Abstract
Purpose
Balloon-assisted maturation (BAM) is emerging as a salvage management for arteriovenous fistula maturation failure (AVF MF). However, BAM is a relatively new, yet controversial technique for AVF maturation. Therefore, we evaluated the effectiveness of BAM for AVF MF.
Methods
Between January 2012 and December 2014, 249 AVFs were created. The total MF rate was 24.8%. But, only 110 AVFs were enrolled, including 74 brachiocephalic (BC) AVFs and 36 radiocephalic (RC) AVFs. The follow-up period was 12 months. Among those, there were 42 MFs (22 BC AVFs and 20 RC AVFs) and 68 maturation successes (MS) (52 BC AVFs and 16 RC AVFs). BAM was involved in MF group. We compared the clinical characteristics, AVF flows, and AVF flow ratios of MF and MS groups. Also, we evaluated the etiology, management, and result of MF.
Results
There was no difference in clinical characteristics between MF and MS groups. In MF group, 39 balloon angioplasties (BAs) for 42 AVF MFs were performed. Number of BA was 1.45 ± 0.57 and duration of BA was 21.30 ± 21.24 weeks. BAM rate was 46.2%. For 1 year after AVF creation, AVF flows of MS group were significantly larger than those of MF group (P < 0.05) but there was no difference in AVF flow ratio between MF and MS groups (P > 0.05).
Conclusion
BA for AVF MF is a relatively applicable and effective modality. Although a large volume study is necessary, we suggest BAM is an effective salvage management for AVF MF.
Keywords: Balloon angioplasty, Treatment failure, Renal dialysis, Arteriovenous fistula
INTRODUCTION
The arteriovenous fistula (AVF) is the access of choice for hemodialysis (HD), but its success as an access is limited by a high rate of maturation failure (MF) [1]. Therefore, an upsurge of new techniques and studies has emerged in an effort to increase maturation and salvage rates in AVFs [2]. Balloon-assisted maturation (BAM) is a recent, innovative, yet controversial method for developing AVF maturation [2,3]. The use of BAM is becoming increasingly popular, despite the limited number of evidence-based studies and lack of randomized prospective trials [2]. This method has been used in effort to increase successful primary maturation as defined by the National Kidney Foundation - Disease Outcomes Quality Initiative (NKF-DOQI) [2,4]. For that, the AVF MF is subjected to a series of staged, serial long-segment angioplasty dilations until it reaches the desired diameter and flow rate [3]. A successful BAM can rapidly speed up the maturation process and reduce the need for a tunneled dialysis catheter and prosthetic grafts [3]. Therefore, we evaluated the effectiveness of BAM for AVF MF in our early period experience. This research was approved by the Institutional Review Board of Incheon St. Mary's Hospital (OC15RISI0137).
METHODS
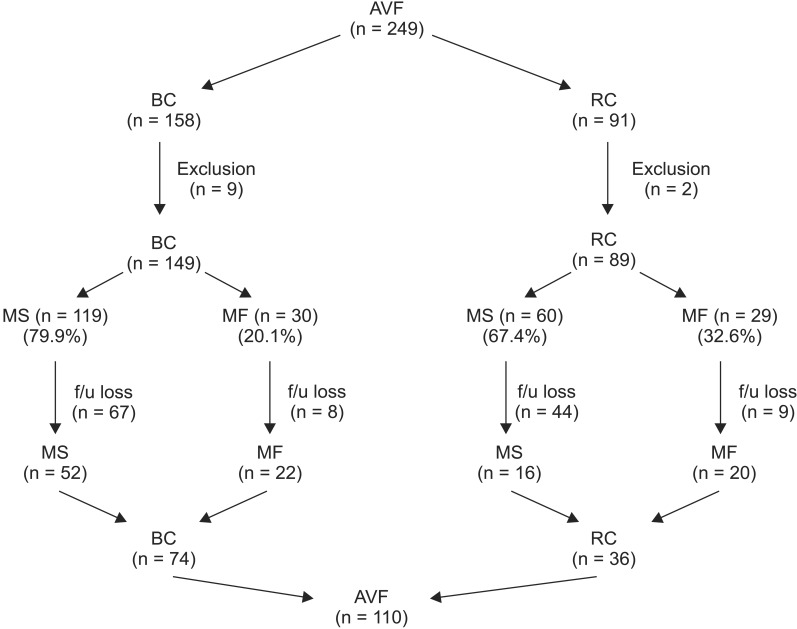
Between January 2012 and December 2014, a total of 249 AVFs were created. Among the 249 cases, there were 11 cases of exclusion that had to receive AVF recreations due to acute complications or we could not decide MF or MS because patients had been transferred to other hospitals immediately on AVF creations (Fig. 1). Eleven cases of exclusion included 9 BC AVFs and 2 RC AVFs. Therefore,, there were 59 cases of MF including 30 of 149 BC AVFs and 29 of 89 RC AVFs (Fig. 1). Also, the total MF rate was 24.8%. However, only 110 AVFs including 74 brachiocephalic (BC) AVFs and 36 radiocephalic (RC) AVFs followed for 1 year were enrolled (Fig. 1). Among these cases, there were 42 cases of MF (22 BC AVFs and 20 RC AVFs) and 68 cases of maturation success (MS) (52 BC AVFs and 16 RC AVFs) (Fig. 1); and, BAM was involved in MF group. We compared the clinical characteristics including age, sex, comorbidity, and etiology of end stage renal disease (ESRD), AVF flows, and AVF flow ratios of the MF and MS groups. Also, we evaluated etiology, management, and result of MF in MF group.
Fig. 1. Arteriovenous fistula created in our hospital over 3 years. AVF, arteriovenous fistula; BC, brachiocephalic; RC, radiocephalic; MS, maturation success; MF, maturation failure; f/u, follow-up.
We examined preoperatively the vessel status using duplex ultrasonography or arm venography. Duplex ultrasonography was mostly used for the preemptive AVF creations, and arm venography was mostly used for the nonpreemptive AVF creations. This trend was due to the conditions at our hospital. Thereafter, if a diameter of a cephalic vein at wrist was more than 2.5 mm, we performed RC AVFs. Also, if the diameter of a cephalic vein at the wrist was less than 2.5 mm, we performed BC AVFs. We did not include sex, DM, and age into the criteria for AVF creation. The MF rate of BC AVF was 20.1% and that of RC AVF was 32.6% (Fig. 1).
All operations including AVF creation, balloon angioplasty (BA), and branched cephalic vein ligation (BCVL), were performed by the same vascular surgeon. All enrolled patients had construction of their AVF at our institution and were instructed to return for follow-up at our outpatient office for evaluation of maturation at 4 and 8 weeks. Those who were not maturing were subjected to BAMs at 2-week intervals. In the literature, AVF MF was defined as a surgically created AVF that failed to properly grow to become usable for the purpose of HD in 8 to 12 weeks after its creation [5]. The Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend that prompt vascular interventions, such as BA and BCVL, should be performed if the AVF fails to mature by 6 weeks after creation [6]. Thus, our criteria for AVF MF was AVF with physical examination findings or duplex ultrasonography findings of nonmaturation by 6 weeks after creation or AVF with a flow volume of less than 600 mL/min measured with a transonic flowmeter (HD03, Transonic Systems Inc., Ithaca, NY, USA) in a trial cannulation at 8 weeks after creation. If AVF was included in more than 1 of 2 criteria, we defined it as AVF MF. Physical examination at 6 weeks was determined clinically by look-listen-feel steps by a vascular surgeon and nephrologist [6]. Also, duplex ultrasonography findings of nonmaturation were a diameter of less than 6 mm, depth of more than 6 mm, or flow of less than 600 mL/min [6].
We performed vascular interventions, such as BA and BCVL starting at 8 weeks after their creation in 2-week intervals until successful cannulation and desired flow rate (600 mL/min) were reached. We checked results by physical examination or duplex ultrasonography at outpatient clinic at 2 weeks after vascular interventions. If their results met our criteria, we attempted cannulation. But, if their results were inferior to our criteria, we attempted reinterventions.
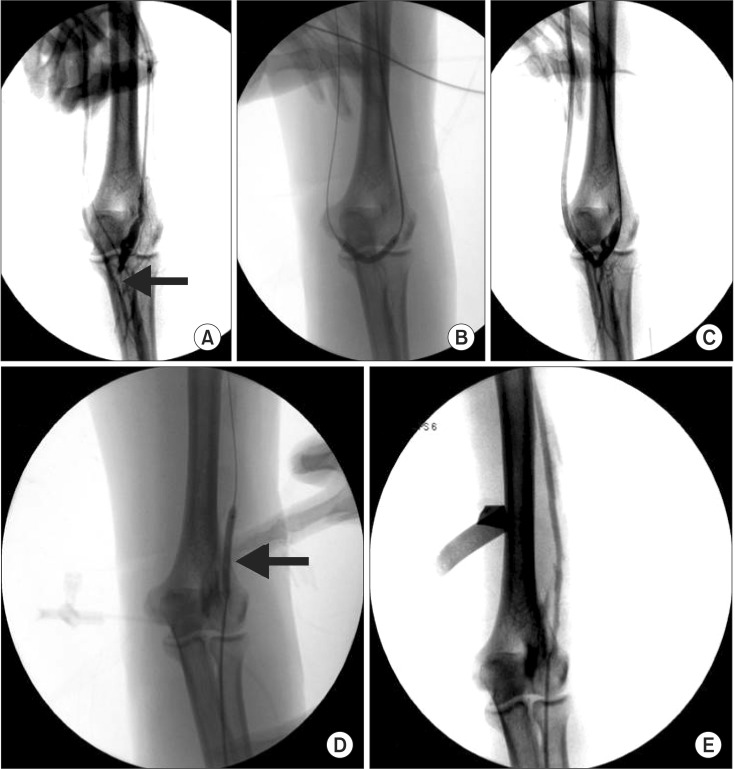
The BA for BAM procedure was performed under a standard protocol using local anesthesia and fluoroscopy guidance (Fig. 2). The C-arm (ARCADIS Avantic, Siemens AG, Erlangen, Germany) was used in all cases to provide excellent visualization of the entire fistula. All procedures were performed in the operation room, with the same vascular team.
Fig. 2. Balloon angioplasty (BA) for balloon assisted maturation of arteriovenous fistula (AVF) maturation failure. (A) Juxtaanastomotic stenosis (JAS) of AVF. Arrow indicates JAS lesion. (B) BA for JAS lesion. (C) Postballooning fistulography shows improvement of JAS lesion. (D) BA for cephalic vein stenosis (CVS) lesion. Arrow indicates inflated balloon. (E) Postballooning fistulography shows improvement of CVS lesion.
The fistula was then cannulated using an 18 gauge angiocathneedle directly or a micropuncture needle and sheath. A 0.035-inch Glidewire (Terumo Medical Corp., Somerset, NJ, USA)and 5-Fr sheath were then inserted and positioned into the proximal artery or distal vein during retrograde and antegrade cannulation, respectively [7]. Serial dilatations were then performed using a 4- to 6-mm Mustang balloon dilatation catheter (Boston Scientific, Natick, MA, USA) depending on vein caliber and surgeon preference (Fig. 2). Mostly, we used a balloon 1 to 2 mm larger than the estimated vein caliber [8]. Each balloon dilatation was performed multiple times with full insufflation, between 2.5 and 3.0 MPa (or 2533125 and 3039750 Pa), for 50 seconds [5].
Patients were instructed to return for follow-up for physical examination and AVF flow measurement with a transonic flowmeter (HD03) at 4 to 6 weeks postoperatively. Subsequent BAs were performed as necessary, at 2-week intervals following each procedure. Interval BA procedures were performed until successful HD using the AVF or clinical evidence of maturation on follow-up [8]. We checked AVF flows with a transonic flowmeter by 1- to 3-month intervals postoperatively, and followed up on enrolled patients for 1 year retrospectively.
Statistical analysis was done by Student t-test, chi-square test, Mann-Whitney test, and Fisher exact test using the IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA). A P-value < 0.05 was considered statistically significant. Data were presented as mean ± standard deviation.
RESULTS
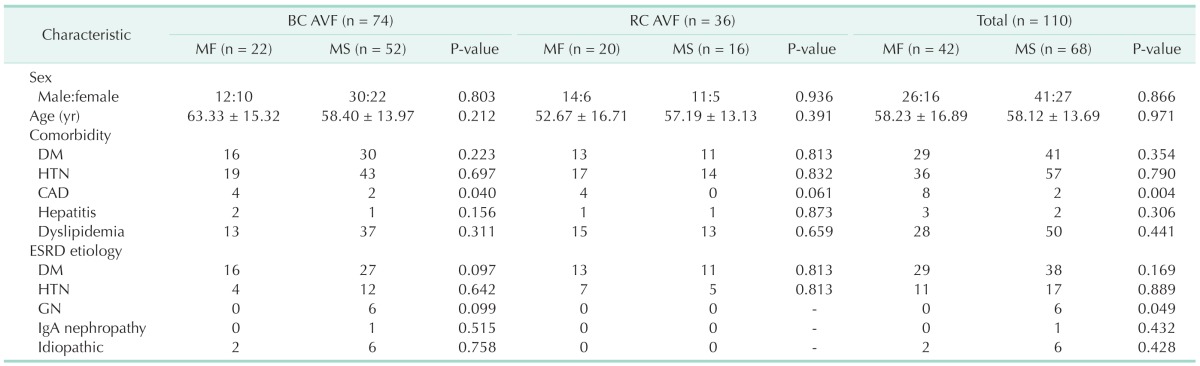
Between MF and MS groups, sexual distribution, age, comorbidities, and etiologies of ESRD were statistically insignificant in BC AVF, RC AVF, and total AVF groups, separately (P > 0.05) (Table 1).
Table 1. Baseline clinical characteristics.
BC, brachiocephalic; AVF, arteriovenous fistula; RC, radiocephalic; MF, maturation failure; MS, maturation success; DM, diabetes mellitus; HTN, hypertension; CAD, coronary artery disease; ESRD, end stage renal disease; GN, glomerulonephritis.
The 42 of 110 enrolled patients were MF. For 42 AVF MFs, MF etiologies were juxtaanastomotic stenosis (JAS) only in 23 patients, JAS and cephalic vein stenosis (CVS) in 7 patients, JAS and branched cephalic vein (BCV) in 7 patients, BCV only in 3 patients, and CVS only in 2 patients (Table 2). Managements for MF were BA only in 32 patients, BA and BCVL in 7 patients, and BCVL only in 3 patients (Table 2). BA to BAM numbers were 1.45 ± 0.57 (Table 2). BA duration (week) after BAM was 21.30 ± 21.24 (Table 2). BA(n) to BAM means numbers of BA needed until AVF MF reaches MS (BAM). And, BA duration means an interval between balloon angioplasties performed after AVF MF reaches MS (BAM). So, we needed to do 1.45 ± 0.57 BAs until AVF MF reached BAM. At 21.30 ± 21.24 weeks after BAM, we needed to do an additional BA during follow-up period. Results of management for MF were 22 fails (52.4%) including 4 ruptures, 5 occlusions, and 13 HDs with low access flow (<600 mL/min), and 20 successes (47.6%) with 18 (46.2%) by BAM (Table 2). Complications including rupture and occlusion related with BAs were 9 cases. Four cases of ruptures included 1 case of anastomosis site rupture and 3 cases of vein rupture (Table 2). Complication rate was 21.4%. In BC AVF and RC AVF groups, MF characteristics including etiology of MF, management for MF, BA number to BAM, BA duration after BAM, and result of management for MF, also showed similar aspects with those in total AVF groups (Table 2). Between BC AVF and RC AVF groups, there was statistically no difference in MF characteristics (P > 0.05) (Table 2).
Table 2. Arteriovenous fistula maturation failure Characteristics.
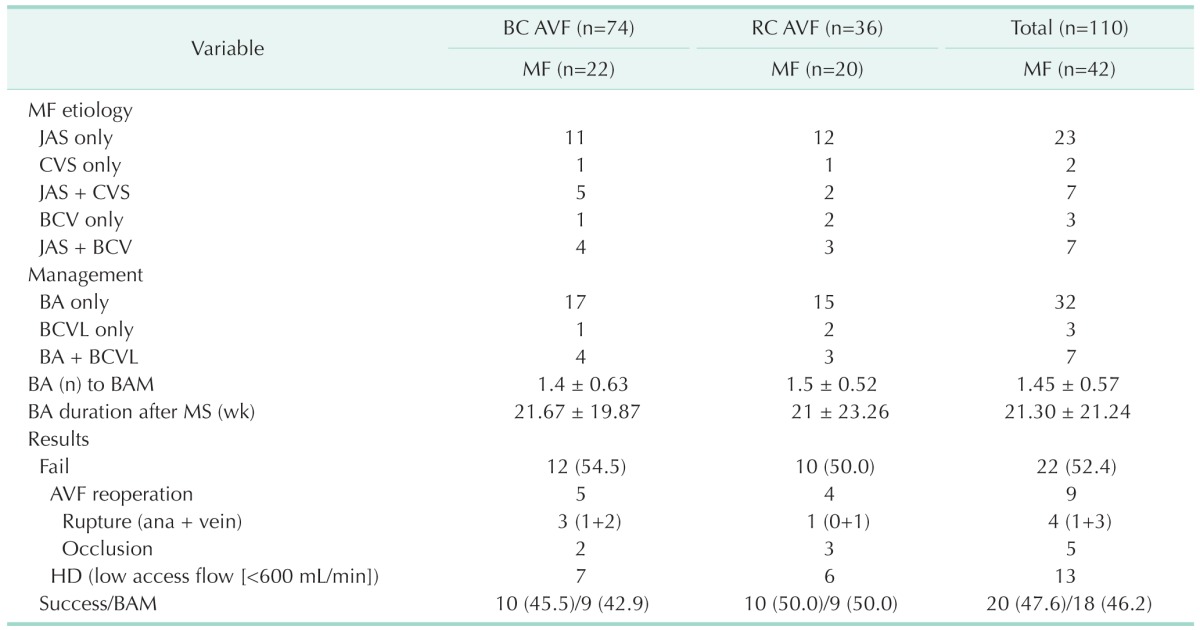
Values are presented as number, mean ± standard deviation, or number (%).
BC, brachiocephalic; AVF, arteriovenous fistula; RC, radiocephalic; MF, maturation failure; JAS, juxtaanastomotic stenosis; CVS, cephalic vein stenosis; BCV, branched cephalic vein; BA, balloon angioplasty; BCVL, branched cephalic vein ligation; BAM, balloon assisted maturation; MS, maturation success; ana, anastomosis; HD, hemodialysis.
In total AVFs, BA durations (week) after BAM were insignificant at 21.30 ± 21.24 in MF group and 34.13 ± 30.36 in MS group (P = 0.213). In BC AVF group, BA durations (week) after BAM were insignificant at 21.67 ± 19.78 in MF group and 36.43 ± 32.03 in MS group (P = 0.275). In RC AVF group, BA durations (week) after BAM were insignificant at 21.00 ± 23.26 in MF group and 31.78 ± 28.51 in MS group (P = 0.176).
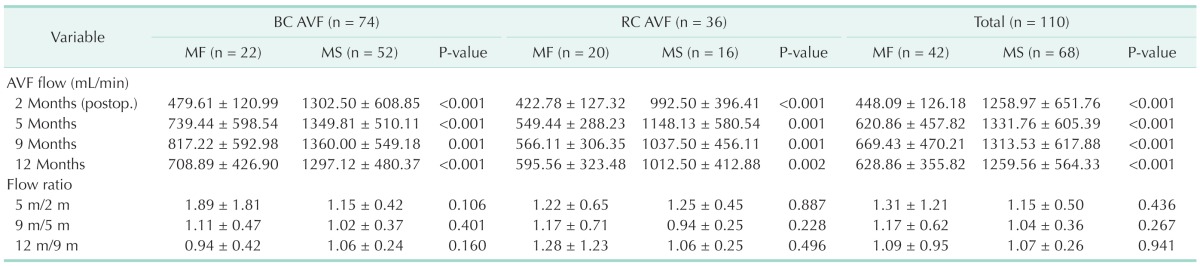
The AVF flows of MF group were significantly less than that of MS group respectively at 2, 5, 9, and 12 months after AVF creation (P < 0.05) (Table 3). And, AVF flows of MF group were also significantly less than those of MS group after AVF creation in BC AVF and RC AVF groups (P < 0.05) (Table 3).
Table 3. Arteriovenous fistula flow during 1 year after creation.
Values are presented as mean ± standard deviation.
BC, brachiocephalic; AVF, arteriovenous fistula; RC, radiocephalic; MF, maturation failure; MS, maturation success.
In MF groups, AVF flow (mL/min) before BA for BAM was 448.09 ± 126.18, and AVF flows after BA for BAM were 620.86 ± 457.82, 669.43 ± 470.21, 628.86 ± 355.82 at 3, 7, and 10 months, respectively in total AVF groups (Table 3). Also, in BC AVF and RC AVF groups, AVF flows before BAs for BAM were less than 600 mL/min and those after BAs for BAM were more than 600 mL/min in MF group (Table 3). In total AVF groups, AVF flow ratios were 1.31 ± 1.21 vs. 1.15 ± 0.50, 1.17 ± 0.62 vs. 1.04 ± 0.36, 1.09 ± 0.95 vs. 1.07 ± 0.26 between MF and MS groups at 5 months by 2 months, 9 months by 5 months, 12 months by 9 months, respectively, and AVF flow ratio was insignificant between MF and MS groups (P > 0.05) (Table 3). In BC AVF and RC AVF groups, AVF flow ratios also showed similar aspects with those in total AVF groups (Table 3).
DISCUSSION
Since the implementation of NKF-DOQI recommendations in 1997, more patients have undergone creation of AVFs as their primary access of HD [8,9,10]. Although these recommendations have identified AVF as the superior method of vascular access, it is not flawless [2,8]. Primary AVF maturation rates within the recommended 4–6 weeks, without assistance, have been reported as low as 23%–53% [2,8,11,12]. While the exact mechanism of MF is unclear, advancements in assisted maturation techniques and an understanding of the underlying physiology in AVF development will play a role in improved AVF maturation and survival [8]. But, BAM continues to be a controversial method for improving and expediting development of AVF maturation [2]. Roy-Chaudhury et al. [13] attribute AVF failure to the use of angioplasty, by causing significant endothelial and smooth muscle cell injury, thus promoting smooth muscle cell activation, increased cytokine activation, and promoting neo-intimal hyperplasia, medial hypertrophy, and vascular remodeling. In contrast, De Marco Garcia et al. [14] concluded that focal angioplasty injury to the venous endothelium helps the venous wall reorganize into a fibrous conduit based on large diameter segments with smooth lining on postprocedural imaging. And, a few studies have reported evaluating the usefulness of BAMs in an effort to meet the growing need for AVF within the NKF-DOQI guidelines [2]. The BAM technique addresses the issues related to poor function in addition to facilitating diameter maturation by combining angioplasty, healing, and AVF remodeling into a sequential process [15]. BAM focuses on dilating the usable segment of the AVF to a sufficiently large diameter, thereby facilitating cannulation [15]. Each sequential dilatation increases the vein diameter by 2 to 4 mm, and they are performed 2 to 4 weeks apart to allow for healing [15]. The NKF-DOQI currently classifies more likely maturation as an AVF that, within 6 weeks of creation, has a blood flow greater than 600 mL/min, depth less than 6 mm, and minimum diameter of 6 mm [11]. Miller et al. [4] reported a case series of staged BA maturation with secondary patency at 12 months as high as 77%. Similarly, De Marco Garcia et al. [14] reported a case series involving serial BAMs along with primary angioplasty of the vein before AVF creation. A successful AVF was established in 85.4% of patients, wherein success was defined as the ability to use the AVF for HD without revision for 90 days [2,14].
In our study, we defined AVF MFs as AVFs with physical examination findings or duplex ultrasonography findings of nonmaturation at 4 to 6 weeks after creation or AVFs with access flow less than 600 mL/min at trial cannulation at 8 weeks after creation [2,11,16]. We checked AVF flows with a transonic flowmeter (HD03) with trial cannulation from 8 weeks after creation instead of a duplex ultrasonography [11]. We also checked at least every 3 months. We believe that a merit of a transonic flowmeter is that we can frequently check AVF flow at a low cost when an HD will be done in a patient.
The KDOQI guidelines recommend that prompt vascular interventions, such as BA and BCVL, should be performed if the AVF fails to mature by 6 weeks after creation [6]. Also, if the AVF failed to mature by 6 weeks after creation, prompt interventions, such as percutaneous transluminal angioplasty and accessory vein ligation, were recommended at 6 to 8 weeks after creation in the literature [8,15,16]. So, if AVF failed to mature by 6 weeks after creation, we performed vascular interventions for all AVF MFs at 6 to 8 weeks after creation.
In our result, the success rate (46.2%) of BAM was lower than that (>80%) in the literature [14,17,18]. We believe that the first reason was that cutoff values of access flow (<600 mL/min) might be higher than that in the literature [11]. So, if cutoff values of access flow were <400 mL/min, the success rate of BAM might be >80%. The second reason was that we followed up every 2 weeks after BA, but additional BA was inapplicable in many patients because of cost and permission of patient.
Until now, definite criteria of access flow for maturation or intervention in AVF have not been as well established [11]. But, a study found that combining venous diameter (>0.4 cm) and flow volume (>500 mL/min) at 1 month after AVF creation increased the predictive power of adequate fistula maturation to 95% [11]. Fistulae maintain patency at lower flows than grafts but access flows less than 350 mL/min are likely to produce recirculation and inadequate delivery of dialysis [6,11]. So, values of 400 to 650 mL/min have been proposed [6,11]. Higher values increase sensitivity, but lose specificity [11]. Some fistulae can maintain patency for years at flows less than 400 mL/min, but with high-efficiency/high-flux dialysis, the treatment time requires extension [11]. We therefore need to confirm adequate criteria of access flow for maturation or intervention in AVF. Thus, we evaluated and suggested criteria of access flow for maturation as 600 mL/min.
The complication rate (21.4%) was very high. We think that the reason was technical problems during the early period. Most complications occurred during the beginning period. Nowadays, we have few complications related with BAs for BAM. We believe further effort is required. However, we feel that the timing of BA for BAM was appropriate according to the literature [6,8,15,16].
In our results, AVF flows of MS group were significantly larger than those of MF group (P < 0.05). Yet, both additional BA duration after AVF maturation and AVF flow ratio during follow-up period were insignificant between MF and MS groups (P > 0.05). We suggest that BAM is an effective salvage management for AVF MF.
All newly created AVFs must be physically examined by using a thorough systemic approach by a knowledgeable professional 4 to 6 weeks postoperatively to ensure appropriate maturation for cannulation [11]. If an AVF fails to mature by 6 weeks, a fistulogram or other imaging study should be obtained to determine the cause of the problem [11]. Then, prompt correction, such as BAM or ligation of side branches, should be undertaken [11].
In conclusion, although larger studies and prospective trials are necessary to confirm the elements of MS and the efficacy of BAM, BA for AVF MF is a relatively applicable and effective modality and, we suggest BAM as an effective salvage management for AVF MF.
ACKNOWLEDGEMENTS
This article has been presented with oral presentation at the 67th Congress of the Korean Surgical Society in Seoul, Korea from 5-7 November 2015.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Powell S, Chan T. Endovascular techniques for cannulation difficulties in dialysis access. J Vasc Access. 2014;15(Suppl 7):S96–S100. doi: 10.5301/jva.5000254. [DOI] [PubMed] [Google Scholar]
- 2.DerDerian T, Hingorani A, Ascher E, Marks N, Jimenez R, Aboian E, et al. To BAM or not to BAM?: a closer look at balloon-assisted maturation. Ann Vasc Surg. 2013;27:104–109. doi: 10.1016/j.avsg.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Samett EJ, Hastie J, Chopra PR, Pradhan S, Ahmad I, Chiramel T, et al. Augmented balloon-assisted maturation (aBAM) for nonmaturing dialysis arteriovenous fistula. J Vasc Access. 2011;12:9–12. doi: 10.5301/jva.2010.6018. [DOI] [PubMed] [Google Scholar]
- 4.Miller GA, Goel N, Khariton A, Friedman A, Savransky Y, Trusov I, et al. Aggressive approach to salvage non-maturing arteriovenous fistulae: a retrospective study with follow-up. J Vasc Access. 2009;10:183–191. doi: 10.1177/112972980901000309. [DOI] [PubMed] [Google Scholar]
- 5.Nassar GM. Endovascular management of the "failing to mature" arteriovenous fistula. Tech Vasc Interv Radiol. 2008;11:175–180. doi: 10.1053/j.tvir.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S248–S273. doi: 10.1053/j.ajkd.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 7.Ascher E, Hingorani A, Marks N. Duplex-guided balloon angioplasty of failing or nonmaturing arterio-venous fistulae for hemodialysis: a new office-based procedure. J Vasc Surg. 2009;50:594–599. doi: 10.1016/j.jvs.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 8.DerDerian T, Hingorani A, Boniviscage P, Carollo A, Ascher E. Acute complications after balloon-assisted maturation. Ann Vasc Surg. 2014;28:1275–1279. doi: 10.1016/j.avsg.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Ascher E, Gade P, Hingorani A, Mazzariol F, Gunduz Y, Fodera M, et al. Changes in the practice of angioaccess surgery: impact of dialysis outcome and quality initiative recommendations. J Vasc Surg. 2000;31(1 Pt 1):84–92. doi: 10.1016/s0741-5214(00)70070-x. [DOI] [PubMed] [Google Scholar]
- 10.Song D, Moon C. Arteriovenous fistula creation through basilic vein transposition in an upper extremity. J Korean Surg Soc. 2008;74:286–291. [Google Scholar]
- 11.Vascular Access 2006 Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.McLafferty RB. Techniques to enhance arteriovenous fistula maturation. Perspect Vasc Surg Endovasc Ther. 2009;21:41–45. doi: 10.1177/1531003509333229. [DOI] [PubMed] [Google Scholar]
- 13.Roy-Chaudhury P, Spergel LM, Besarab A, Asif A, Ravani P. Biology of arteriovenous fistula failure. J Nephrol. 2007;20:150–163. [PubMed] [Google Scholar]
- 14.De Marco Garcia LP, Davila-Santini LR, Feng Q, Calderin J, Krishnasastry KV, Panetta TF. Primary balloon angioplasty plus balloon angioplasty maturation to upgrade small-caliber veins (<3 mm) for arteriovenous fistulas. J Vasc Surg. 2010;52:139–144. doi: 10.1016/j.jvs.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Chawla A, DiRaimo R, Panetta TF. Balloon angioplasty to facilitate autogenous arteriovenous access maturation: a new paradigm for upgrading small-caliber veins, improved function, and surveillance. Semin Vasc Surg. 2011;24:82–88. doi: 10.1053/j.semvascsurg.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Kim JK, Jeong JH, Song YR, Kim HJ, Lee WY, Kim KI, et al. Obesity-related decrease in intraoperative blood flow is associated with maturation failure of radiocephalic arteriovenous fistula. J Vasc Surg. 2015;62:1010–1017.e1. doi: 10.1016/j.jvs.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Voormolen EH, Jahrome AK, Bartels LW, Moll FL, Mali WP, Blankestijn PJ. Nonmaturation of arm arteriovenous fistulas for hemodialysis access: a systematic review of risk factors and results of early treatment. J Vasc Surg. 2009;49:1325–1336. doi: 10.1016/j.jvs.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 18.Nikolic B. Hemodialysis fistula interventions: diagnostic and treatment challenges and technical considerations. Tech Vasc Interv Radiol. 2008;11:167–174. doi: 10.1053/j.tvir.2008.09.003. [DOI] [PubMed] [Google Scholar]