Abstract
Handheld, point-of-care glucometers are commonly used in NHP for clinical and research purposes, but whether these devices are appropriate for use in NHP is unknown. Other animal studies indicate that glucometers should be species-specific, given differences in glucose distribution between RBC and plasma; in addition, Hct and sampling site (venous compared with capillary) influence glucometer readings. Therefore, we compared the accuracy of 2 human and 2 veterinary glucometers at various Hct ranges in rhesus macaques (Macaca mulatta), sooty mangabeys (Cercocebus atys), and chimpanzees (Pan troglodytes) with that of standard laboratory glucose analysis. Subsequent analyses assessed the effect of hypoglycemia, hyperglycemia, and sampling site on glucometer accuracy. The veterinary glucometers overestimated blood glucose (BG) values in all species by 26 to 75 mg/dL. The mean difference between the human glucometers and the laboratory analyzer was 7 mg/dL or less in all species. The human glucometers overestimated BG in hypoglycemic mangabeys by 4 mg/dL and underestimated BG in hyperglycemic mangabeys by 11 mg/dL; similar patterns occurred in rhesus macaques. Hct did not affect glucometer accuracy, but all samples were within the range at which glucometers generally are accurate in humans. BG values were significantly lower in venous than capillary samples. The current findings show that veterinary glucometers intended for companion-animal species are inappropriate for use in the studied NHP species, whereas the human glucometers showed clinically acceptable accuracy in all 3 species. Finally, potential differences between venous and capillary BG values should be considered when comparing and evaluating results.
Abbreviations: BG, blood glucose; ISO, International Organization for Standardization
There are several indications in NHP medicine for using handheld, point-of-care glucose meters (glucometers), which rapidly and easily measure blood glucose (BG). For example, naturally occurring type 2 diabetes has been reported in more than 40 NHP species, including the 3 evaluated in the current study—rhesus macaques, sooty mangabeys, and chimpanzees.18,24,26 In addition, because glucometers require very small volumes of blood, they are useful in small-bodied NHP that have increased susceptibility to hypoglycemia, such as neonates and many New World species. Furthermore, glucometers are commonly used in NHP biomedical research related to obesity and diabetes.17,22,23,38 Human glucometers as well as veterinary glucometers intended for use with small companion animals are commercially available; however, the literature contains no information regarding the accuracy of these glucometers in NHP.
The accuracy of glucometers in humans has been evaluated extensively under various conditions, and many studies show that commercially available human glucometers can yield glucose measurements that differ significantly from the actual values.33 For example, a recent study of 43 glucometers found a bias range of –14.1% to12.4%, with widely varying limits of agreement.8 In addition, criteria for acceptable accuracy vary widely between professional societies and standards organizations, and these requirements are rarely met by currently available glucometers.33,39 For these reasons, there is increasing concern regarding the reliability of glucometers in human medicine, especially when highly accurate measurements are needed to maintain tight glucose control or guide treatment decisions.
Glucometer performance has also been assessed in dogs, cats, horses, birds, deer, sheep, cattle, alpacas, ferrets, and rabbits.34 Results from these studies show that glucometers intended for humans have decreased accuracy when used in animal species, suggesting that similar findings might be observed in NHP species. Veterinary-specific glucometers were recently developed for companion animals, but how well these glucometers perform in NHP is unknown.
Differences in glucometer accuracy between species have been attributed, at least in part, to species-specific differences in glucose distribution.1 Glucometers measure glucose in whole blood, but because glucose reference ranges typically are based on plasma levels, most glucometers perform a calculation and report the estimated plasma glucose level.39 The distribution of glucose between plasma and RBC varies between species.10 Humans have a much higher ratio of intracellular glucose (glycosylated hemoglobin) to plasma glucose than do dogs and other domestic animals. For example, the cell:plasma glucose ratio (×100) is 67.1 in humans and 34.2 in canines.10 Although veterinary species-specific glucometers use the same enzyme technology and equipment as do human glucometers, species-specific algorithms are needed to calculate and report plasma glucose levels.1 The glucose distribution in NHP has not been evaluated widely, but one study found that the cell:plasma ratio (×100) is 70.8 in rhesus macaques and 77.7 in olive baboons.10 These results suggest that glucometer accuracy might differ among NHP species, particularly between Asian and African species.
In addition, Hct affects the accuracy of glucometers in humans and animals.9,13,21,25,29,32,37,39 Glucometers are designed to operate within a specific range of Hct values. When Hct is lower or higher than the established range, glucometer-reported BG values are generally higher or lower than the actual BG value, respectively.12,21,27,37 The sooty mangabey's normal Hct ranges from 43% to 49.9% and thus is higher than that of humans, chimpanzees, rhesus macaques, canines, and felines, potentially decreasing the accuracy of both veterinary and human glucometers in the mangabeys by significantly underestimating the actual BG value.5,6,11,14,19,35
Several human studies have reported that glucometers may be less accurate during hypoglycemia, generally overestimating the BG level.15,28,33,36 Accuracy during hyperglycemia has been less frequently reported, but diabetes-induced perfusion disturbances and ketoacidosis, factors often associated with hyperglycemia, are both known to interfere with glucometer accuracy.39
Glucose levels differ between collection sites, with arterial blood having the highest glucose level.39 Arterial blood is delivered to capillaries, where glucose is absorbed by tissues as an energy source, thus resulting in the lowest glucose level in venous circulation.39 Capillary and venous glucose levels have been reported to correlate well in human studies, but it is important to understand the differences between these sampling methods when interpreting glucometer readings.4
The primary aims of the current study were to: 1) compare the accuracy of 2 veterinary and 2 human glucometers in 3 NHP species with inherently different Hct ranges; 2) determine the accuracy of 2 human glucometers during hypoglycemia and hyperglycemia in rhesus macaques and sooty mangabeys; and 3) determine whether glucometer performance differs between capillary and venous sampling sites. Accuracy was defined as the absolute and actual mean difference in BG levels measured by each glucometer and a standard laboratory glucose analyzer. We hypothesized that, due to interspecies differences in cell and plasma glucose distribution, human glucometers would report NHP glucose levels more accurately than would veterinary-specific glucometers. Furthermore, sooty mangabeys have a naturally higher HCT range and potentially higher cell:plasma glucose ratio than those of Asian monkeys and African apes; therefore, we hypothesized that human glucometers would be less accurate in sooty mangabeys than in rhesus macaques and chimpanzees. In addition, we expected glucometers to be less accurate during hypoglycemia and hyperglycemia compared with euglycemia in both rhesus macaques and sooty mangabeys. Finally, we hypothesized that the mean difference between both human glucometers and the laboratory analyzer would be significantly smaller in venous samples compared with capillary samples. Data from this study will be useful to investigators seeking information regarding glucometer use in NHP for both clinical and research purposes and the interpretation of BG values obtained by using these devices.
Materials and Methods
Animals.
The study population comprised 80 rhesus macaques (mean age [range], 7.8 y [0.7 to 24.5 y]); 24 male, 56 female), 50 sooty mangabeys (9.6 y [0.9 to 21.1 y]; 13 male, 37 female), and 12 chimpanzees (31.5 y [18.1 to 50.4 y]; 2 male, 10 female) maintained at the Yerkes National Primate Research Center (Lawrenceville, GA). All study animals were maintained in social groups and housed in indoor–outdoor enclosures. Rhesus macaques and sooty mangabeys received LabDiet 5038 (PMI Nutrition International, St Louis, MO), and chimpanzees were fed fiber-enriched LabDiet 5050 (PMI Nutrition International). Routine enrichment provided to all animals included fresh produce, climbing structures, foraging devices, and other manipulanda. Blood was collected from animals that had been food-fasted overnight (in most cases) as part of annual health exams and clinical evaluations. Because blood samples were collected opportunistically, the duration of fasting before collection varied among animals. Most study animals were fed between 1500 and 1600 the day before blood collection; on the day of blood collection, food was offered after each animal was fully recovered from morning anesthesia, therefore, food was withheld from the majority of study animals for 17 to 20 h prior to blood collection. Before examination and blood collection for all experiments, the study NHP were sedated with either ketamine HCl (10mg/kg IM) or tiletamine HCl–zolazepam HCl (3 to 5mg/kg IM). Procedures involving these animals were approved by the IACUC of Emory University and were conducted in accordance with USDA Animal Welfare Regulations, the Guide for the Care and Use of Laboratory Animals, and institutional policies.2,16 The facilities and animal resources program at YNPRC are fully AAALAC-accredited.
Experiment 1: Comparison of human and veterinary glucometers.
To determine whether human or veterinary glucometers were more suitable for use in NHP species, approximately 2 mL of venous blood was collected from the femoral or saphenous vein of animals sedated for annual health exams or clinical evaluations. Immediately after blood collection, venous BG was measured by using 2 human (Accu-Chek Aviva Plus, Roche Diagnostics, Basel, Switzerland [glucometer HA]; FreeStyle Freedom Lite, Abbott Laboratories, Abbott Park, IL [glucometer HB]) and 2 veterinary (AlphaTrak2, Abbott Animal Health, Abbott Park, IL [glucometer VA]; GlucoPet, Animal Diabetes Management, Janesville, WI [glucometer VB]) glucometers. The veterinary glucometers were coded for canine use. The remainder of the whole blood was divided equally between 2-mL vacuum phlebotomy tubes, one containing EDTA (3.6 mg) and one containing sodium fluoride–potassium oxalate (5 mg–4 mg; gray-top tube) and chilled until processed. Gray-top tubes containing the glycolysis inhibitor sodium fluoride were centrifuged at 1560 × g for 10 min at 4 °C. Plasma was removed and stored at –80 °C until analyzed by using a laboratory glucose analyzer (model 2300 STAT Plus, Yellow Springs Instrument, Yellow Springs, OH). The times of blood collection and processing were recorded. For Hct determination in the macaques and mangabeys, EDTA-anticoagulated blood was placed into nonheparinized microhematocrit tubes and centrifuged at 14,500 × g for 5 min; the Hct was read by using a microhematocrit reader card. Hct was calculated for the chimpanzees by using an automated hematology analyzer (model xs-1000i, Sysmex, Hialeah, FL).
Experiment 2: Effects of hypoglycemia and hyperglycemia.
To compare the accuracy of the human glucometers during different glycemic states, rhesus macaques were screened during clinical exams for hypoglycemia or hyperglycemia by using either human glucometer with a capillary (fingerstick) blood sample. Sooty mangabeys were screened for inclusion based on venous BG according to both human glucometers during experiment 1. For both rhesus macaques and sooty mangabeys, hypoglycemia was defined as a BG value of less than 60 mg/dL, whereas BG greater than 100 mg/dL was considered hyperglycemia. Rhesus macaques and sooty mangabeys with BG of 60 to 100 mg/dL were considered euglycemic controls. These ranges were selected in light of previously reported reference values and diagnostic criteria for diabetes in Old World NHP.7,35,40 For both species, the final categorization regarding glycemic state was established according to the venous BG value determined by a laboratory glucose analyzer (Yellow Springs Instrument). Chimpanzees were not evaluated in the current experiment because of low sample size for each of the glycemic states. In addition, veterinary glucometers were not evaluated in light of the results from experiment 1.
Approximately 1.5 mL of venous blood was collected from the femoral or saphenous vein of each animal. Immediately after blood collection, venous BG was measured using both human glucometers. The remaining whole blood was divided between a serum microtainer tube for reference laboratory serum chemistry (model SA010 Superchem, Antech Diagnostics, Chapel Hill, NC) and a gray-top vacuum phlebotomy tube containing sodium fluoride for subsequent BG analysis by using the laboratory glucose analyzer (Yellow Springs Instrument). Blood in gray-top tubes was processed and stored as described in experiment 1.
Experiment 3: Effect of capillary compared with venous sampling site.
Capillary and venous blood samples collected in experiment 2 were used also to investigate glucometer accuracy at different sampling sites. Venous samples were collected immediately after capillary measurement during the same anesthetic event. For capillary sampling, the distal portion of a digit was swabbed clean with 70% ethanol and gauze before being lanced with a 25-gauge hypodermic needle. Occasionally, due to error messages, more than one glucometer strip was needed for successful measurement of each sample. The number of strips used for each sample with each glucometer was recorded for later comparison.
Laboratory glucose analyzer.
Glucometer results were compared with the actual BG value determined by the laboratory glucose analyzer (Yellow Springs Instrument), a bench-top, glucose oxidase analyzer. The laboratory analyzer is a well-established standard for the measurement of BG and has historically been used to evaluate the accuracy of glucometers.3,39 Approximately 1 h before analysis, plasma samples were removed from storage at –80 °C and allowed to thaw at room temperature. On each day of sample measurement, operational checks including membrane integrity testing and linearity testing with control solutions were performed according to the manufacturer's instructions. The laboratory analyzer was programmed to autocalibrate after every fifth sample measurement. All samples were analyzed in duplicate, and the mean of the 2 values was used for data analysis.
Statistical analyses.
By using statistical software (JMP 11; SAS Institute, Cary, NC), all data were first evaluated to confirm normality and equality of variance. Linear mixed-effects models then were performed, with NHP species, glucometer, and glycemic state (hypoglycemia, euglycemia, and hyperglycemia) as fixed effects; qualitative hemolysis score and time (in minutes) in gray-top tubes as covariates; and individual subjects as a random effect. Primary outcomes were absolute and actual mean difference between each glucometer and the reference method. Tukey HSD tests were used for posthoc comparisons. Pearson correlation analysis was used to compare capillary and venous glucometer readings, with +1 representing perfect positive correlation, –1 representing perfect negative correlation, and 0 denoting the absence of correlation. A P value less than 0.05 was considered significant for all analyses. Only rhesus macaques and sooty mangabeys with euglycemic BG values were included in the analysis for experiment 1; all glycemic states were included in experiment 2. Because neither sex nor age affected glucometer accuracy, pooled data are presented for each species.
The clinical implication of the data from the human and veterinary glucometer in all 3 species was assessed by using error grid analysis. The Parkes error grid compares reference glucose analyzer values (x axis) with glucometer values (y axis) to indicate the clinical consequences of results.30 The plot is divided into 5 risk zones (A through E), according to 100 human physicians’ classification of the disagreement between 2 values.30 The ideal zone is A, where the 2 values would result in identical treatment decisions, whereas a glucometer value in zone E represents a significantly inaccurate glucometer reading that might result in a life-threatening treatment decision, such as administration of insulin to a hypoglycemic patient.
Results
Experiment 1.
Table 1 displays the mean BG values and ranges obtained by using the 2 human and 2 veterinary glucometers and the laboratory reference glucose analyzer for all 3 NHP species. Blood glucose values from the 2 veterinary glucometers were 30 to 50 units higher than those from the 2 human glucometers among rhesus macaques and sooty mangabeys and 30 to 70 units higher in chimpanzees. The actual mean differences in BG values between each glucometer and the laboratory reference analyzer are shown in Figure 1 A through C. In all 3 NHP species, the actual mean BG differences of the 2 veterinary glucometers were significantly different from each other and from the 2 human glucometers (P < 0.0001 for all comparisons). Specifically, glucometer VB significantly overestimated the true glucose value in all 3 NHP species by 25 to 35 mg/dL, whereas glucometer VA significantly overestimated BG by approximately 45 mg/dL in rhesus macaques and mangabeys and greater than 75 mg/dL in chimpanzees. The actual mean differences in BG between both human glucometers and the laboratory reference analyzer were 7 mg/dL or less and did not significantly differ from each other or across NHP species. Similarly, no significant differences between NHP species were observed when BG values were expressed as an absolute mean difference between each human glucometer and the reference analyzer (data not shown).
Table 1.
Comparison of human and veterinary glucometers across 3 NHP species
| Blood glucose (mg/dL) |
Blood glucose (mg/dL) from laboratory glucose analyzer |
||||
| Glucometer | Mean ± SE | Range | Mean ± SE | Range | |
| Rhesus macaques (n = 38) | HB | 69.8 ± 1.42 | 55–91 | 76.8 ± 1.46 | 62.3–99.1 |
| HA | 71.1 ± 1.18 | 59–90 | |||
| VB | 111.5 ± 1.75 | 90–135 | |||
| VA | 120.6 ± 2.32 | 91–158 | |||
| Sooty mangabeys (n = 27) | HB | 72.3 ± 2.56 | 50–104 | 76.2 ± 2.06 | 60.4–97.7 |
| HA | 71.1 ± 2.28 | 48–90 | |||
| VB | 102.1 ± 2.77 | 78–124 | |||
| VA | 122.6 ± 3.68 | 88–158 | |||
| Chimpanzees (n = 12) | HB | 107.2 ± 4.17 | 72–127 | 106.8 ± 4.69 | 69.2–126.5 |
| HA | 112.3 ± 5.2 | 75–138 | |||
| VB | 137.8 ± 5.6 | 109–178 | |||
| VA | 184 ± 7.27 | 121–209 | |||
Figure 1.
Comparison of human and veterinary glucometers in (A) rhesus macaques, (B) sooty mangabeys, and (C) chimpanzees. The veterinary glucometers (VA and VB) significantly overestimated venous BG by 25 to 75 units, whereas both human glucometers (HA and HB) were within 2 to 7 units of the actual BG value for each species, with no interspecies differences. Data are given as mean ± SE; within each species, different lowercase letters denote values that differ significantly (P < 0.05).
Parkes error grid.
The distribution in the Parkes error grid (Figure 2) was similar for all 3 NHP species. The majority of measurements from both human glucometers fell within zone A, whereas most of the measurements from both veterinary glucometers were within zone B with some results in zones C and D, indicating that the BG values obtained from the human glucometers might lead to safer and more accurate clinical decisions in these species, compared with the BG data obtained by using the veterinary glucometers.
Figure 2.
Parkes error-grid analysis. Risk zones: A, clinically accurate measurements, no effect on clinical action; B, altered clinical action, little or no effect on clinical outcome; C, altered clinical action, likely to affect clinical outcome; D, altered clinical action, could have significant clinical risk; and E, altered clinical action, could have dangerous consequences.30 Most of the measurements from the 2 human glucometers (HA and HB) fall within zone A. The majority of the measurements from the 2 veterinary glucometers (VA and VB) fall within zone B, with some results in zones C and D.
Hct.
The mean Hct was significantly (P < 0.001) higher in sooty mangabeys than in rhesus macaques and chimpanzees (Table 2). The Hct values obtained for the 3 NHP species in this study were similar to those previously reported in the literature, and a linear regression analysis revealed no significant effect of Hct on the absolute and actual mean differences between each glucometer and the laboratory glucose analyzer.5,14,35
Table 2.
Hct values of rhesus macaques, sooty mangabeys, and chimpanzees
| Hct (mean ± 1 SD) |
||
| This study | Published valuea | |
| Rhesus macaques | 40.67 ± 2.68 | 41.8 ± 2.9 |
| Sooty mangabeys | 45.84 ± 2.6b | 43.0 ± 3.3 to 49.9 ± 9.4 |
| Chimpanzees | 42.27 ± 9.34 | 41.69 ±2.63 to 46.5 ± 3.09 |
Reference values for rhesus macaques, sooty mangabeys, and chimpanzees obtained from references 5, 35, and 14, respectively.
Significantly (P < 0.05) different from values for rhesus macaques and chimpanzees.
Experiment 2.
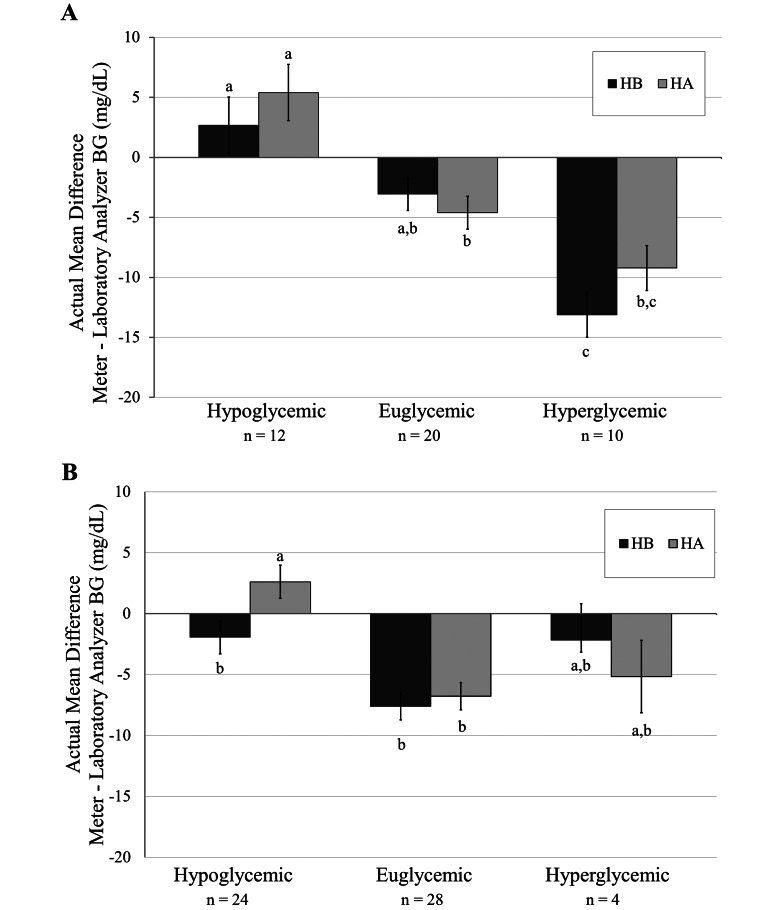
Results from experiment 1 showed that the veterinary glucometers tested were unsuitable for use in these NHP species, therefore only the human glucometers were used for the remainder of the study. Euglycemic (BG, 71.2 to 97.7 mg/dL; n = 20), hypoglycemic (BG, 35.2 to 59.6 mg/dL; n = 12), and hyperglycemic (BG, 103 to 442 mg/dL; n = 10) sooty mangabeys were identified. Overall, the actual mean differences in BG values between each human glucometer and the laboratory reference (Figure 3 A) were small (less than 15 mg/dL) during all 3 glycemic states. Unlike glucometer HA, glucometer HB significantly underestimated the reference BG value by 13.1 ± 1.9 mg/dL in hyperglycemic mangabeys compared with euglycemic mangabeys. The actual mean difference between the BG readings obtained by using the glucometer compared with the laboratory analyzer differed significantly (P < 0.05) between hypoglycemic and euglycemic sooty mangabeys when glucometer HA—but not glucometer HB—was used; however the difference between the absolute mean values was nonsignificant (data not shown, P = 0.34).
Figure 3.
Human glucometer accuracy during hypoglycemia, euglycemia, and hyperglycemia in (A) sooty mangabeys and (B) rhesus macaques. Both human glucometers underestimated the BG value in euglycemic and hyperglycemic sooty mangabeys and rhesus macaques, but a significant difference between the euglycemic and hyperglycemic states was seen only for mangabeys when glucometer HB was used. Glucometer HA significantly overestimated the BG value in the hypoglycemic state as compared with the euglycemic state in both macaques and mangabeys. Data are given as mean ± SE; within each species, different lowercase letters denote values that differ significantly (P < 0.05).
Euglycemic (BG, 70.1 to 99.1 mg/dL; n = 28), hypoglycemic (BG, 26.9 to 58.8 mg/dL; n = 24), and hyperglycemic (BG, 108.5 to 120 mg/dL; n = 4) rhesus macaques were identified. In rhesus macaques, both human glucometers had similar accuracy during hypoglycemic and hyperglycemic states compared with the euglycemic state (Figure 3 B). Similar to mangabeys, the actual mean difference in BG readings from the glucometer compared with laboratory analyzer was significant (P < 0.0001) between hypoglycemic and euglycemic rhesus macaques when glucometer HA was used; however the absolute mean values did not differ between the devices (data not shown, P = 0.38).
Experiment 3.
BG values from capillary- and venous-derived samples correlated significantly (P < 0.05) when both human glucometers were used in rhesus macaques. However, the strength of the correlation coefficient for glucometer HA (correlation coefficient, 0.97; strong correlation) was stronger than that of glucometer HB (correlation coefficient, 0.68; moderate correlation). The actual mean difference between both human glucometers and the laboratory analyzer were significantly (P < 0.01) smaller for venous samples compared with capillary samples (Figure 4 A). Specifically, the capillary glucometer readings were higher than the actual venous BG value by 10.9 ± 2.8 mg/dL (mean ± SE), whereas glucometer readings from venous blood slightly underestimated the actual venous BG value by 1.2 ± 2.8 mg/dL. When each human glucometer was evaluated individually (Figure 4 B), a similar pattern was observed, and capillary and venous BG values measured by using glucometer HB differed significantly (P < 0.05); a significant difference was not observed with glucometer HA.
Figure 4.
Comparison of capillary and venous sampling sites for BG measurement in rhesus macaques. (A) Capillary BG values were significantly higher than venous BG values. (B) When evaluating each glucometer individually, a significant difference between the capillary and venous BG values was seen only with glucometer HB. Within each graph, data are given as mean ± SE; within each species, different lowercase letters denote values that differ significantly (P < 0.05).
Strip use.
As a secondary outcome in experiment 3, we compared the number of strips used by the 2 human glucometers between capillary and venous samples. Regardless of glucometer type, significantly (P < 0.05) more strips were required for capillary compared with venous sampling, with a mean of 1.2 ± 0.0 and 1.0 ± 0.0 strips used per sample, respectively (Figure 5). This difference was driven primarily by the use by glucometer HA compared with glucometer HB, in that glucometer HA required the most strips for capillary measurement. The difference between venous and capillary sampling with glucometer HA did not reach significance (P = 0.07), but 16.6% of the capillary readings required more than 1 strip to complete a measurement, compared with 5.3% of the venous readings.
Figure 5.
Number of strips used per BG measurement, according to sampling site and glucometer. Significantly more strips were required for capillary compared with venous sampling. Data are given as mean ± SE; within each species, different lowercase letters denote values that differ significantly (P < 0.05).
Serum chemistry values.
To compare the laboratory analyzer we used with an (off-site) reference laboratory, BG values obtained by using the laboratory analyzer were compared with those from serum chemistry panels. No significant difference in BG values was found between the laboratory analyzer and the off-site reference laboratory. The mean absolute difference in BG between the laboratory analyzer and the reference laboratory was 4.2 ± 1.0 mg/dL.
Discussion
To the our knowledge, this study is the first to evaluate the accuracy of both veterinary and human glucometers in NHPs. Glucometer studies have been performed in humans and other veterinary species, often demonstrating unacceptable levels of inaccuracy.33,34 Glucometers are frequently used in NHP in both research and clinical settings. Due to species-associated differences in BG distribution, the glucometer most appropriate for each species should be identified. As expected, our findings showed that human glucometers are more accurate for use in NHP than are veterinary glucometers and that values obtained from venous samples are significantly lower than those from capillary samples. According to this data, human glucometers are more appropriate than are veterinary glucometers for use in NHP; even higher accuracy might be obtained with glucometers specifically designed for use in NHP. We were unable to definitively demonstrate that human glucometers are less accurate during both hypoglycemia and hyperglycemia in rhesus macaques and sooty mangabeys, but our results suggested that this difference might emerge if sample size increased. In contrast to our expectation that human glucometers would be less accurate in sooty mangabeys than in rhesus macaques and chimpanzees, we found no significant interspecies differences in glucometer accuracy.
In this study, the 2 veterinary glucometers, which were calibrated for canine use, were less accurate than both of the human glucometers in all 3 NHP species. Using veterinary glucometers on feline settings would likely increase the inaccuracy, due to the very low cell:plasma ratio of glucose distribution in felines compared with canines, humans, and NHP.1 Mean venous BG values obtained by each human glucometer were within 2 to 7 mg/dL of the actual BG value for each NHP species, with no interspecies differences. As illustrated with the Parkes error grid, the small mean differences seen with the human glucometers would be unlikely to significantly affect clinical treatment decisions. Although the chart is designed for human use, the threshold values that are used to determine the consequences of treatment decisions are applicable to NHP. The majority of the measurements from the 2 veterinary glucometers fell within zone B, but the several values that fell within zones C and D would have directed decisions likely to have negative clinical consequences.
In humans, glucometers commonly overestimate BG during hypoglycemia, potentially adversely affecting clinical treatment plans, including the administration of supplemental glucose.15,28,33 One study reported that accuracy is similar between the different glycemic states but mentioned that this level of inaccuracy could be much more dangerous in cases of hypoglycemia than in euglycemia or hyperglycemia.20 In our study, the human glucometers both overestimated BG in hypoglycemic mangabeys, with glucometer HA providing the greater overestimation. In addition, the human glucometers underestimated BG in hyperglycemic mangabeys; this underestimation was greater with glucometer HB. The overestimation of low glucose levels and underestimation of high glucose levels can cause a true abnormal or critical value to be missed. The differences seen in this study generally were small, but mean values for the hyperglycemic mangabeys were 9 to 13 mg/dL lower than the actual value. This level of inaccuracy might affect the treatment plan for an animal that is nearing the threshold of the diabetic range. This level of inaccuracy might also falsely represent clinical improvement when monitoring and managing disease progression. A similar pattern was observed among rhesus macaques; however the difference did not achieve statistical significance, most likely due to the low number of hyperglycemic rhesus monkeys.
Several factors, including Hct, can affect the accuracy of glucometer measurement. Glucose is found within the aqueous portion of a blood sample.39 Because RBC lipid membranes and hemoglobin exclude water, plasma has relatively higher water content than does whole blood and consequently a higher glucose concentration.39 Although the specific distribution ratio differs among species, plasma glucose value is higher than the RBC-associated glucose level in humans and the previously discussed veterinary species.10 Decreased RBC concentration (that is, Hct) causes less displacement of plasma, making relatively more glucose molecules available to react with enzymes during measurement.27 Conversely, increased Hct causes more displacement of plasma, making relatively fewer glucose molecules available to react with enzymes during measurement.27 In the present study, we expected the glucometers to be less accurate in sooty mangabeys, due to their inherently higher Hct, than in the other 2 NHP species. Although we did find a species-specific difference in Hct ranges, with mangabeys having a significantly higher Hct than rhesus macaques and chimpanzees, Hct did not significantly alter glucometer accuracy. For all subjects, Hct values were within the range in which the glucometers are expected to function accurately, according to the manufacturer's recommendations. Some human studies, however, have found that Hct levels outside a range of 35% to 45% may affect accuracy.31 We might similarly have noted a significant effect if additional anemic and polycythemic animals had been included.
Sampling site and fasting status can affect the accuracy of glucometer measurements. As expected, capillary and venous BG measurements collected at the same time point significantly differed. BG values from venous samples were a mean of 12 mg/dL lower than capillary samples, but the difference varied, with as large as a 50 mg/dL difference between capillary and venous values in some animals. In humans, this difference is most substantial after a meal or insulin dose.3 Although the difference might be small in a fasted patient, the postprandial difference might be considerably larger, with as much as a 30% discrepancy between capillary and venous values.3 This difference might not fully disappear until 4.5 h postprandially; however, venous and capillary glucose levels might still differ even after overnight fasting and is intensified by oxygenation changes, pH differences, and perfusion disturbances.3,39 Unless an animal was fasted intentionally, the fasting status of NHP patients is often unknown, especially when emergent or critical cases require immediate treatment. For this reason, clinicians working with NHP should be cautious when comparing capillary and venous samples. An animal might have been hypoglycemic on initial venous blood work, and evaluating subsequent treatment effectiveness by using serial capillary measurements might falsely indicate a clinical improvement. Comparing values obtained from different sites can be misleading and confusing, leading some users to question the accuracy of the measurement equipment.
Glucometer measurements can be influenced by delays in sample processing and analysis. Whole blood that is allowed to sit at room temperature can lose glucose at a rate of 5% to 7% hourly due to blood cell metabolism.39 Such glycolysis is generally not problematic when glucometer measurement is performed immediately after blood collection but can result in falsely decrease values obtained from central laboratory devices when analysis is delayed. To separate cells and serum, samples for serum chemistry should be centrifuged as soon as possible. In this study, we also used the glycolytic inhibitor sodium fluoride to delay glycolysis in the samples measured by using the laboratory analyzer.
In humans, the importance of selecting an appropriate glucometer has been well established. Several organizations have published criteria for evaluating glucometer accuracy in humans, with some creating different criteria for defining hypoglycemic and hyperglycemic ranges.39 In 2013, the International Organization for Standardization (ISO) updated their accuracy criteria requiring 95% of glucometer measurements to be within 15 mg/dL of actual BG values under 100 mg/dL and within 15% for BG values of 100 mg/dL and higher.8 According to our results, both of the human glucometers we used in this study meet the ISO standards in all 3 NHP species. Interestingly, many currently available human glucometers fail to meet the accuracy criteria when used in human patients. For example, one study found that 18 of the 34 (52.9%) BG monitoring systems evaluated did not meet the European minimal conformity standard ISO 15197:2013.8 Other human glucometers may provide clinically accurate BG measurement in NHP, but it is important to critically evaluate each glucometer individually. Human glucometers that are shown to be highly inaccurate for use in humans will likely also be unacceptably inaccurate for use in NHP.
In addition to accuracy, ease of use is an important consideration for the selection of a glucometer. In this study, samples sometimes required the use of multiple test strips, due to error messages. Glucometer strips often cost more than USD$1 each, and multiple errors can increase the cost of BG monitoring. Error messages can result from damaged test strips, insufficient blood sample, incorrect timing of sample application, electronic errors, temperature extremes, and several other factors. In this study, significantly more strips were required for capillary compared with venous sampling. Subjectively, this difference seemed to be due mainly to insufficient blood sample volume or difficulty positioning the portion of the test strip that wicks blood for collection of the sample.
Because the samples evaluated in this study were collected opportunistically, one limitation was the inability to ensure an equal distribution of hypoglycemic, euglycemic, and hyperglycemic samples. With additional hypoglycemic and hyperglycemic animals, we might have seen more significant effects related to the glycemic state. Furthermore, because of the opportunistic nature of sample collection, we were unable to ensure a consistent fasting period among animals. Prior studies show that fasting can affect blood glucose readings between capillary and venous sampling sites; therefore, knowledge of the exact prandial status of each animals may have reduced variance in these analyses.3 In addition, not all samples could be processed immediately after collection, but the potential effects of this delay were mitigated by use of the glycolytic inhibitor and by chilling samples prior to processing. Ideally, multiple instruments of each brand of glucometer would be evaluated to account for interinstrument variation, but all meters were checked regularly by using control solutions, as recommended by the manufacturers.
In this study, the human glucometers produced clinically acceptable results in the 3 species tested and would likely be more appropriate than veterinary glucometers in other NHP species. It is important to note that interspecies differences in glucose distribution between plasma and RBC can affect the measurement of glucose in whole blood by glucometers. Although a small level of inaccuracy may be clinically acceptable, the glucometers used for research purposes might affect study data, and any level of inaccuracy is undesirable. Ideally, the glucose distribution in NHP should be defined and used to develop a glucometer specifically programmed for each NHP species. Until NHP-species–specific glucometers are available, a human glucometer that has proven accuracy in humans and is used according to manufacturer recommendations is an acceptable choice for easy and rapid blood glucose evaluation. In addition, it is important to be aware of the effects that sampling site (venous compared with capillary), Hct, and other physiologic factors, including blood pressure and pH status, might have on BG measurement.39
Collection of data needed to calculate NHP-species–specific glucometer algorithms is underway. Future research directions might include repeating the current study in additional primate species, such as New World monkeys, and evaluating BG accuracy in more NHP with abnormally high or low Hct values. These studies may provide useful information about the effects of such abnormalities on glucose measurement.
Acknowledgments
This work was supported by a NIH-NCRR base grant (P51OD11132) to YNPRC. We thank the YNPRC field station veterinarians, veterinary technicians, and colony management personnel for their assistance with this work.
References
- 1.AlphaTRAK Meter. [Internet]. 2014. Veterinarian brochure: AlphaTRAK Blood Glucose Monitoring System. Package insert. [Cited 27 August 2013]. Available at: http://www.alphatrakmeter.com/pdf/AlphaTRAK_AT2-2063_VeterinaryBrochure_FINAL_1.0.pdf
- 2.Animal Welfare Regulations. 2013. 9 CFR § 1.1–§ 4.11.
- 3.Arabadjief D, Nichols JH. 2006. Assessing glucose meter accuracy. Curr Med Res Opin 22:2167–2174. [DOI] [PubMed] [Google Scholar]
- 4.Boyd R, Leigh B, Stuart P. 2005. Capillary versus venous bedside blood glucose estimations. Emerg Med J 22:177–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Qin S, Ding Y, Wei L, Zhang J, Li H, Bu H, Lu Y, Cheng J. 2009. Reference values of clinical chemistry and hematology parameters in rhesus monkeys (Macaca mulatta). Xenotransplantation 16:496–501. [DOI] [PubMed] [Google Scholar]
- 6.Choi SY, Hwang JS, Kim IH, Hwang DY, Kang HG. 2011. Basic data on the hematology, serum biochemistry, urology, and organ weights of beagle dogs. Lab Anim Res 27:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox JG, Anderson LC, Loew FM, Quimby FW, 2002. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press. [Google Scholar]
- 8.Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. 2012. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol 6:1060–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghys T, Goedhuys W, Spincemaille K, Gorus F, Gerlo E. 2007. Plasma-equivalent glucose at the point-of-care: evaluation of Roche Accu-Chek Inform and Abbott Precision PCx glucose meters. Clin Chim Acta 386:63–68. [DOI] [PubMed] [Google Scholar]
- 10.Higgins PJ, Garlick RL, Bunn HF. 1982. Glycosylated hemoglobin in human and animal red cells. Role of glucose permeability. Diabetes 31:743–748. [DOI] [PubMed] [Google Scholar]
- 11.Hollowell JG, van Assendelft OW, Gunter EW, Lewis BG, Najjar M, Pfeiffer CCenters for Disease Control and Prevention, National Center for Health Statistics. 2005. Hematological and iron-related analytes—reference data for persons aged 1 year and over: United States, 1988 to 1994. Vital Health Stat 11:1–156. [PubMed] [Google Scholar]
- 12.Holtzinger C, Szelag E, DuBois JA, Shirey TL, Presti S. 2008. Evaluation of a new POCT bedside glucose meter and strip with hematocrit and interference corrections. Point Care 7:16–21. [Google Scholar]
- 13.Hussain K, Sharief N. 2000. The inaccuracy of venous and capillary blood glucose measurement using reagent strips in the newborn period and the effect of haematocrit. Early Hum Dev 57:111–121. [DOI] [PubMed] [Google Scholar]
- 14.Ihrig M, Tassinary LG, Bernacky B, Keeling ME. 2001. Hematologic and serum biochemical reference intervals for the chimpanzee (Pan troglodytes) categorized by age and sex. Comp Med 51:30–37. [PubMed] [Google Scholar]
- 15.Inoue S, Egi M, Kotani J, Morita K. 2013. Accuracy of blood-glucose measurements using glucose meters and arterial blood gas analyzers in critically ill adult patients: systematic review. Crit Care 17:R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.Ishizaka T, Sato T, Kato K, Ohba M, Kimotsuki T, Yasuda M. 2003. Subcutaneous continuous glucose monitoring and dose adjustment decreases glycosylated hemoglobin in spontaneously diabetic cynomolgus monkeys. Contemp Top Lab Anim Sci 42:36–40. [PubMed] [Google Scholar]
- 18.Jones AC, Herndon JG, Courtney CL, Collura L, Cohen JK. 2014. Clinicopathologic characteristics, prevalence, and risk factors of spontaneous diabetes in sooty mangabeys (Cercocebus atys). Comp Med 64:200–210. [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn CM, Line S. 2010. The Merck veterinary manual, 10th ed. Whitehouse Station (NJ): Merck. [Google Scholar]
- 20.Kanji S, Buffie J, Hutton B, Bunting PS, Singh A, McDonald K, Fergusson D, McIntyre LA, Hebert PC. 2005. Reliability of point-of-care testing for glucose measurement in critically ill adults. Crit Care Med 33:2778–2785. [DOI] [PubMed] [Google Scholar]
- 21.Karon BS, Griesmann L, Scott R, Bryant SC, Dubois JA, Shirey TL, Presti S, Santrach PJ. 2008. Evaluation of the impact of hematocrit and other interference on the accuracy of hospital-based glucose meters. Diabetes Technol Ther 10:111–120. [DOI] [PubMed] [Google Scholar]
- 22.Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD. 2007. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 15:1666–1674. [DOI] [PubMed] [Google Scholar]
- 23.Kavanagh K, Zhang L, Wagner JD. 2009. Tissue-specific regulation and expression of heat-shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones 14:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhar CW, Fuller GA, Dennis PM. 2013. A survey of diabetes prevalence in zoo-housed primates. Zoo Biol 32:63–69. [DOI] [PubMed] [Google Scholar]
- 25.Lane SL, Koenig A, Brainard BM. 2015. Formulation and validation of a predictive model to correct blood glucose concentrations obtained with a veterinary point-of-care glucometer in hemodiluted and hemoconcentrated canine blood samples. J Am Vet Med Assoc 246:307–312. [DOI] [PubMed] [Google Scholar]
- 26.LeRoith D, Taylor SI, Olefsky JM. 2004. Diabetes mellitus: a fundamental and clinical text, 3rd ed. Philadelphia (PA): Lippincott Williams and Wilkins. [Google Scholar]
- 27.Mann EA, Salinas J, Pidcoke HF, Wolf SE, Holcomb JB, Wade CE. 2008. Error rates resulting from anemia can be corrected in multiple commonly used point-of-care glucometers. J Trauma 64:15–20; discussion 20–11. [DOI] [PubMed] [Google Scholar]
- 28.Ngerncham S, Piriyanimit S, Kolatat T, Wongsiridej P, Inchgarm L, Kitsommart R, Vutrapongwatana P, Jeerapaet K. 2012. Validity of 2 point-of-care glucometers in the diagnosis of neonatal hypoglycemia. Indian Pediatr 49:621–625. [DOI] [PubMed] [Google Scholar]
- 29.Paul AE, Shiel RE, Juvet F, Mooney CT, Mansfield CS. 2011. Effect of hematocrit on accuracy of 2 point-of-care glucometers for use in dogs. Am J Vet Res 72:1204–1208. [DOI] [PubMed] [Google Scholar]
- 30.Pfutzner A, Klonoff DC, Pardo S, Parkes JL. 2013. Technical aspects of the Parkes Error Grid. J Diabetes Sci Technol 7:1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramljak S, Lock JP, Schipper C, Musholt PB, Forst T, Lyon M, Pfutzner A. 2013. Hematocrit interference of blood glucose meters for patient self-measurement. J Diabetes Sci Technol 7:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao LV, Jakubiak F, Sidwell JS, Winkelman JW, Snyder ML. 2005. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clin Chim Acta 356:178–183. [DOI] [PubMed] [Google Scholar]
- 33.Rebel A, Rice MA, Fahy BG. 2012. Accuracy of point-of-care glucose measurements. J Diabetes Sci Technol 6:396–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selleri P, Di Girolamo N, Novari G. 2014. Performance of 2 portable meters and a benchtop analyzer for blood glucose concentration measurement in rabbits. J Am Vet Med Assoc 245:87–98. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Chennareddi L, Greene-Hartsfield EZ, Villinger F, Cohen JK, Herndon JG. 2014. Hematology and serum chemistry values of sooty mangabeys (Cercocebus atys): comparison with rhesus monkeys. J Med Primatol 43:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonmez A, Yilmaz Z, Uckaya G, Kilic S, Tapan S, Taslipinar A, Aydogdu A, Yazici M, Yilmaz MI, Serdar M, Erbil MK, Kutlu M. 2010. The accuracy of home glucose meters in hypoglycemia. Diabetes Technol Ther 12:619–626. [DOI] [PubMed] [Google Scholar]
- 37.Tang Z, Lee JH, Louie RF, Kost GJ. 2000. Effects of different hematocrit levels on glucose measurements with handheld meters for point-of-care testing. Arch Pathol Lab Med 124:1135–1140. [DOI] [PubMed] [Google Scholar]
- 38.Tardif SD, Power ML, Ross CN, Rutherford JN, Layne-Colon DG, Paulik MA. 2009. Characterization of obese phenotypes in a small nonhuman primate, the common marmoset (Callithrix jacchus). Obesity (Silver Spring) 17:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonyushkina K, Nichols JH. 2009. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol 3:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wagner JD, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, Jr, Kaplan JR. 2006. Old world nonhuman primate models of type 2 diabetes mellitus. ILAR J 47:259–271. [DOI] [PubMed] [Google Scholar]