Abstract
The debate on how best to manage patients with metal-on-metal (MOM) hip implants continues. With over 1 million patients affected worldwide, the impact is far reaching. The majority of the aggressive failures of MOM hip implants have been dealt with by revision hip surgery, leaving patients with a much more indolent pattern of failure of devices that have been in situ for more than 10 years. The longer-term outcome for such patients remains unknown, and much debate exists on how best to manage these patients. Regulatory guidance is available but remains open to interpretation due to the lack of current evidence and long-term studies. Metal ion thresholds for concern have been suggested at 7 ppb for hip resurfacing arthroplasty and below this level for large diameter total hip arthroplasties. Soft tissue changes including pseudotumours and muscle atrophy have been shown to progress, but this is not consistent. New advanced imaging techniques are helping to diagnose complications with metal hips and the reasons for failure, however these are not widely available. This has led to some centres to tackle difficult cases through multidisciplinary collaboration, for both surgical management decisions and also follow-up decisions. We summarise current evidence and consider who is at risk, when revision should be undertaken and how patients should be managed.
Keywords: Metal on metal hip, Management, Multi-disciplinary, Revision, Decision
Core tip: Evidence supporting the management of metal on metal hips is lacking, and guidance is open to interpretation. Until supporting evidence is available, an evidence based multi-disciplinary approach on a case-by-case basis is considered a safe method to help surgeons make decisions and potentially improve patient outcomes.
INTRODUCTION
Considerable debate continues to surround the use and management of patients with failing metal-on-metal (MOM) hip implants. Over a million patients worldwide have been implanted with a MOM device[1], and according to the United Kingdom National Joint Registry (NJR), their use peaked in 2006 [hip resurfacing arthroplasty (HRA)] and 2008 [large diameter total hip replacement (LDTHR)][2]. However, due to several concerns of catastrophic soft tissue reactions leading to early failures and associated complications, medical device alerts were published[3], and MOM hips were subsequently withdrawn from use by the British Hip Society in 2012.
It is clear that there are evolving and changing patterns of behaviour in the failure of MOM hips[4]. Many of the early, aggressive failures have been dealt with by revision hip surgery, and we now see a much more indolent pattern of failure in patients who have had devices in situ for more than 10 years.
This spectrum of patients from the well functioning, that require only monitoring, to the poorly functioning, which require revision continues to evoke debate among surgeons, especially since the bulk of patients fall between these two extremes Figures 1 and 2. Uncertainties surround thresholds for investigation, revision surgery and methods for surveillance[5]. Guidance from international regulatory agencies exists, but tend to reflect the needs of local health authorities, which accounts for some of the variation seen in the guidance[3,6,7].
Figure 1.

Diagram demonstrating the spectrum of concern for patients with metal-on-meta hip implants. The decision on how to manage patients at the extremes of the spectrum is relatively straightforward. However the majority of patients fall into to the middle category, where the management is uncertain or difficult. MRI: Magnetic resonance imaging; MARS: Metal artefact reduction sequence.
Figure 2.
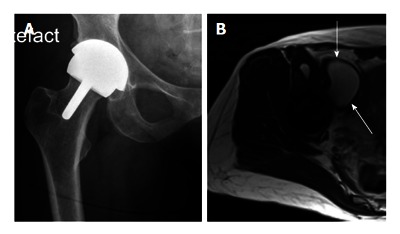
Middle of the spectrum - A typical patient with moderate problems. This 58-year-old very active lady with a right hip resurfacing arthroplasty (A: X-ray AP hip) implanted 8 years ago. She has minimal symptoms and moderately raised blood metal ion levels (Cobalt 13 ppb, Chromium 7 ppb). A magnetic resonance imaging scan (B: Axial T2 weighted image) has revealed a 6 cm cystic pseudotumour anterior to the hip (arrows).
This review examines the literature on current clinical dilemmas facing surgeons and their patients with MOM hip replacements, and summarises current clinical guidance for how and when patients should be managed.
MOM hip implants
MOM hip implants consist of two broad types, the HRA and the LDTHR. Since their inception in 1937[4], they have gone through several key design changes and modifications, with the expected fluctuations in their use. Their use flourished in the 1990s with the introduction of the modern HRA and subsequently accounted for approximately a third of all hip replacements being implanted in the United States in 2008[1].
The proposed benefits for using MOM bearings were to reduce the occurrence of polyethylene disease (aseptic loosening) and to allow the use of large diameter femoral head components to reduce the occurrence of hip dislocation[4]. However, the inception of highly cross-linked polyethylene and improved ceramic bearing design, have diminished the perceived advantages of MOM over other bearing surfaces[8,9].
Besides this, metal debris and corrosion products have led to inflammatory reactions within the soft tissues surrounding MOM hip implants and subsequently their early failure and need for revision[10-13]. This has led to the subsequent fall in use of MOM hips and intervention from regulators[3].
Metal debris - A cause for concern?
Metal implants are considered biologically inert, however wear debris is not and is thought to evoke an immune response[14]. The release of material from metallic implants occurs by wear, corrosion and mechanical factors such as fretting and third body wear. Cobalt and chromium are the major constituents of alloy metal implants, and are the main cause for concern.
Metal particulate and ionic wear debris from the hip is released into the peri-prosthetic tissues and transported systemically throughout the body[15,16]. Studies have demonstrated a peak in blood cobalt levels at 6-mo post implantation and chromium levels at 9-mo, followed by a steady decline over time[17,18]. Following revision of a MOM implant to an alternative bearing, blood ion levels reduce but do not normalise in the post-operative period[19,20].
Component design and positioning has been shown to be associated with increased wear and as a result raised metal ion levels[21-25]. Blood cobalt and chromium ion levels in patients with unexplained painful MOM hips are double those of well-functioning MOM hips[13].
Wear debris can accumulate locally as seen by studies of joint fluid surrounding MOM hip implants[26-28]. The level of chromium is greater in joint fluid compared to cobalt, whereas the converse is true for blood analysis[28]. However it is believed that cobalt is the species with greatest reactivity causing local tissue inflammatory reactions due to its ready solubility[29-31].
WHO IS AT RISK?
Local soft tissue reactions
Pseudotumours are well described in patients with MOM hip implants, and can be either solid or cystic. Reported prevalence in both symptomatic and asymptomatic patients ranges from 0.1% to 69%[10,11,32-37]. The precise aetiology is not known, however the term aseptic lymphocytic vasculitis-associated lesion (ALVAL) is used to describe the histological features associated with metal hips[38]. It has been suggested that a delayed type IV hypersensitivity reaction to metal ions is the potential cause, however this has been challenged[12]. Pseudotumours were, however, shown to correlate with elevated blood and hip aspirate metal ion levels suggesting a relation to excessive implant wear[12,39].
Recent evidence regarding the natural history of soft tissues abnormalities is conflicting. Studies report varying degrees of progression in size and grade of pseudotumours, however limitations in sample size, implant type and imaging modality do not readily allow the generalisability of the results[40-42]. It appears that when disease progression does occur, it is slow and therefore serial imaging annually is sufficient to identify change. The potential to cause local pressure effects causing necrosis and compression of nearby structures such as the iliac vessels, femoral vessels and the sciatic nerve is also a concern.
Muscle atrophy is now becoming an increasing concern, and is driving the debate regarding the timing of revision surgery in order to prevent irreversible damage. A recent publication demonstrated progressive muscle atrophy using serial magnetic resonance imaging (MRI) scanning in a mixed cohort of patients[43].
Systemic effects
Several cases of systemic effects from metal hip implants have been reported, including cardiac, endocrine, neurological and dermatological complications, however this remains a relatively rare occurrence[44]. There is a mixture of cases reported in both fractured ceramic hips and in primary MOM hip patients[44]. Removal of the implant led to reduced metal ion levels and symptomatic improvement in several of these cases. Additionally, chronic low dose exposure over several years revealed a negative effect on cardiac function and bone density[45], however these were subtle and sub-clinical. Recent cases of cardiac toxicity have been further highlighted and novel diagnostic techniques are being explored[46,47].
MANAGEMENT - WHEN SHOULD PATIENTS BE REVISED?
The local and systemic effects of metal particulate and ionic debris from MOM hips have led to increased rates of revision hip surgery. It has also led to significant levels of patient anxiety, not to mention the physical and financial burden of a failed metal implant on the patient and the health services.
The British Hip Society was the first to publish their guidance through the Medicines and Healthcare Products Regulatory Agency (MHRA)[3]. The MOM task force (American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and The Hip Society) in the United States[48], the European Hip Society (EHS) (2012)[6], and most recently the European Commission’s Scientific Committee on Emerging and Newly Identified Health Risks, have also published guidance for surgeons[49]. However, uncertainties remain over decision making because of the difficulty - for any guideline - to define or quantify clinical symptoms, imaging findings and clinically important thresholds for blood metal ion results.
Role of metal ions
The MHRA currently recommends 7 ppb as the threshold for concern beyond which further investigations are recommended to diagnose complications associated with MOM hip implants. The Food and Drug Administration (FDA) does not currently set an action level, and SCENIHR acknowledge that the level for concern lies between 2-7 ppb based on questions raised regarding the current available evidence.
The population background level of cobalt in blood has been shown to be 0.5 ppb. There is a correlation seen with wear rates, where 2 ppb can be expected with wear rates of 2 cubic mm per year[50,51].
Since the 7 ppb level was derived from research based on hip resurfacings[51], it has been postulated that this may not apply to stemmed implants. A study including a variety of implant types demonstrated improved sensitivity and specificity with a threshold cobalt level of 4.5 ppb[50].
Various groups have argued for a blood metal ion threshold for revision. The prevalence of patients with blood metal ion levels over 25 ppb was 2.6% in HRA patients, and 3.1% in total Hip Replacement (THR) patients[52]. The sensitivity and specificity of the 7 ppb cut-off level have been reported to be 52% and 89%, respectively, indicating that the 7 ppb has relative poor ability to identify MOM failures. The lowering of the cut-off level to 5 ppb increases the sensitivity to 63% and lowers specificity to 86%[51]. The Finland group demonstrated that 25 ppb was 99% specific compared to 93% specificity at 7 ppb, however more notably revised patients with metal ions over 25 ppb had a significantly lower oxford hip score 12 mo after revision compared to those with ions less than 25 ppb. The re-revision rate was also higher in those patients with metal ions over 25 ppb[52].
Based on current literature 7 ppb remains a safe level for concern in patients with a HRA implant, whereas the presence of a taper (LDTHR) would prompt a lower threshold for concern.
Role of diagnostic imaging
The MHRA advise metal artefact reduction sequence MRI (MARS MRI) or ultrasound scan as part of the investigation algorithm[3]. MARS MRI appears more appropriate, due to excellent sensitivity and specificity for detection of both superficial and deep lesions[53-55], and also muscle atrophy. Ultrasound is a satisfactory modality for identifying tendon abnormalities[54]. Current MARS MRI techniques do suffer from metal artefact that limits the diagnosis of osteolysis, however, improved techniques are being developed[56]. Currently computed tomography (CT) scanning is ideal for visualising osteolysis if it is suspected on plain radiographs[57].
In patients where the cause of pain is unexplained, single-photon emission CT (SPECT-CT) has been recommended[58]. SPECT-CT was shown to be clinically valuable in diagnosing the cause of pain and influenced management decisions in over half of patients with unexplained pain following a MOM hip arthroplasty despite inconclusive conventional investigations.
Pseudotumours
There is a lack of evidence surrounding the need for revision secondary to pseudotumours, particularly regarding the outcome following revision surgery and the long-term natural history of pseudotumours. This is reflected in the current guidance by the limited detail in how to interpret MRI findings.
It has been shown that revision for pseudotumour is associated with significant post-operative complications[59]. In addition, recurrence after revision with excision is possible and may be as high as 30%. If pseudotumours, cystic or solid, are large enough to cause pressure necrosis or stretch of soft tissues, then this is usually an indication for revision surgery (Figure 3).
Figure 3.
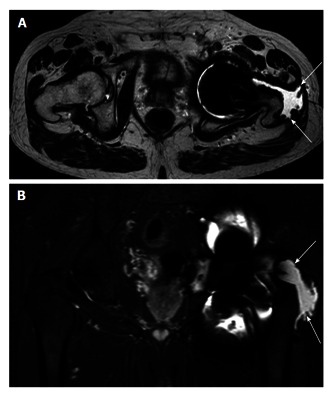
Axial (A) and coronal (B) magnetic resonance imaging. Example of abductor stripping secondary to a pseudotumour (marked by arrows). The pseudotumour can be seen traversing the posterior hip around the greater tuberosity onto its lateral aspect, which is now void of abductor tendon insertion.
Large pseudotumours with intra-pelvic extensions along the psoas sheath or arising wholly within the pelvis are of particular concern. These have the potential for compression of neurovascular structures including the iliac vessels. In addition, surgical excision becomes more difficult and often a multi-disciplinary surgical approach with vascular surgeons is required (Figure 4).
Figure 4.
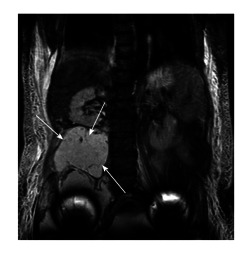
A 58-year-old patient with bilateral large diameter total hip replacement metal-on-meta implanted 9-years ago, moderate hip symptoms and raised metal ion levels (Cobalt 17 ppb, Chromium 13 ppb). She presented to the general surgeons with abdominal pain and distension. Coronal magnetic resonance imaging scan (above) demonstrated a large cystic pseudotumour extending into the pelvis up to the level of the L2 vertebra and abutting the right kidney in the retroperitoneal space (arrows). The cystic pseudotumour was drained prior to surgical excision with both orthopaedic and vascular surgeons present.
Osteolysis
Osteolysis surrounding MOM hip implants is a further concern that needs to be addressed[60], and one should be vigilant in the presence of very high metal ions. Progressive osteolysis may be an indication for early intervention if the potential for peri-prosthetic fracture is apparent.
Muscle atrophy
There is growing evidence supporting early revision to a non-MOM hip implant to prevent irreversible damage[38,61]. Campbell et al[62] observed that patients can expect a good outcome if their soft tissues remain intact. A recent study demonstrated progressive muscle atrophy over a period of 12 mo using serial MRI, and noted an association with high metal ion levels[43]. Liddle et al[63] highlight the degree of misdiagnosis possible when planning for revision of MOM hip implants. They describe that pre-operative imaging can underestimate the degree of soft tissue abnormalities seen at revision surgery including a high rate of severe abductor muscle atrophy and stripping of the tendinous attachment[63]. If progressive and destructive soft tissue change is possible, predicting those patients that are likely to fail is paramount so that revision can be undertaken early to ensure a better outcome (Figure 3).
Broadly however, a decision to revise should not be based on a single investigation, instead the decision should take into account patient symptoms, activity level, implant type, metal ion levels and imaging findings.
WHEN SHOULD PATIENTS BE FOLLOWED UP?
Current guidance stratifies patients by risk depending on the type of implant they have in situ. Small diameter THR and hip resurfacing arthroplasty is considered low risk, where as the large diameter THR and the DePuy ASR implants are considered high risk[3]. A recent publication went one step further and stratified all current generation MOM hip implants into low, medium and high risk categories[5], based on registry and regulatory advice. More recently however the Regulators state that low risk implants that are functioning well should be monitored according to local hospital protocols, whereas high-risk implants require follow-up for the life of the implant. The Birmingham Hip Resurfacing (Smith and Nephew, London, United Kingdom) has been the best performing hip resurfacing, however concerns have always existed regarding their use in female patients, and patients with small diameter femoral heads (< 48 mm). As a result of these concerns the MHRA have released further guidance advising against their use in this population and additional advice on the management of patients with these implants in situ[64].
However the majority of guidelines do not offer detail on what constitutes follow up, and more importantly which patients require more frequent monitoring. A pragmatic approach would be to take this on a case-by-case basis where the frequency of follow up needs to be tailored to the individual based on the implant risk stratification and the patients clinical status.
Based on the literature, with particular reference to the natural history of soft tissue changes, annual follow up would suffice for those with a medium to high risk implant. Follow up should consist of a history, clinical examination, functional scoring, blood metal ions measurement and X-ray. If clinical concern exists then cross sectional imaging with MARS MRI would be indicated. For low risk implants in individuals with a low risk profile, then less intensive follow-up would be indicated, such as annual questionnaires and 5-yearly clinical review.
One must be mindful of applying a simplistic approach based on implant risk stratification alone, since certain aspects of the patients clinical and surgical history would suggest a heightened risk even in the best performing hip implants. Low risk implants in patients with hip symptoms, evidence of soft tissue abnormality or high metal ions would require closer monitoring. In addition, excessive acetabular cup inclination can lead to edge loading and early failure[65,66], and also female patients with small femoral head size hip resurfacing arthroplasties and females with primary hip dysplasia have worse long term outcomes[48].
HOW - MULTI-DISCIPLINARY TEAM APPROACH
Some clinical cases are straightforward and decision-making is relatively easy. However, in many instances surgeons experience considerable uncertainty in decision-making because of the lack of guidelines or the difficulty in applying guidelines in complex cases. This gap has led to the use of a multidisciplinary teams (MDT) approach to help interpret the guidance published by the regulatory agencies, with the aim of using surgical experience, tacit knowledge, and evidence-based current best practice to reduce the uncertainty surrounding the management of patients with MOM hip implants[5].
This highlights the need for a more collaborative approach between surgeons, regulators and industry representatives to improve the available evidence and the guidance offered to aid the management of patients with MOM hip implants.
Role of retrievals
In a recent commentary by Jacobs et al[67], the importance of implant retrieval analysis by centres with access to large retrieval cohorts was emphasized as significant in understanding mechanisms of failure and also for developing future preclinical testing models. This reflects the number of developments established through retrieval analysis and includes the relationship with cup position and edge loading[68], the correlation of wear rates with blood metal ion levels[66] and the role of frictional torque and fretting currents in LDTHR[69].
CONCLUSION
The management of patients with MOM hip implants continues to cause concern and difficulties for patients and surgeons alike. The evidence is lacking in certain scenarios, and regulatory guidance can be interpreted differently. When considering which patient requires revision, no single investigation or aspect of the history should be taken in isolation. Decisions should be taken on a case-by-case basis, with consideration given to all aspects of the patient’s clinical history and investigation results. A multi-disciplinary approach with shared decision-making, tacit knowledge and surgical experience appears to be a safe and practical approach to improving patient’s outcomes.
Footnotes
Conflict-of-interest statement: The authors declare that they no conflict of interest; Skinner JA and Hart AJ have a consultancy agreement with Depuy and Stryker for metal on metal hip retrieval work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 29, 2015
First decision: September 30, 2015
Article in press: March 9, 2016
P- Reviewer: Agilli M, Luo XH S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
References
- 1.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 2.Borroff M, Green M, Gregg P, MacGregor A, Porter M, Tucker K, Wishart N. 11th Annual Report 2014 - National Joint Registry for England, Wales and Northern Ireland. 2014. [Google Scholar]
- 3.Ludgate S, Haque F, Cacou C. Medical Device Alert: All metalon-on-metal (MoM) hip replacements. [Updated 2012 Aug 9] Available from: http//www.mhra.gov.uk/home/groups/dtsbs/documents/medicaldevicealert/con155767.pdf.
- 4.Haddad FS, Thakrar RR, Hart AJ, Skinner JA, Nargol AV, Nolan JF, Gill HS, Murray DW, Blom AW, Case CP. Metal-on-metal bearings: the evidence so far. J Bone Joint Surg Br. 2011;93:572–579. doi: 10.1302/0301-620X.93B4.26429. [DOI] [PubMed] [Google Scholar]
- 5.Berber R, Pappas Y, Khoo M, Miles J, Carrington R, Skinner J, Hart A. A new approach to managing patients with problematic metal hip implants: the use of an Internet-enhanced multidisciplinary team meeting: AAOS exhibit selection. J Bone Joint Surg Am. 2015;97:e20. [PubMed] [Google Scholar]
- 6.Hannemann F, Hartmann A, Schmitt J, Lützner J, Seidler A, Campbell P, Delaunay CP, Drexler H, Ettema HB, García-Cimbrelo E, et al. European multidisciplinary consensus statement on the use and monitoring of metal-on-metal bearings for total hip replacement and hip resurfacing. Orthop Traumatol Surg Res. 2013;99:263–271. doi: 10.1016/j.otsr.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Food and Drug Administration U. Metal-on-Metal Hip Implants - Advice for Surgeons. [Updated 2014 May 10] Available from: http//www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241667.htm. [Google Scholar]
- 8.Hart AJ, Sabah SA, Henckel J, Lloyd G, Skinner JA. Lessons learnt from metal-on-metal hip arthroplasties will lead to safer innovation for all medical devices. Hip Int. 2015;25:347–354. doi: 10.5301/hipint.5000275. [DOI] [PubMed] [Google Scholar]
- 9.Graves S, Davidson D, de Steiger R, Lewis P, Stoney J. Annual Report 2014 - Australian orthopaedic association national joint replacement registry. 2014. [Google Scholar]
- 10.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 11.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;26:511–518. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Kwon YM, Thomas P, Summer B, Pandit H, Taylor A, Beard D, Murray DW, Gill HS. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28:444–450. doi: 10.1002/jor.21015. [DOI] [PubMed] [Google Scholar]
- 13.Hart AJ, Sabah S, Henckel J, Lewis A, Cobb J, Sampson B, Mitchell A, Skinner JA. The painful metal-on-metal hip resurfacing. J Bone Joint Surg Br. 2009;91:738–744. doi: 10.1302/0301-620X.91B6.21682. [DOI] [PubMed] [Google Scholar]
- 14.Wooley PH, Nasser S, Fitzgerald RH. The immune response to implant materials in humans. Clin Orthop Relat Res. 1996;(326):63–70. doi: 10.1097/00003086-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, Solomon L. Widespread dissemination of metal debris from implants. J Bone Joint Surg Br. 1994;76:701–712. [PubMed] [Google Scholar]
- 16.Merritt K, Brown SA. Release of hexavalent chromium from corrosion of stainless steel and cobalt-chromium alloys. J Biomed Mater Res. 1995;29:627–633. doi: 10.1002/jbm.820290510. [DOI] [PubMed] [Google Scholar]
- 17.Daniel J, Ziaee H, Pradhan C, McMinn DJ. Six-year results of a prospective study of metal ion levels in young patients with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2009;91:176–179. doi: 10.1302/0301-620X.91B2.21654. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JJ, Skipor AK, Campbell PA, Hallab NJ, Urban RM, Amstutz HC. Can metal levels be used to monitor metal-on-metal hip arthroplasties? J Arthroplasty. 2004;19:59–65. doi: 10.1016/j.arth.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Ball ST, Severns D, Linn M, Meyer RS, Swenson FC. What happens to serum metal ion levels after a metal-on-metal bearing is removed? J Arthroplasty. 2013;28:53–55. doi: 10.1016/j.arth.2013.06.040. [DOI] [PubMed] [Google Scholar]
- 20.Ebreo D, Khan A, El-Meligy M, Armstrong C, Peter V. Metal ion levels decrease after revision for metallosis arising from large-diameter metal-on-metal hip arthroplasty. Acta Orthop Belg. 2011;77:777–781. [PubMed] [Google Scholar]
- 21.De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 22.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, Nargol AV. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91:1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 23.Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90:1143–1151. doi: 10.1302/0301-620X.90B9.20785. [DOI] [PubMed] [Google Scholar]
- 24.Hart AJ, Skinner JA, Henckel J, Sampson B, Gordon F. Insufficient acetabular version increases blood metal ion levels after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2011;469:2590–2597. doi: 10.1007/s11999-011-1930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthies AK, Henckel J, Cro S, Suarez A, Noble PC, Skinner J, Hart AJ. Predicting wear and blood metal ion levels in metal-on-metal hip resurfacing. J Orthop Res. 2014;32:167–174. doi: 10.1002/jor.22459. [DOI] [PubMed] [Google Scholar]
- 26.De Smet K, De Haan R, Calistri A, Campbell PA, Ebramzadeh E, Pattyn C, Gill HS. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am. 2008;90 Suppl 4:202–208. doi: 10.2106/JBJS.H.00672. [DOI] [PubMed] [Google Scholar]
- 27.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J Bone Joint Surg Br. 2010;92:38–46. doi: 10.1302/0301-620X.92B1.22770. [DOI] [PubMed] [Google Scholar]
- 28.Davda K, Lali FV, Sampson B, Skinner JA, Hart AJ. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J Bone Joint Surg Br. 2011;93:738–745. doi: 10.1302/0301-620X.93B6.25804. [DOI] [PubMed] [Google Scholar]
- 29.Hart AJ, Quinn PD, Lali F, Sampson B, Skinner JA, Powell JJ, Nolan J, Tucker K, Donell S, Flanagan A, et al. Cobalt from metal-on-metal hip replacements may be the clinically relevant active agent responsible for periprosthetic tissue reactions. Acta Biomater. 2012;8:3865–3873. doi: 10.1016/j.actbio.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Hart AJ, Quinn PD, Sampson B, Sandison A, Atkinson KD, Skinner JA, Powell JJ, Mosselmans JF. The chemical form of metallic debris in tissues surrounding metal-on-metal hips with unexplained failure. Acta Biomater. 2010;6:4439–4446. doi: 10.1016/j.actbio.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Goode AE, Perkins JM, Sandison A, Karunakaran C, Cheng H, Wall D, Skinner JA, Hart AJ, Porter AE, McComb DW, et al. Chemical speciation of nanoparticles surrounding metal-on-metal hips. Chem Commun (Camb) 2012;48:8335–8337. doi: 10.1039/c2cc33016d. [DOI] [PubMed] [Google Scholar]
- 32.Canadian Hip Resurfacing Study Group. A survey on the prevalence of pseudotumors with metal-on-metal hip resurfacing in Canadian academic centers. J Bone Joint Surg Am. 2011;93 Suppl 2:118–121. doi: 10.2106/JBJS.J.01848. [DOI] [PubMed] [Google Scholar]
- 33.Sabah SA, Mitchell AW, Henckel J, Sandison A, Skinner JA, Hart AJ. Magnetic resonance imaging findings in painful metal-on-metal hips: a prospective study. J Arthroplasty. 2011;26:71–76, 76.e1-2. doi: 10.1016/j.arth.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;93:2164–2171. doi: 10.2106/JBJS.J.01884. [DOI] [PubMed] [Google Scholar]
- 35.Matthies AK, Skinner JA, Osmani H, Henckel J, Hart AJ. Pseudotumors are common in well-positioned low-wearing metal-on-metal hips. Clin Orthop Relat Res. 2012;470:1895–1906. doi: 10.1007/s11999-011-2201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, Cobb JP, Skinner JA, Mitchell AW. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94:317–325. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 37.Chang EY, McAnally JL, Van Horne JR, Statum S, Wolfson T, Gamst A, Chung CB. Metal-on-metal total hip arthroplasty: do symptoms correlate with MR imaging findings? Radiology. 2012;265:848–857. doi: 10.1148/radiol.12120852. [DOI] [PubMed] [Google Scholar]
- 38.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 39.Bosker BH, Ettema HB, Boomsma MF, Kollen BJ, Maas M, Verheyen CC. High incidence of pseudotumour formation after large-diameter metal-on-metal total hip replacement: a prospective cohort study. J Bone Joint Surg Br. 2012;94:755–761. doi: 10.1302/0301-620X.94B6.28373. [DOI] [PubMed] [Google Scholar]
- 40.Almousa SA, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. The natural history of inflammatory pseudotumors in asymptomatic patients after metal-on-metal hip arthroplasty. Clin Orthop Relat Res. 2013;471:3814–3821. doi: 10.1007/s11999-013-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebreo D, Bell PJ, Arshad H, Donell ST, Toms A, Nolan JF. Serial magnetic resonance imaging of metal-on-metal total hip replacements. Follow-up of a cohort of 28 mm Ultima TPS THRs. Bone Joint J. 2013;95-B:1035–1039. doi: 10.1302/0301-620X.95B8.31377. [DOI] [PubMed] [Google Scholar]
- 42.van der Weegen W, Brakel K, Horn RJ, Hoekstra HJ, Sijbesma T, Pilot P, Nelissen RG. Asymptomatic pseudotumours after metal-on-metal hip resurfacing show little change within one year. Bone Joint J. 2013;95-B:1626–1631. doi: 10.1302/0301-620X.95B12.32248. [DOI] [PubMed] [Google Scholar]
- 43.Berber R, Khoo M, Cook E, Guppy A, Hua J, Miles J, Carrington R, Skinner J, Hart A. Muscle atrophy and metal-on-metal hip implants: a serial MRI study of 74 hips. Acta Orthop. 2015;86:351–357. doi: 10.3109/17453674.2015.1006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bradberry SM, Wilkinson JM, Ferner RE. Systemic toxicity related to metal hip prostheses. Clin Toxicol (Phila) 2014;52:837–847. doi: 10.3109/15563650.2014.944977. [DOI] [PubMed] [Google Scholar]
- 45.Prentice JR, Clark MJ, Hoggard N, Morton AC, Tooth C, Paley MN, Stockley I, Hadjivassiliou M, Wilkinson JM. Metal-on-metal hip prostheses and systemic health: a cross-sectional association study 8 years after implantation. PLoS One. 2013;8:e66186. doi: 10.1371/journal.pone.0066186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan AH, Verma R, Bajpai A, Mackey-Bojack S. Unusual case of congestive heart failure: cardiac magnetic resonance imaging and histopathologic findings in cobalt cardiomyopathy. Circ Cardiovasc Imaging. 2015;8:pii: e003352. doi: 10.1161/CIRCIMAGING.115.003352. [DOI] [PubMed] [Google Scholar]
- 47.Samar HY, Doyle M, Williams RB, Yamrozik JA, Bunker M, Biederman RW, Shah MB. Novel Use of Cardiac Magnetic Resonance Imaging for the Diagnosis of Cobalt Cardiomyopathy. JACC Cardiovasc Imaging. 2015;8:1231–1232. doi: 10.1016/j.jcmg.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 48.Kwon YM, Lombardi AV, Jacobs JJ, Fehring TK, Lewis CG, Cabanela ME. Risk stratification algorithm for management of patients with metal-on-metal hip arthroplasty: consensus statement of the American Association of Hip and Knee Surgeons, the American Academy of Orthopaedic Surgeons, and the Hip Society. J Bone Joint Surg Am. 2014;96:e4. doi: 10.2106/JBJS.M.00160. [DOI] [PubMed] [Google Scholar]
- 49.Epstein M, Emri I, Hartemann P, Hoet P, Leitgeb N, Martinez L, Proykova A, Rizzo L, Rodriguez-Farre E, Rushton L, et al. The safety of Metal-on-Metal joint replacements with a particular focus on hip implants. [Updated 2014 Oct 14] Available from: http//ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_042.pdf. [Google Scholar]
- 50.Sidaginamale RP, Joyce TJ, Lord JK, Jefferson R, Blain PG, Nargol AV, Langton DJ. Blood metal ion testing is an effectivescreening tool to identify poorly performing metal-on-metal bearingsurfaces. Bone Joint Res. 2013;2:84–95. doi: 10.1302/2046-3758.25.2000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hart AJ, Sabah SA, Bandi AS, Maggiore P, Tarassoli P, Sampson B, A Skinner J. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J Bone Joint Surg Br. 2011;93:1308–1313. doi: 10.1302/0301-620X.93B10.26249. [DOI] [PubMed] [Google Scholar]
- 52.Hart AJ, Sabah SA, Sampson B, Skinner JA, Powell JJ, Palla L, Pajamäki KJ, Puolakka T, Reito A, Eskelinen A. Surveillance of Patients with Metal-on-Metal Hip Resurfacing and Total Hip Prostheses: A Prospective Cohort Study to Investigate the Relationship Between Blood Metal Ion Levels and Implant Failure. J Bone Joint Surg Am. 2014;96:1091–1099. doi: 10.2106/JBJS.M.00957. [DOI] [PubMed] [Google Scholar]
- 53.Nawabi DH, Hayter CL, Su EP, Koff MF, Perino G, Gold SL, Koch KM, Potter HG. Magnetic resonance imaging findings in symptomatic versus asymptomatic subjects following metal-on-metal hip resurfacing arthroplasty. J Bone Joint Surg Am. 2013;95:895–902. doi: 10.2106/JBJS.K.01476. [DOI] [PubMed] [Google Scholar]
- 54.Siddiqui IA, Sabah SA, Satchithananda K, Lim AK, Cro S, Henckel J, Skinner JA, Hart AJ. A comparison of the diagnostic accuracy of MARS MRI and ultrasound of the painful metal-on-metal hip arthroplasty. Acta Orthop. 2014;85:375–382. doi: 10.3109/17453674.2014.908345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garbuz DS, Hargreaves BA, Duncan CP, Masri BA, Wilson DR, Forster BB. The John Charnley Award: Diagnostic accuracy of MRI versus ultrasound for detecting pseudotumors in asymptomatic metal-on-metal THA. Clin Orthop Relat Res. 2014;472:417–423. doi: 10.1007/s11999-013-3181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayter CL, Koff MF, Shah P, Koch KM, Miller TT, Potter HG. MRI after arthroplasty: comparison of MAVRIC and conventional fast spin-echo techniques. AJR Am J Roentgenol. 2011;197:W405–W411. doi: 10.2214/AJR.11.6659. [DOI] [PubMed] [Google Scholar]
- 57.Robinson E, Henckel J, Sabah S, Satchithananda K, Skinner J, Hart A. Cross-sectional imaging of metal-on-metal hip arthroplasties. Can we substitute MARS MRI with CT? Acta Orthop. 2014;85:577–584. doi: 10.3109/17453674.2014.964618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berber R, Henckel J, Khoo M, Wan S, Hua J, Skinner J, Hart A. Clinical Usefulness of SPECT-CT in Patients with an Unexplained Pain in Metal on Metal (MOM) Total Hip Arthroplasty. J Arthroplasty. 2015;30:687–694. doi: 10.1016/j.arth.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 59.Grammatopoulos G, Pandit H, Kwon YM, Gundle R, McLardy-Smith P, Beard DJ, Murray DW, Gill HS. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg Br. 2009;91:1019–1024. doi: 10.1302/0301-620X.91B8.22562. [DOI] [PubMed] [Google Scholar]
- 60.Randelli F, Banci L, Favilla S, Maglione D, Aliprandi A. Radiographically undetectable periprosthetic osteolysis with ASR implants: the implication of blood metal ions. J Arthroplasty. 2013;28:1259–1264. doi: 10.1016/j.arth.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Daniel J, Holland J, Quigley L, Sprague S, Bhandari M. Pseudotumors associated with total hip arthroplasty. J Bone Joint Surg Am. 2012;94:86–93. doi: 10.2106/JBJS.J.01612. [DOI] [PubMed] [Google Scholar]
- 62.Campbell P, Shimmin A, Walter L, Solomon M. Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplasty. 2008;23:1080–1085. doi: 10.1016/j.arth.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 63.Liddle AD, Satchithananda K, Henckel J, Sabah SA, Vipulendran KV, Lewis A, Skinner JA, Mitchell AW, Hart AJ. Revision of metal-on-metal hip arthroplasty in a tertiary center: a prospective study of 39 hips with between 1 and 4 years of follow-up. Acta Orthop. 2013;84:237–245. doi: 10.3109/17453674.2013.797313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Medicines and Healthcare products Regulatory Agency. Metal-on-metal (MoM) hip replacements - guidance on implantation and patient management MHRA 2015. [Accessed 2016 Jan 5] Available from: http//www.gov.uk/drug-device-alerts/metal-on-metal-mom-hip-replacements-guidance-on-implantation-and-patient-management. [Google Scholar]
- 65.Hart AJ, Ilo K, Underwood R, Cann P, Henckel J, Lewis A, Cobb J, Skinner J. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93:315–320. doi: 10.1302/0301-620X.93B3.25545. [DOI] [PubMed] [Google Scholar]
- 66.Hart AJ, Muirhead-Allwood S, Porter M, Matthies A, Ilo K, Maggiore P, Underwood R, Cann P, Cobb J, Skinner JA. Which factors determine the wear rate of large-diameter metal-on-metal hip replacements? Multivariate analysis of two hundred and seventy-six components. J Bone Joint Surg Am. 2013;95:678–685. doi: 10.2106/JBJS.J.01447. [DOI] [PubMed] [Google Scholar]
- 67.Jacobs JJ, Wimmer MA. An important contribution to our understanding of the performance of the current generation of metal-on-metal hip replacements. J Bone Joint Surg Am. 2013;95:e53. doi: 10.2106/JBJS.M.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Underwood RJ, Zografos A, Sayles RS, Hart A, Cann P. Edge loading in metal-on-metal hips: low clearance is a new risk factor. Proc Inst Mech Eng H. 2012;226:217–226. doi: 10.1177/0954411911431397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panagiotidou A, Meswania J, Osman K, Bolland B, Latham J, Skinner J, Haddad FS, Hart A, Blunn G. The effect of frictional torque and bending moment on corrosion at the taper interface : an in vitro study. Bone Joint J. 2015;97-B:463–472. doi: 10.1302/0301-620X.97B4.34800. [DOI] [PubMed] [Google Scholar]


