Abstract
Cystic fibrosis has historically been considered a pulmonary disease, but with the increasing life expectancy of these patients, gastrointestinal manifestations are becoming more important. Furthermore, nutritional status is closely linked to pulmonary function and, thus, overall mortality. This article discusses gastrointestinal manifestations (which involve nutritional, pancreatic, hepatobiliary, and, in particular, gastrointestinal tract issues) of cystic fibrosis as well as management of the disease. In addition, the article discusses studies that have been critical to our understanding of gastrointestinal manifestations of cystic fibrosis.
Cystic fibrosis is the result of a defect in the cystic fibrosis transmembrane regulator (CFTR), which is responsible for the excretion of salt. The defect results in viscous secretions in multiple organ systems. For decades, cystic fibrosis was thought to only be a disease of childhood, given the low life expectancy associated with it. Largely because of improvements in nutrition, the average life expectancy of patients with cystic fibrosis is now well into adulthood. This article discusses the various gastrointestinal manifestations of cystic fibrosis, which involve pancreatic, nutritional, gastrointestinal tract, and hepatobiliary issues.
Clinical Manifestations
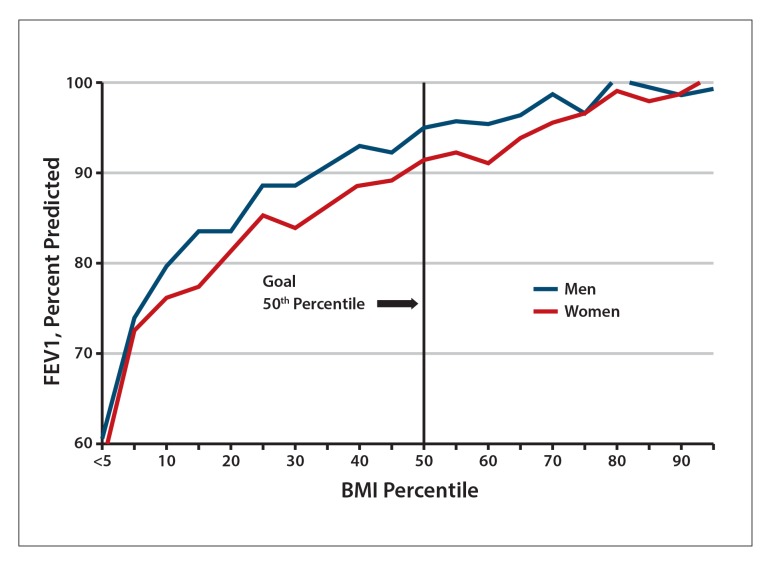
Defects in the CFTR result in multisystemic disease involving lung, liver, and gastrointestinal disease as well as pancreatic insufficiency. The majority of cystic fibrosis mutations cause lung disease, which is closely tied to growth and nutritional status.1 Having a body mass index (BMI) greater than or equal to 50% of a patient’s age has been shown to correlate with the predicted percentage of forced expiratory volume in 1 second (FEV1) greater than or equal to 90%, which is an important marker of lung function2 (Figure). For cystic fibrosis patients age 20 years or older, it is recommended that women maintain a BMI at or above 22 and that men maintain a BMI at or above 23.
Figure.
Predicted percentage of FEV1 vs BMI percentile in cystic fibrosis patients age 6 to 20 years.
BMI, body mass index; FEV1, forced expiratory volume in 1 second.
Adapted from Cystic Fibrosis Foundation.25
Nutritional failure in cystic fibrosis is multifactorial. Malabsorption of fat, protein, and fat-soluble vitamins is a result of insufficient production of pancreatic enzymes, which can be exacerbated by bile salt abnormalities in the presence of concurrent liver disease. Progressive pulmonary infection can lead to increased work of breathing, reduced appetite, and increased caloric needs from inflammatory catabolism. Other factors that affect nutrition include cystic fibrosis-related diabetes mellitus, altered motility of the gastrointestinal tract, and small bowel bacterial overgrowth.
Pancreatic insufficiency results in malabsorption and maldigestion of nutrients and fat-soluble vitamins. In fact, cystic fibrosis derives its name from the cysts and fibrosis noted in the pancreas of patients with the disease. Pancreatic enzyme replacement therapy (PERT) and optimization of nutritional deficiencies can prevent growth failure and improve other outcomes in patients with cystic fibrosis, including quality of life, resistance to infection, and chronic lung disease, which can lead to longer life expectancy.3,4 The type of the genetic mutation causing cystic fibrosis determines whether a patient is pancreatic-sufficient or pancreatic-insufficient, although approximately 85% of patients are pancreatic-insufficient by age 1 to 2 years.4 Pancreatic sufficiency in the setting of cystic fibrosis is a risk factor for recurrent pancreatitis, and recurrent pancreatitis can often be a presentation for the diagnosis of cystic fibrosis (Table 1).
Table 1.
Pancreatic Function and Mutations
| Pancreatic-Sufficient Dominant CF Mutations | Variable Pancreatic-Sufficient CF Mutations |
|---|---|
| G551S P574H R117H R334W R347H R352Q T3381 |
G85E R347P 3849 + 10kb C → T A455E 2789 5G → A |
CF, cystic fibrosis.
Adapted from Borowitz D et al.17
Gastrointestinal tract manifestations of cystic fibrosis are related to mucous inspissation and dysmotility and include meconium ileus (MI), constipation, distal intestinal obstruction syndrome (DIOS), gastroesophageal reflux disease (GERD), and small bowel bacterial overgrowth. DIOS is caused by inspissated intestinal contents that completely or partially block the small intestinal lumen, most commonly at the ileocecal junction.5 This is thought to be related to a cascade of intestinal inflammation in the setting of the defect in the CFTR.6
Intussusception occurs in approximately 1% of patients with cystic fibrosis.5 The intussusception is usually caused by inspissated bowel contents that serve as a lead point for the intussusception.
Hepatobiliary disease in cystic fibrosis occurs as a patchy biliary disease and is thought to have a prevalence of approximately 10% to 15% and peaks in pre-adolescence.7 Screening consists of annual liver transaminase measurement; if the levels are elevated, an abdominal ultrasound (US) is obtained and done annually thereafter if cystic fibrosis-related liver disease is suspected. US findings of the liver can show coarse echotexture, steatosis, and progressive portal hypertension. End-stage liver disease can result in liver transplant. The risk of gallstones is also higher in cystic fibrosis.
Management
Pancreatic Enzyme Replacement Therapy
The pancreas is responsible for secreting enzymes that aid in the absorption of nutrients, including fat-soluble vitamins (vitamins A, D, E, and K). In order to counteract this malabsorption, PERT is used. Lipase is the enzyme responsible for fat absorption. PERT is based on the dosage of lipase in the supplement and is dosed at 500 to 2500 units lipase per kilogram body weight per meal or less than 10,000 units lipase per kilogram body weight per day. Chronic administration of PERT in excess doses can result in fibrosing colopathy, which is characterized by ileocecal inflammation with lower gastrointestinal bleeding, abdominal pain, and obstructive abdominal symptoms. Thus, the Cystic Fibrosis Foundation issued a consensus statement regarding PERT dosing, as noted above.
Nutritional Management
According to the Cystic Fibrosis Foundation, higher energy intake is needed for improved weight gain. In order to achieve an energy intake of110% to 200% of the energy intake of the healthy population, the Cystic Fibrosis Foundation recommends a high-calorie, unlimited-fat meal.8,9
For cystic fibrosis patients age 1 to 12 years, intensive behavioral and nutritional counseling is recommended to promote weight gain.10-13 For children with growth deficits and adults who are having difficulty maintaining weight gain, oral or enteral nutritional supplements are recommended.
In addition, fat-soluble vitamin supplements are routinely administered.4 The fat-soluble vitamins A, D, E, and K are supplemented in all children with cystic fibrosis. Supplements are started at diagnosis, including in asymptomatic infants and those without pancreatic insufficiency. Standard supplements tend to overestimate vitamin A and underestimate vitamins D and K. This can be problematic, for example in cystic fibrosis patients who also have cystic fibrosis—related liver disease, as vitamin A toxicity can affect the liver. Furthermore, the risk of vitamin A toxicity may change, as the amount and type of vitamin A in cystic fibrosis-specific formulations can differ.
Vitamin A deficiency is rare except at the time of diagnosis. Vitamin A is necessary for vision, gene expression, growth, and immune function. The available forms of vitamin A include preformed vitamin A (retinol, retinoic acid) and provitamin A (alpha-carotene, beta carotene). Vitamin A levels are monitored via serum retinol and retinol-binding protein. Vitamin A toxicity is possible in cystic fibrosis patients and is marked by bone mineral loss and liver abnormalities.
Vitamin D deficiency is common in cystic fibrosis. Vitamin D helps the body use calcium obtained from a person’s diet, and deficiency can lead to poor bone mineralization.14 Vitamin D3 (cholecalciferol) is contained in most supplements and is the form produced in the skin by sunlight. The Cystic Fibrosis Foundation recommends vitamin D3 vs vitamin D2 (ergocalciferol) because a small study showed that it achieved better target 25-hydroxyvi- tamin D. Levels are checked annually at the end of winter. Bone disease in cystic fibrosis patients results from decreased mineral density, which worsens with age, severity of lung disease, and malnutrition. There is also an increase in fracture rates and kyphosis in young adults with cystic fibrosis. In addition to vitamin D deficiency, chronic corticosteroid use and reduced weight-bearing activity can contribute to bone disease.
Vitamin E is an antioxidant, and its deficiency could contribute to inflammation and lung disease in cystic fibrosis patients. Vitamin E deficiency leads to delayed stretch reflexes, cerebellar ataxia, and peripheral neuropathy. Some studies have shown a correlation between vitamin E status, polyunsaturated fatty acid (PUFA) status, and inflammation in cystic fibrosis. Eight different forms of vitamin E exist, with the most common being alpha tocopheryl acetate. The recommended intake for cystic fibrosis patients is 20 times the generally recommended intake. Serum vitamin E levels are influenced by serum lipid levels.
Vitamin K deficiency is associated with coagulation abnormalities and bone disease. Vitamin K is found in green vegetables, and vitamin K status is monitored by a serum prothrombin time. As intestinal bacteria are a source of vitamin K, supplementation should be provided during courses of antibiotics, a common occurrence in cystic fibrosis patients.
Essential fatty acids are long-chain PUFAs and include omega-3 and omega-6 fatty acids. Deficiency may contribute to an inflammatory state, including scaly dermatitis, alopecia, and growth failure, and tends to be more common in infants.4 Routine supplementation is not recommended at this time. However, a systematic review showed that supplementation with omega-3 fatty acids improved several markers of lung disease. The most common fatty acid abnormalities in patients with cystic fibrosis are lin- oleic acid and docosahexaenoic acid (DHA) deficiencies.15 A study looking at DHA supplementation in cystic fibrosis patients has shown improvement in inflammatory markers but inconsistent improvement in FEV1.16
Cystic fibrosis patients are prone to hyponatremic dehydration under conditions of heat stress secondary to sodium losses through sweat. Sodium chloride supplementation can be used, especially in warm months or climates and in infants.
The Cystic Fibrosis Foundation recommends a trial of zinc supplementation for children younger than age 2 years who are not growing well. Dermatitis can be a symptom of zinc deficiency.17 Iron deficiency has been noted in several studies of cystic fibrosis patients and is monitored yearly by serum hemoglobin and hematocrit levels.
Oral supplements are used in cystic fibrosis but are often less effective because they may displace ordinary food.18,19 Studies have shown that calorie-protein supplements are not superior to monitoring and dietary advice from a health professional and nutritionist.18,19 Enteral nutrition is generally started via gastrostomy tube for patients with growth failure. Although there are no randomized, controlled trials to support this, enteral nutrition is thought to improve and maintain lung function in patients with cystic fibrosis. For toddlers, a combination of daytime feeds, daytime meals, and nighttime continuous feeds is used.
Pancreatitis is treated with supportive care. It is important to ensure that constipation does not worsen with the use of narcotics to treat pancreatitis-related abdominal pain.
In terms of the treatment of gastrointestinal manifestations, it is important to treat GERD symptoms, especially in patients with progressive lung disease and those who have undergone lung transplant. Importantly, the use of acid suppression enhances the use of PERT, as pancreatic enzymes require a bicarbonate-rich environment under normal physiologic conditions, which does not exist with cystic fibrosis, where pancreatic bicarbonate secretion is impaired.20
Patients with cystic fibrosis are thought to be at an increased risk for GERD-related anatomic changes that occur to the lungs over time, perhaps also exacerbated by intestinal dysmotility. A retrospective study in 2013 showed that Nissen fundoplication helped improve lung function and nutritional status in cystic fibrosis patients, especially in those with milder pulmonary disease.21 Although fundoplication is not routinely recommended, it is important to address and control GERD symptoms with proper acid blockade in cystic fibrosis patients.
MI is a condition diagnosed in the newborn period and characterized by inspissated meconium in the intestine of newborns with cystic fibrosis. Approximately 10% of patients with cystic fibrosis present as neonates with MI. This condition is treated by a radiocontrast enema or surgical intervention. MI is thought to be a precursor to DIOS later in life.22 DIOS is defined as acute complete or incomplete obstruction of the ileocecum by inspissated intestinal contents (Table 2). DIOS must be distinguished from constipation and is characterized by gradual onset of fecal impaction of the colon, starting at the sigmoid and extending proximally. DIOS can occur at any age, but it is more common in those with pancreatic insufficiency.5 It is important to note that DIOS can mimic appendicitis. Finally, treatment of constipation is important, as it can be a cause of abdominal discomfort and rectal prolapse, and it can result in decreased appetite.
Table 2.
ESPGHAN Cystic Fibrosis Working Group Definition for DIOS in Patients With Cystic Fibrosis
| Criteria | Explanation |
|---|---|
| 1 | Complete intestinal obstruction as evidenced by vomiting of bilious material and/or fluid levels in the small intestine on abdominal radiography |
| 2 | Fecal mass in the ileocecum |
| 3 | Abdominal pain and/or distension |
Complete DIOS = 1 and 2 and 3. Incomplete/impending DIOS: 2 and 3 only.
DIOS, distal intestinal obstruction syndrome; ESPGHAN, European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
Adapted from Houwen RH et al.22
As patients with cystic fibrosis are living longer, it has become clear that cystic fibrosis carries a higher prevalence of intestinal cancers diagnosed at a younger age than that in the general population.23,24 However, the reasons for this are unclear at this time.
Ursodeoxycholic acid is used to treat cystic fibrosis—related liver disease and can result in normalization of liver transaminases, but it is not known if this treatment affects histologic changes in the liver associated with cystic fibrosis. Liver biopsies are not routinely obtained in cystic fibrosis-related liver disease unless another diagnosis is being considered that would change management. Furthermore, cystic fibrosis-related liver disease is patchy and can be missed on routine liver biopsy.
Conclusion
Cystic fibrosis is a disease in which nutritional status is important. Thus, it is vital to monitor nutritional status regularly, look at growth and overall digestive issues such as signs of malabsorption, and screen for nutritional deficiencies. The recognition and treatment of gastrointestinal manifestations also enhance these outcomes and are closely tied to overall well-being and life expectancy.
Footnotes
The author has no relevant conflicts of interest to disclose.
References
- 1.Peterson ML, Jacobs DR, Jr, Milla CE. Longitudinal changes in growth parameters are correlated with changes in pulmonary function in children with cystic fibrosis. Pediatrics. 2003;112(3 pt 1):588–592. doi: 10.1542/peds.112.3.588. [DOI] [PubMed] [Google Scholar]
- 2.Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax. 2002;57(7):596–601. doi: 10.1136/thorax.57.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brady MS, Rickard K, Yu PL, Eigen H. Effectiveness of enteric coated pancreatic enzymes given before meals in reducing steatorrhea in children with cystic fibrosis. J Am Diet Assoc. 1992;92(7):813–817. [PubMed] [Google Scholar]
- 4.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Clinical Practice Guidelines on Growth and Nutrition Subcommittee; Ad Hoc Working Group. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108(5):832–839. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Colombo C, Ellemunter H, Houwen R, Munck A, Taylor C, Wilschanski M. ECFS. Guidelines for the diagnosis and management of distal intestinal obstruction syndrome in cystic fibrosis patients. J Cyst Fibros. 2011;10(suppl 2):S24–S28. doi: 10.1016/S1569-1993(11)60005-2. [DOI] [PubMed] [Google Scholar]
- 6.Werlin SL, Benuri-Silbiger I, Kerem E, et al. Evidence of intestinal inflammation in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;51(3):304–308. doi: 10.1097/MPG.0b013e3181d1b013. [DOI] [PubMed] [Google Scholar]
- 7.Sokol RJ, Durie PR. Cystic Fibrosis Foundation Hepatobiliary Disease Consensus Group. Recommendations for management of liver and biliary tract disease in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1999;28(suppl 1):S1–S13. doi: 10.1097/00005176-199900001-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ellis JA, Bond SA, Wootton SA. Energy and protein intakes of patients with cystic fibrosis. J Hum Nutr Diet. 1992;5:333–342. [Google Scholar]
- 9.Hanning RM, Blimkie CJ, Bar-Or O, Lands LC, Moss LA, Wilson WM. Relationships among nutritional status and skeletal and respiratory muscle function in cystic fibrosis: does early dietary supplementation make a difference? Am J Clin Nutr. 1993;57(4):580–587. doi: 10.1093/ajcn/57.4.580. [DOI] [PubMed] [Google Scholar]
- 10.Powers SW, Jones JS, Ferguson KS, Piazza-Waggoner C, Daines C, Acton JD. Randomized clinical trial of behavioral and nutrition treatment to improve energy intake and growth in toddlers and preschoolers with cystic fibrosis. Pediatrics. 2005;116(6):1442–1450. doi: 10.1542/peds.2004-2823. [DOI] [PubMed] [Google Scholar]
- 11.Powers S, Byars K, Mitchell M, Patton S, Schindler T, Zeller M. A randomized pilot study of behavioral treatment to increase caloric intake in toddlers with cystic fibrosis. Child Health Care. 2003;32:297–311. [Google Scholar]
- 12.Stark LJ, Opipari-Arrigan L, Quittner AL, Bean J, Powers SW. The effects of an intensive behavior and nutrition intervention compared to standard of care on weight outcomes in CF. Pediatr Pulmonol. 2011;46(1):31–35. doi: 10.1002/ppul.21322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stark LJ, Opipari LC, Spieth L, et al. Contribution of behavioral therapy to dietary treatment in cystic fibrosis: a randomized controlled study with 2-year follow-up. Behavior Therapy. 2003;34:237–258. [Google Scholar]
- 14.Paccou J, Zeboulon N, Combescure C, Gossec L, Cortet B. The prevalence of osteoporosis, osteopenia, and fractures among adults with cystic fibrosis: a systematic literature review with meta-analysis. Calcif Tissue Int. 2010;86(1):1–7. doi: 10.1007/s00223-009-9316-9. [DOI] [PubMed] [Google Scholar]
- 15.Aldámiz-Echevarría L, Prieto JA, Andrade F, et al. Persistence of essential fatty acid deficiency in cystic fibrosis despite nutritional therapy. Pediatr Res. 2009;66(5):585–589. doi: 10.1203/PDR.0b013e3181b4e8d3. [DOI] [PubMed] [Google Scholar]
- 16.Van Biervliet S, Devos M, Delhaye T, Van Biervliet JP, Robberecht E, Christophe A. Oral DHA supplementation in DeltaF508 homozygous cystic fibrosis patients. Prostaglandins Leukot Essent Fatty Acids. 2008;78(2):109–115. doi: 10.1016/j.plefa.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(3):246–259. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Luder E, Kattan M, Thornton JC, Koehler KM, Bonforte RJ. Efficacy of a nonrestricted fat diet in patients with cystic fibrosis. Am J Dis Child. 1989;143(4):458–464. doi: 10.1001/archpedi.1989.02150160084017. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd-Still JD, Smith AE, Wessel HU. Fat intake is low in cystic fibrosis despite unrestricted dietary practices. JPEN J Parenter Enteral Nutr. 1989;13(3):296–298. doi: 10.1177/0148607189013003296. [DOI] [PubMed] [Google Scholar]
- 20.Heijerman HG, Lamers CB, Bakker W. Omeprazole enhances the efficacy of pancreatin (pancrease) in cystic fibrosis. Ann Intern Med. 1991;114(3):200–201. doi: 10.7326/0003-4819-114-3-200. [DOI] [PubMed] [Google Scholar]
- 21.Sheikh SI, Ryan-Wenger NA, McCoy KS. Outcomes of surgical management of severe GERD in patients with cystic fibrosis. Pediatr Pulmonol. 2013;48(6):556–562. doi: 10.1002/ppul.22630. [DOI] [PubMed] [Google Scholar]
- 22.Houwen RH, van der Doef HP, Sermet I, et al. ESPGHAN Cystic Fibrosis Working Group. Defining DIOS and constipation in cystic fibrosis with a multicentre study on the incidence, characteristics, and treatment of DIOS. J Pediatr Gastroenterol Nutr. 2010;50(1):38–42. doi: 10.1097/MPG.0b013e3181a6e01d. [DOI] [PubMed] [Google Scholar]
- 23.Neglia JP, FitzSimmons SC, Maisonneuve P, et al. Cystic Fibrosis and Cancer Study Group. The risk of cancer among patients with cystic fibrosis. N Engl J Med. 1995;332(8):494–499. doi: 10.1056/NEJM199502233320803. [DOI] [PubMed] [Google Scholar]
- 24.Maisonneuve P, FitzSimmons SC, Neglia JP, Campbell PW, 3rd, Lowenfels AB. Cancer risk in nontransplanted and transplanted cystic fibrosis patients. J Natl Cancer Inst. 2003;95(5):381–387. doi: 10.1093/jnci/95.5.381. [DOI] [PubMed] [Google Scholar]
- 25. Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: Annual Data Report 2009. Bethesda, Maryland; 2011.