Abstract
Body temperature is a common physiologic parameter measured in both clinical and research settings, with rectal thermometry being implied as the ‘gold standard.’ However, rectal thermometry usually requires physical or chemical restraint, potentially causing falsely elevated readings due to animal stress. A less stressful method may eliminate this confounding variable. The current study compared 2 types of digital rectal thermometers—a calibrated digital thermometer and a common digital thermometer—with an implantable subcutaneous transponder microchip. Microchips were implanted subcutaneously between the shoulder blades of 16 ferrets (8 male, 8 female), and temperatures were measured twice from the microchip reader and once from each of the rectal thermometers. Results demonstrated the microchip temperature readings had very good to good correlation and agreement to those from both of the rectal thermometers. This study indicates that implantable temperature-sensing microchips are a reliable alternative to rectal thermometry for monitoring body temperature in ferrets.
One of the most basic and fundamental physiologic parameters, measured in both clinical and research settings, is body temperature. Body temperature varies with the location of measurement, with the goal of using a site that yields readings that approximate the core body temperature as closely as possible. Measurement of hypothalamic or deep body sites accurately determines core body temperature.7 Generally this level of monitoring can only be obtained by using some form of invasive technique, such as urinary bladder catheterization, tympanic membrane probes, or pulmonary artery catheterization.5,10,14 Due to the impractical and potentially dangerous nature of conducting an invasive technique on every animal, rectal thermometry has become the ‘gold-standard’ technique for obtaining an estimated core body temperature. Limitations of rectal thermometry are that it can be time- and labor-intensive and might require some form of physical or chemical restraint, depending on the species.7 The use of physical or chemical restraint has been shown to induce stress responses in animals, thus potentially altering the core body temperature and leading to an inaccurate measurement.1,12 Inaccurate temperature measurements can delay treatment or result in unnecessary treatment, both of which can be stressful to animals and possibly confound research data.
Less invasive thermometry methods, such as implantable temperature-sensing microchips, have been developed and appear to be advantageous and potentially less stressful than is rectal thermometry. Advantages of implantable temperature-sensing microchips include decreased use of chemical and physical restraint, reduced stress, and a decreased risk of injury to personnel. Subcutaneous temperature-sensing microchips have been proven successful in a variety of species, including cats, pigs, common marmosets, owl monkeys, guinea pigs, rabbits, large animals, and rodents.3,4,7,11,13,15,16,18 The use of implantable temperature-sensing subcutaneous microchips in ferrets has yet to be evaluated for accuracy, reliability, and repeatability. The objective of the present study was to evaluate the correlation between standard rectal thermometry and implantable temperature-sensing subcutaneous microchips in ferrets to determine whether microchip transponders might be used as an alternative method to accurately measure body temperature.
Materials and Methods
Animals.
This study used 16 (8 male, 8 female) purpose-bred domestic ferrets (Mustela putorius furo) that ranged in age from 5.3 to 6 mo and weighed between 0.80 and 1.68 kg (Marshall BioResources, North Rose, NY). Prior to arrival, the ferrets were neutered or spayed, vaccinated with 2 doses (2 wk apart) of a modified live distemper vaccine starting at 9 wk of age, and vaccinated with killed rabies vaccine (Imrab-3, Merial, Duluth, GA) at 12 wk of age. Ferrets were free from overt clinical signs of illness and appeared healthy on arrival. Ferrets were housed within the Uniformed Services University of the Health Sciences (USUHS) Central Animal Facility, an AAALAC-accredited facility. Animals underwent a 4-wk acclimation period prior to use. The ferrets in the current study were part of an IACUC-approved protocol establishing an animal model for lymphatic filariasis. Experimental methods associated with the current study were performed prior to any manipulations for the lymphatic filariasis protocol. All research was conducted in compliance with the Animal Welfare Act and Department of Defense regulations and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals.9
Ferrets were housed in same-sex pairs. The environment was maintained at 68 to 72 °F (20 to 22.2 °C), with a relative humidity of 30 to 70%. The ferrets were maintained on a 12:12-h light:dark light cycle and had unrestricted access to a commercial diet (Teklad Certified Global Diet 2072C, Harlan Teklad, Madison, WI) and fresh water. The ferrets were provided with a variety of toys for enrichment and had at least 1 h of free playtime in a designated exercise pen several times each week.
Equipment.
The equipment used in this study included 2 types of rectal thermometers and implantable temperature-sensing subcutaneous microchips. The primary rectal digital thermometer (Welch Allyn Sure Temp Plus 690V, Skaneateles Falls, NY) was battery-powered and handheld, and operated within a temperature range of 80 to 110 °F ± 0.2 °F (26.7 to 43.3 °C ± 0.1 °C). This device was chosen as the ‘gold standard’ reference because it can be recalibrated to manufacturer's specifications. In fact, this device was originally calibrated by the manufacturer and, less than 2 wk before the device's first use, the University's Medical Maintenance Division used the manufacturer's calibration key to verify and certify the accuracy of the manufacturer's specifications. The second rectal digital thermometer (model 15-737, MABIS Healthcare, Waukegan, IL) was battery-powered and handheld, and operated within a temperature range of 90 to 111.9 °F ± 0.2 °F (32.2 to 44.3 °C ± 0.1 °C); it represents a style used commonly for rectal thermometry. This device was originally calibrated by the manufacturer but was not verified for accuracy prior to use. The implantable temperature-sensing subcutaneous microchip (IPTT-300 Microchip, Bio Medic Data Systems, Seaford, DE) is a biocompatible glass capsule coated with a polypropylene polymer to prevent migration. Each microchip, measuring 14 mm × 2 mm and weighing 120 mg, was preloaded in a sterile, single-use, 12-gauge delivery syringe. Microchips had a factory-calibrated range of 90 to 110 °F (32 to 43 °C) and were not verified for accuracy prior to use. Temperature measurements were obtained by using a compatible reader (DAS-7007S Straight Wireless Handheld Reader, Bio Medic Data Systems).
Neither the common rectal thermometer nor the microchips were verified for accuracy prior to use as both have narrow temperature ranges and thus cannot be tested using the ‘gold-standard’ thermometer calibration methods of immersion in either a circulating hot-water bath (212 °F [100 °C]) or an ice-water bath (32 °F [0 °C]). As such, we assumed that the common rectal thermometer and the microchips were performing within manufacturer's published specifications.
Experimental design.
Microchips were implanted subcutaneously between the scapulae of the ferrets 3 wk prior to the first temperature measurements, to allow for the resolution of any local inflammation due to implantation. The microchip was scanned prior to implantation to ensure readability and to obtain the microchip number. Each ferret was assigned a separate microchip number, which was recorded to correspond to the ferrets’ individual ear tag number. Ferrets were manually restrained for microchip implantation. On each ferret, a 3 cm × 3 cm area between the scapulae was shaved with electric clippers and wiped with 70% ethyl alcohol solution. The skin was tented, the needle of the delivery syringe containing the microchip was inserted subcutaneously, and the microchip was implanted. The syringe was withdrawn, and the insertion site held closed for a few seconds to ensure skin closure and hemostasis. Ferrets were then scanned by using the microchip reader to verify correct implantation and functioning. After microchip verification, the ferrets were returned to their home cages.
Two experiments were conducted over a period of 5 d. Each ferret was manually restrained and scanned with the reader device by holding the reader a few millimeters above the shaved area, to confirm the location and readability of the microchip. Each microchip number was verified to match the number initially assigned. The first experiment compared temperature measurements between the calibrated digital thermometer and microchip. Each ferret's body temperature was read first by using the calibrated digital thermometer followed by scanning of the microchip. The rectal temperature was obtained by inserting the calibrated thermometer probe, which was encased in a probe cover and lubricated with bacteriostatic lubricant, approximately 0.25 in. (0.635 cm) into the rectum, where it was held until the unit displayed a reading. The thermometer probe cover was replaced between ferrets. Ferrets were immediately scanned with the microchip reader as previously described until the reader's display indicated the microchip's temperature reading. Ferrets subsequently were returned to their home cage. Only one temperature reading was obtained from each ferret on each day.
The second experiment was conducted 5 d after the conclusion of the first and followed the same process, except the rectal temperature measurement was obtained by using the common digital thermometer. The digital rectal thermometer was sanitized by wiping with 70% ethyl alcohol solution between ferrets. The microchip temperature was obtained immediately after obtaining the rectal temperature, as done in the first experiment 1.
Statistical analysis.
Statistical analysis consisted of obtaining the Shrout and Fleiss intraclass correlation coefficient and 95% limits of agreement as described by Bland and Altman.2,17 The intraclass correlation coefficient assessed the reliability and correlation between each rectal thermometer and the implantable temperature-sensing subcutaneous microchip. The sample size of 16 ferrets had 80% power at a significance level of 5% to reject the null hypothesis that the correlation is poor at an intraclass correlation coefficient 0.2 or less when the true intraclass correlation coefficient is 0.7.20 The statistical analysis of intraclass correlation coefficients was conducted by using SPSS (version 22, IBM, Armonk, NY).
The 95% limits of agreement enabled us to determine the degree of agreement between each rectal thermometer and the implantable temperature-sensing subcutaneous microchip. This method analyzes 2 continuous variables to determine if a new technique agrees sufficiently with the gold standard technique and can serve as an alternative.2 Rectal temperature was the gold standard technique used for analysis. Predetermined criterion for agreement between the methods was within 2 °F (1.2 °C) with a 95% confidence. The limit of agreement of 2 °F (1.2 °C) was used as it is half of the published normal temperature range of 100 to 104 °F (37.7 to 40 °C),6 and clinical decisions to treat a ferret or remove it from study are rarely based solely on a single temperature reading but rather a collection of clinical signs and trend observation. Therefore, if we observe limits of agreement outside the range of ± 2°F, we would expect unacceptably large differences between the rectal thermometer and implantable temperature-sensing subcutaneous microchip in at least 5% of the study population. Statistical analysis for 95% limits of agreement was conducted by using STATA (version 12, StataCorp, College Station, TX).
Results
This study evaluated both a calibrated rectal thermometer and a common digital rectal thermometer and compared them individually with a subcutaneously implanted temperature-sensing microchip. The microchip showed very good to good correlation with both the calibrated rectal thermometer (intraclass correlation coefficient, 0.743; 95% confidence interval, 0.401 to 0.902) and common digital rectal thermometer (intraclass correlation coefficient, 0.603; 95% confidence interval, 0.046 to 0.855). According to previous studies that calculated the reliability between measurements, an intraclass correlation coefficient of less than 0.40 signifies poor correlation, whereas 0.40 to 0.59 signifies fair correlation, 0.60 to 0.74 indicates good correlation, and greater than 0.74 implies excellent correlation.19,21
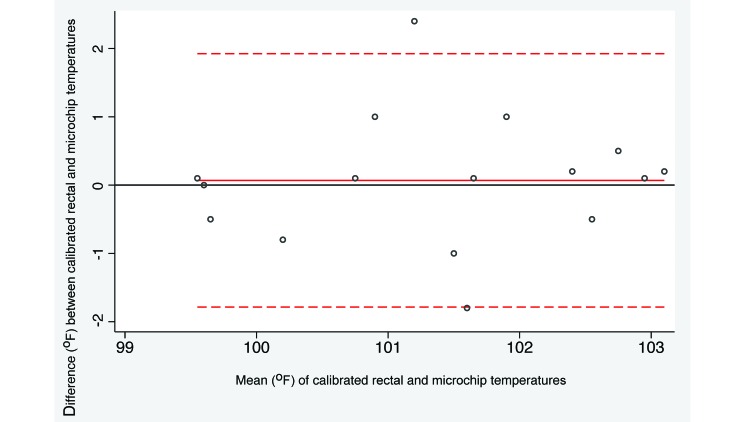
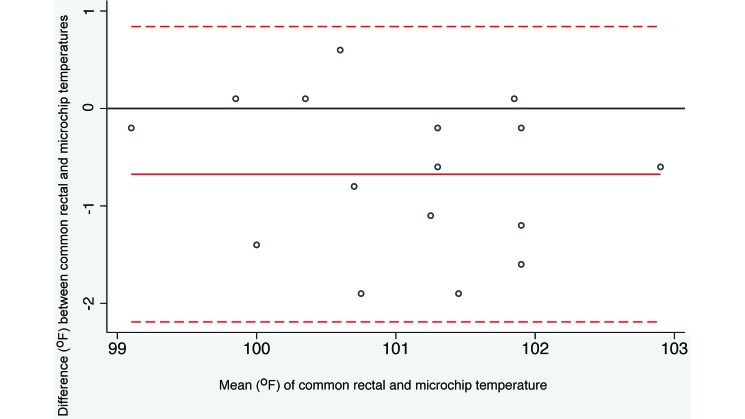
Bland–Altman analysis of the recorded temperatures is presented in Table 1. The microchip agreed sufficiently with both rectal thermometers to be accepted as an alternative technique. The average difference between the calibrated rectal thermometer and the microchip was 0.07 °F (P = 0.775, paired t test) and 95% limits of agreement were –1.82 and 1.96 (Figure 1). Because both limits are less than 2 in absolute value, differences between the 2 methods of more than 2 °F in either direction are unlikely. Furthermore, the common rectal thermometer is comparable to the microchip despite a significantly (P = 0.0033) higher temperature recording (0.675 °F) for the microchip compared with the common rectal thermometer. The 95% limits of agreement were –2.19 and 0.84 (Figure 2), indicating that microchip temperatures more than 2 °F different from the corresponding rectal temperature might occur with some frequency, but differences greater than 2.2 °F would be rare.
Table 1.
Summary statistics for implantable microchip and rectal thermometry
| Thermometry method | Temperature (°F) |
Mean difference between methods | 95% Agreement limits (°F) | |
| Range | Average | |||
| Microchip | 99.5–103.0 | 101.4 | 0.069 | −1.82 to +1.96 |
| Calibrated rectal | 99.4–103.2 | 101.4 | Not applicable | |
| Microchip | 99.2–103.2 | 101.4 | −0.675 | −2.19 to +0.84 |
| Common rectal | 99.0–102.6 | 100.7 | Not applicable | |
Figure 1.
Bland–Altman plot illustrating the difference between calibrated rectal and microchip transponder thermometry. The difference in temperature methods is plotted relative to the pairwise mean. The horizontal reference line at 0 represents no difference between the methods. The dotted lines represent the limits of agreement (mean ± 1.96 SD). Negative values represent rectal temperatures that were lower than the corresponding microchip temperatures.
Figure 2.
Bland–Altman plot illustrating the difference between common rectal and microchip transponder thermometry. The difference in temperature methods is plotted relative to the pairwise mean. The horizontal reference line at 0 represents no difference between the methods. The dotted lines represent the limits of agreement (mean ± 1.96 SD). Negative values represent rectal temperatures that were lower than the corresponding microchip temperatures.
Discussion
This study demonstrates that implantable temperature-sensing microchips have very good to good agreement with both calibrated and common rectal thermometry. Our results indicate that, compared with common rectal thermometers, microchips might overstate the body temperature in approximately 4% to 5% of ferrets. In fact, the common rectal thermometer might underestimate body temperatures, given that the microchip yielded an average difference <0.06 °F compared with the calibrated rectal thermometer, whereas the average difference between the readings from the microchip and common rectal thermometer was just over 0.5 F, indicating that the microchip-provided temperatures are closer to those of the calibrated thermometer. This finding may reflect the ability to recalibrate the calibrated thermometer to manufacturer's specifications, whereas the common rectal thermometer cannot be recalibrated.
The use of implantable temperature-sensing microchips has several advantages compared with rectal thermometry in ferrets. The temperature recording time is considerably faster with the microchips than with either type of rectal thermometers. The average time for a temperature reading with the microchips was 3 s at most. The calibrated rectal thermometer and the common rectal thermometer required an average of 15 s and 10 s, respectively, to provide a digital reading (data not shown). A faster reading time decreases the amount of time a ferret is subject to restraint and thus might decrease handling-associated stress. The longer times required to obtain readings from the calibrated and common rectal thermometers caused many of the ferrets to resist the physical restraint, which might have falsely increased temperature readings. Restraint resistance during rectal thermometer readings might be minimized through habituation to rectal thermometry. In addition, the small diameter of the ferret's anus may contribute to discomfort, thereby increasing resistance to rectal thermometry. This observation is particularly relevant for the calibrated thermometer, which has a larger probe diameter than did the common rectal thermometer. Marked physical restraint was unnecessary when using the microchips; ferrets required minimal restraint and were held with a softer grip for readings. Decreased physical restraint may minimize stress, allowing a more accurate point-in-time temperature reading. Eliminating stress-induced temperature elevations would benefit the reliability of data from studies requiring multiple temperature readings within a brief time period.
The implantable microchips also provide a very practical method for ferret identification. Ferrets were identified by the USDA silver ID clip in the right ear. This clip is very small and often difficult to read. In addition, it might easily be ripped from the ferret's ear. The microchips are preloaded with a programmable identification number, allowing for consistent ferret identification in the absence of the USDA silver ID chip. There was no microchip loss during the current study, with all microchips readable for 4 mo after implantation or longer. The duration of microchip functionality was not evaluated in the current study but was observed anecdotally, because these ferrets were part of another study that required repeated identification confirmation. All of the microchips that were scanned 4 mo after implantation were functional and provided a reading within 3 s.
The ferrets tolerated the microchips with no clinical signs of local inflammation at the implantation site. None of the ferrets developed any long-term sequelae (for example, hematomas, abscesses). Further studies are needed to accurately assess long-term sequelae after microchip implantation. Overall, the microchip implantation was a relatively easy procedure and was comparable to microchip implantation in dogs and cats. The physical implantation of the microchip took only a few seconds, provided the ferret was restrained sufficiently, but the entire process took several minutes because the implantation site had to be shaved and prepped with alcohol. In dogs and cats, microchip implantation is typically accomplished without hair clipping or alcohol prepping. Despite the minimally increased implantation time, the additional 2 steps of clipping and prepping provided a clean and accessible site for microchip readings.
A negative aspect of using implantable microchips is the close proximity required to scan the ferret with the microchip reader. The reader had to be held within millimeters of the ferrets’ back and almost directly over the microchip implantation site to obtain a reading. Shaving of the implantation site initially allowed for consistent placement and decreased scanning time. Throughout this and the subsequent study, none of the microchips migrated from the implantation site, thereby increasing the ease of microchip location. The need for close reader proximity may increase scanning time when a ferret is not habituated to the process and will not remain immobile. Scanning time is decreased when 2 people (scanner and restrainer) are involved in the scanning process, but this brief restraint period might increase stress and falsely elevate the animal's temperature. Habituation could reduce this risk.
Another concern with the microchip is the potential incompatibility for use with MRI. Functionality of the microchip with MRI was not evaluated in the current study. A literature search yielded no studies that evaluated the post MRI functionality of the transponder that we used; the sole study we identified that used the same model of microchips in mice undergoing MRI22 did not test the functionality of the microchip thereafter. Similar transponder microchips used in small animal practice have demonstrated continued microchip functionality after MRI scans.8
In conclusion, the current study supports the use of implantable temperature-sensing microchips as a reliable alternative to rectal thermometry in healthy ferrets for obtaining an estimate of core body temperature, consistent with their use in other species.3,4,7,11,15,18 Microchips were easily placed and well-tolerated in ferrets and produced temperature readings comparable to those from rectal thermometry. Minimal physical restraint and rapid measurements likely decreased the ferrets’ stress level, allowing accurate approximations of core body temperature to enhance both animal wellbeing and the quality of research data. The microchip's functionality under hyperthermic (for example, febrile disease) and hypothermic (for example, prolonged anesthesia) states and after MRI should be evaluated in subsequent experiments.
Acknowledgments
Research was performed at Uniformed Services University of the Health Sciences (Bethesda, MD) and was funded by the Uniformed Services University of the Health Sciences. There is no objection to its presentation or publication. The opinions, interpretations, and conclusions herein are those of the author and are not necessarily endorsed by the Uniformed Services University of the Health Sciences or the Department of the Defense. Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 2011 edition.
Special thanks to Dr. Edward Mitre, So-Young Kim, and Dr Belinda Jackson for allowing the simultaneous use of their ferrets in this study. We also thank MAJ Amanda Christy for animal technical assistance.
References
- 1.Aydin C, Grace CE, Gordon CJ. 2011. Effect of physical restraint on the limits of thermoregulation in telemetered rats. Exp Physiol 96:1218–1227. [DOI] [PubMed] [Google Scholar]
- 2.Bland JM, Altman DG. 1986. Stastical Methods for Assessing Agreement Between Two Methods of Clinical Measurement. Lancet 327:307–310. [PubMed] [Google Scholar]
- 3.Chen PH, White CE. 2006. Comparison of rectal, microchip transponder and infrared thermoetry techniques for obtaining body temperature in the laboratory rabbit (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci 45:57–63. [PubMed] [Google Scholar]
- 4.Cilia J, Piper DC, Upton N, Hagan JJ. 1998. A comparison of rectal and subcutaneous body temperature measurement in the common marmoset. J Pharmacol Toxicol Methods 40:21–26. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara-Love R. 1991. A comparison of tympanic and pulmonary artery measures of core temperatures. J Post Anesth Nurs 6:161–164. [PubMed] [Google Scholar]
- 6.Fox JG. 1998. Biology and diseases of ferrets, 2nd ed. Baltimore (MD): Wiley-Blackwell. [Google Scholar]
- 7.Goodwin S. 1998. Comparison of body temperatures of goats, horses, and sheep measured with a tympanic infrared thermometer, an implantable microchip transponder, and a rectal thermometer. Contemp Top Lab Anim Sci 37:51 –55. [PubMed] [Google Scholar]
- 8.Haifley KA, Hecht S. 2012. Functionality of implanted microchips following magnetic resonance imaging. J Am Vet Med Assoc 240:577–579. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington DC: National Academies Press. [Google Scholar]
- 10.Jakobsson J, Nilsson A, Carlsson L. 1992. Core temperature measured in the auricular canal: comparison between four different tympanic thermometers. Acta Anaesthesiol Scand 36:819–824. [DOI] [PubMed] [Google Scholar]
- 11.Kort WJ, Hekking-Weijma JM, TenKate MT, Sorm V, VanStrik R. 1998. A microchip implant system as a method to determine body temperature of terminally ill rats and mice. Lab Anim 32:260–269. [DOI] [PubMed] [Google Scholar]
- 12.Livezey GT, Miller JM, Vogel WH. 1985. Plasma norephinephrine, epinephrine and corticosterone stress responses to restraint in individual male and female rats, and their correlations. Neurosci Lett 62:51–56. [DOI] [PubMed] [Google Scholar]
- 13.Lohse L, Uttenthal A, Enoe C, Nielsen J. 2010. A study on the applicability of implantable microchip transponders for body temperature measurements in pigs. Acta Vet Scand 52:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin B. 1995. Tympanic infrared thermometry to determine cat body temperature. Contemp Top Lab Anim Sci 34:89 –92. [PubMed] [Google Scholar]
- 15.Quimby JM, Olea-Popelka F, Lappin MR. 2009. Comparison of digital rectal and microchip transponder thermometry in cats. J Am Assoc Lab Anim Sci 48:402–404. [PMC free article] [PubMed] [Google Scholar]
- 16.Shelton LJ, Jr, White CE, Felt SA. 2006. A comparison of non-contact, subcutaneous, and rectal temperatures in captive owl monkeys (Aotus sp.). J Med Primatol 35:346–351. [DOI] [PubMed] [Google Scholar]
- 17.Shrout PE, Fleiss JL. 1979. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428. [DOI] [PubMed] [Google Scholar]
- 18.Stephens Devalle JM. 2005. Comparison of tympanic, transponder, and noncontact infrared laser thermometry with rectal thermomety in strain 13 guinea pigs (cavia porcellus) Contemp Top Lab Anim Sci 44:35 –38. [PubMed] [Google Scholar]
- 19.Vlach KD, Boles JW, Stiles BG. 2000. Telemetric evaluation of body temperature and physical activity as predictors of mortality in a murine model of staphylococcal entertoxic shock. Comp Med 50:160–166. [PubMed] [Google Scholar]
- 20.Walter SD, Eliasziw M, Donner A. 1998. Sample size and optimal designs for reliability studies. Stat Med 17:101–110. [DOI] [PubMed] [Google Scholar]
- 21.Warn PA, Brampton MW, Sharp A, Morrissey G, Steel N, Denning DW, Priest T. 2003. Infrared body temperature measurement of mice as an early predictor of death in experimental fungal infections Lab Anim 37:126 –131. [DOI] [PubMed] [Google Scholar]
- 22.Wideman RD, Gray SL, Covey SD, Webb GC, Kierffer TJ. 2009. Transplantation of PC1/3-Expressing α-cells improves glucose handling and cold tolerance in leptin-resistant mice. Mol Ther 17:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]