenorphine and norbuprenorphine in rats after intravenous bolus administration of buprenorphine. Eur J Pharm Sci 15:287–293. [DOI] [PubMed] [Google Scholar]
- 15.Guarnieri M, Brayton C, DeTolla L, Forbes-McBean N, Sarabia-Estrada R, Zadnik P. 2012. Safety and efficacy of buprenorphine for analgesia in laboratory mice and rats. Lab Anim (NY) 41:337–343. [DOI] [PubMed] [Google Scholar]
- 16.Gueye PN, Borron SW, Risède P, Monier C, Buneaux F, Debray M, Baud FJ. 2001. Lack of effect of single high doses of buprenorphine on arterial blood gases in the rat. Toxicol Sci 62:148–154. [DOI] [PubMed] [Google Scholar]
- 17.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. 1988. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88. [DOI] [PubMed] [Google Scholar]
- 18.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academics Press. [Google Scholar]
- 19.Johnson RA, deMorais HA. 2012. Respiratory acid-base disorders, p 287–301. In: DiBartola SP, Fluid, electrolyte, and acid-base disorders in small animal practice, 4th ed, St. Louis (MO): Saunders. [Google Scholar]
- 20.Johnson RA, Mitchell GS. 2003. Exercise-induced changes in hippocampal brain-derived neurotrophic factor and neurotrophin-3: effects of rat strain. Brain Res 983:108–114. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Jr, Mitchell GS. 2003. Hippocampal brain-derived neurotrophic factor but not neurotrophin 3 increases more in mice selected for increased voluntary wheel running. Neuroscience 121:1–7. [DOI] [PubMed] [Google Scholar]
- 22.Le Bars D, Gozariu M, Cadden SW. 2001. Animal models of nociception. Pharmacol Rev 53:597–652. [PubMed] [Google Scholar]
- 23.Liles JH, Flecknell PA. 1992. The effects of buprenorphine, nalbuphine, and butorphanol alone or following halothane anaesthesia on food and water consumption and locomotor movement in rats. Lab Anim 26:180–189. [DOI] [PubMed] [Google Scholar]
- 24.Miller AL, Richardson CA. 2011. Rodent analgesia. Vet Clin North Am Exot Anim Pract 14:81–92. [DOI] [PubMed] [Google Scholar]
- 25.Novak CM, Burghardt PR, Levine JA. 2012. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev 36:1001–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raffa RB, Ding Z. 2007. Examination of the preclinical antinociceptive efficacy of buprenorphine and its designation as full or partial agonist. Acute pain: international journal of acute management 9:145–152. [Google Scholar]
- 27.Roughan JV, Flecknell PA. 2000. Effects of surgery and analgesic administration on spontaneous behaviour in singly housed rats. Res Vet Sci 69:283–288. [DOI] [PubMed] [Google Scholar]
- 28.Schaap MW, Uilenreef JJ, Mitsogiannis MD, van't Klooster JG, Arndt SS, Hellebrekers LJ. 2012. Optimizing the dosing interval of buprenorphine in a multimodal postoperative analgesic strategy in the rat: minimizing side-effects without affecting weight gain and food intake. Lab Anim 46:287–292. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder CA, Smith LJ. 2011. Respiratory rates and arterial blood-gas tensions in healthy rabbits given buprenorphine, butorphanol, midazolam, or their combinations. J Am Assoc Lab Anim Sci 50:205–211. [PMC free article] [PubMed] [Google Scholar]
- 30.Shafford HL, Schadt JC. 2008. Effect of buprenorphine on the cardiovascular and respiratory response to visceral pain in conscious rabbits. Vet Anaesth Analg 35:333–340. [DOI] [PubMed] [Google Scholar]
- 31.Shafford HL, Schadt JC. 2008. Respiratory and cardiovascular effects of buprenorphine in conscious rabbits. Vet Anaesth Analg 35:326–332. [DOI] [PubMed] [Google Scholar]