Abstract
Aim
Assuming that genetic variants of the SLC22A2 and SLC31A1 transporter affect patients’ susceptibility to cisplatin-induced ototoxicity, we compared the distribution of 11 SLC22A2 variants and the SLC31A1 variant rs10981694 between patients with and without cisplatin-induced ototoxicity.
Patients & methods
Genotyping was performed in 64 pediatric patients and significant findings were re-evaluated in 66 adults.
Results
The SLC22A2 polymorphism rs316019 (c.808G>T; Ser270Ala) was significantly associated with protection from cisplatin-induced ototoxicity in the pediatric (p = 0.022) and the adult cohort (p = 0.048; both: Fisher’s exact test). This result was confirmed by multiple logistic regression analysis accounting for age which was identified as a relevant factor for ototoxicity as well (rs316019: OR [G/T vs G/G] = 0.12, p = 0.009; age: OR [per year]: 0.84, p = 0.02).
Conclusion
These results identified rs316019 as potential pharmacogenomic marker for cisplatin-induced ototoxicity and point to a critical role of SLC22A2 for cisplatin transport in humans and its contribution to the organ specific side effects of this drug.
Keywords: cisplatin, ototoxicity, polymorphism, SLC22A2, SLC31A1
Background
Cisplatin is one of the most potent anticancer drugs and despite dose-limiting nephro- and ototoxicity it is still recommended as first line treatment for pediatric as well as adult cancers. While nephrotoxicity can be reduced by vigorous hydration, ototoxicity is still of concern in adults and especially in children, who are more sensitive to cisplatin-induced ototoxicity and depend on unaffected hearing as basis for language development, school performance, social integration and an age-based quality of life [1–3].
Permanent bilateral hearing loss was observed at least in 60% of children treated with cisplatin [1, 3, 4]. In adults, persisting ototoxicity may affect up to 50% of patients [5]. Reporting of ototoxicity in clinical trials bases on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) and the WHO Common Toxicity Criteria [6, 7]. These criteria were found to underestimate the incidence of cisplatin ototoxicity and new grading systems were developed, which considered clinical differences in cisplatin-induced hearing loss and their consequences. Brock et al. established a grading system, which is based on standard pure-tone audiologic frequencies at which hearing loss equals or exceeds 40 dB hearing level (HL) [8]. This grading system was further modified by Chang and Chinosornvantana and by the SIOP Boston Ototoxicity Scale [1, 9]. Both grading systems considered 20 dB HL besides 40 dB HL as hearing loss in order to detect mild hearing losses. The Muenster classification, which was applied in this study, further considers tinnitus and minimal hearing losses (>10 to ≤20 dB HL) to already catch beginning ototoxicity classified as grade 1 [8, 10, 11]. Compared to existing scales the acquisition of changes as low as >10 to ≤20 dB HL after the second cisplatin dose by the Muenster classification identifies patients at risk for serious hearing impairment with high sensitivity and predictivity [12].
High cumulative dosages of cisplatin are the most important risk factors predicting cisplatin-induced ototoxicity [5, 13–15]. In addition, bolus-injections of cisplatin, pre-existing renal dysfunction, pre-existing sensorineural hearing loss and cranial radiation significantly increase the risk for cisplatin-induced hearing loss. Children <5 years are significantly more sensitive to cisplatin-induced ototoxicity than older children, while in adults a history of noise exposure further increases the risk of cisplatin-induced ototoxicty [5, 13–17].
Moreover other individual factors exist which allow some patients to tolerate cisplatin exposure without any signs of ototoxicity, while others experience hearing impairment already after the first dose of cisplatin [3, 4, 18]. The hypothesis that genetics may contribute to interpatient variation prompted the search for pharmacogenetic markers associated with patients’ individual risk to experience cisplatin-induced ototoxicity. In a high-throughput genotyping approach of 220 genes encoding Phase I and II metabolizing enzymes the intronic SNPs rs12201199 of the TPMT and rs9332377 of the COMT showed high predictivity for cisplatin-induced hearing loss [19, 20]. By selection of candidate genes on the basis of established or postulated mechanisms of cisplatin ototoxicity minor allele frequencies of nonsynonymous SNPs in the GST genes, rs17999735 in GSTM3 and rs1695 in GSTP1, were associated with protection from cisplatin-induced ototoxicity [21, 22]. Other studies addressed variants in genes involved in drug transport and DNA repair and were able to link the nonsynonymous mutations in the XPC gene rs2228001, in the LRP2 gene rs2075252 and in the CTR1 (SLC31A1) gene rs10981694 to an increased risk for cisplatin-induced ototoxicity [23–26].
Apart from SLC31A1, OCT2 (SLC22A2) was shown to mediate the cellular transport of cisplatin [27–30]. In preclinical models SLC22A2 was already linked to cisplatin-induced oto- and nephro-toxicity [29, 30]. Moreover, the T allele of the SNP rs316019 in the SLC22A2 gene was associated with protection from cisplatin-induced nephrotoxicity [31, 32].
Genetic variants of the SLC22A2 gene had not been analyzed for their relevance on cisplatin-induced ototoxicity so far. Having confirmed previous observations that in mice SLC22A1 and 2 deficiency protects from cisplatin-induced ototoxicity [30], we compared the distribution of 11 SNPs of the SLC22A2 gene between children and adolescents who experienced ototoxicity by cisplatin treatment and those who did not. Because of its previous association with cisplatin-induced ototoxicity we also included the SNP rs10981694 of the SLC31A1 gene in the study [26]. Finally, we validated significant findings in an independent cohort of adult cancer patients treated with cisplatin.
Patients & methods
Patients
A total of 194 children and adolescents, who were scheduled for cisplatin-based chemotherapy at the University Children’s Hospital of Muenster, participated in the exploratory phase of the study. The study was approved by the institutional review board and all patients and/or their guardians gave their written informed consent to participate in the study. Fourteen patients did not receive the scheduled cisplatin in the end and were excluded from further comparisons. To account for known risk factors for hearing loss only patients, who had no hearing impairment before therapy (audiogram grade = 0), who were ≥5 years at the time of therapy, who did not receive cranial irradiation or ototoxic medication in addition to cisplatin and who were devoid of renal impairment before cisplatin therapy, were considered eligible for final comparison. Audiologic examinations were scheduled before therapy, before every course of cisplatin and at the end of treatment. Audiologic follow-up examinations were performed additionally. Patients were only considered eligible, if an evaluable audiogram was performed before and at least within 6 months after completion of therapy at the Department of Phoniatrics and Pedaudiology at University Hospital of Muenster.
Chemotherapy was applied according to the treatment protocols of the German Society of Pediatric Hematology and Oncology at the Department of Pediatric Hematology and Oncology of the University Children’s Hospital of Muenster. The osteosarcoma patients were treated according to the COSS-82/86/91/96 or EURAMOS-1 protocol, neuroblastoma patients according to the NB 90 or 2004 protocol. Patients with germ cell tumors were treated according to the MAKEI 89 or 96 or the MAHO 94 protocol. Depending on protocol and tumor staging, the osteosarcoma protocols prescribed cumulative cisplatin dosages of 240–750 mg/m2, the neuroblastoma protocols cisplatin dosages between 320 and 640 mg/m2, the MAHO and MAKEI protocols cisplatin dosages of 200–620 mg/m2 and the HIT 2000 protocol cumulative cisplatin dosages between 240 and 560 mg/m2 cisplatin. The modes of cisplatin administration for the respective treatment protocols are summarized in Supplementary Table 1 (see online at: www.futuremedicine.com/doi/suppl/10.2217/pgs.14.182).
None of the known SLC22A2 inhibitors [33] were administered in the pediatric population.
In the validation phase, data were used from a cohort of 66 adult cancer patients receiving intravenous infusions of cisplatin (up to 100 mg/m2) once weekly or once every 3-weeks, who were treated at the Erasmus MC–Cancer Institute. Information on dose, schedule, diagnosis and concomitant chemotherapy drugs were reported previously [34]. The study protocols were reviewed and approved by the local review board, and patients provided written informed consent.
Hearing evaluation & ototoxicity
Audiologic examinations performed on the pediatric population included pure-tone audiometry via air and bone (in children younger than 3 years sound-field audiometry), tympanometry and recording of transient click-evoked and distortion product otoacoustic emissions (TEOAE and DPOAE). If necessary, brainstem audiometry was performed. The degree of hearing impairment was determined by using the ‘Muenster classification’ for early detection of cisplatin-induced bilateral high-frequency hearing loss [11]. A pure tone audiogram of grade 0 was necessary to enter the study. The ototoxicity experienced by the adult patients was scored according to the NCI-CTCAE criteria.
Genotyping
EDTA blood samples were obtained from all patients, who consented to participate in the study. DNA was isolated from peripheral blood mononuclear cells according to standard research protocols. Genotyping was carried out using custom-designed genotyping assays according to the manufacturer’s instructions (Applied Biosystems, Darmstadt, Germany). The assay IDs are given in Supplementary Table 2. Allelic discrimination was conducted with an ABI Prism 7700 sequence detection system (Applied Biosystems, Darmstadt, Germany). The PCR setup was carried out on a 384-well plate by a Tecan Genesis 150 robotic system (Tecan Deutschland GmbH, Crailsheim, Germany). For each run two nontemplate and three positive controls were added. Reanalysis of 15% of randomly chosen samples confirmed the results of previous analysis.
Statistics
Single-marker case–control differences were evaluated for the SLC31A1 variant rs10981694 and the SLC22A2 variants rs8177513, rs316019, rs8177509, rs596881, rs653753, rs316024, rs17588242, rs315988, rs316004, rs316000 and rs316002 using the χ2 test, if the total sum was >40 and each frequency >5. At frequencies ≤5 Fisher’s exact test was used. Odds ratios and 95% confidence intervals for the markers in the contingency tables were calculated. To account for confounding factors such as age, probability of ototoxicity was modeled by multiple logistic regression. This analysis was performed with IBM SPSS Statistics 21 for Windows (IBM Corporation, NY, USA).
Linkage, haplotype, deviation from Hardy–Weinberg equilibrium, and pair-wise linkage disequilibrium (LD) analysis were done using Haploview [35]. This program takes one haplotype at a time and compares it between cases and controls.
All inferential statistics were intended to be exploratory (hypothesis generating), not confirmatory, and were interpreted accordingly, that is, p-values are interpreted as metric weight of evidence against the respective null hypothesis of no effect. The local significance level was set to 0.05. No adjustment for multiple testing was performed.
Results
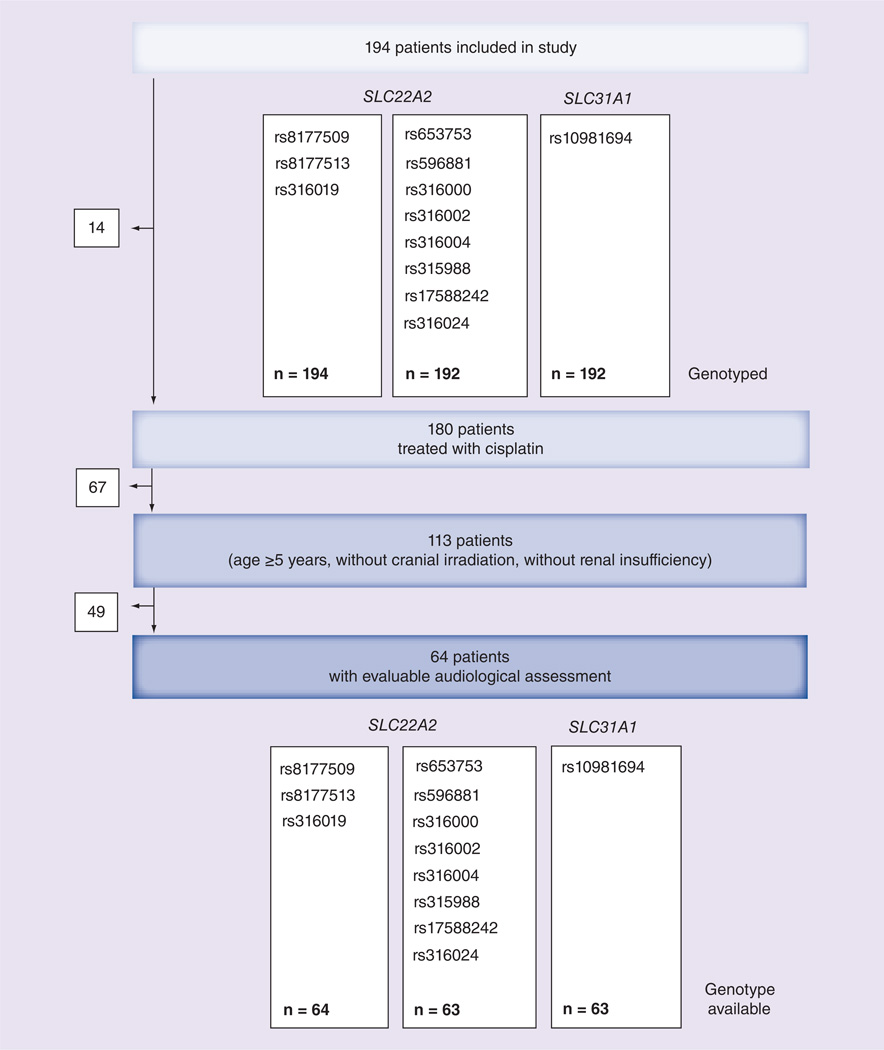
Overall 194 children and adolescents who were initially designed for cisplatin-based chemotherapy were included in the study. While the distribution of the nonsynonymous SNPs rs8177509, rs8177513 and rs316019 was determined in all 194 patients, the noncoding SNPs of the SLC22A2 gene and the SLC31A1 SNP rs10981694 were only analyzed in 192 patients because of insufficient material (Figure 1). Eventually, 180 patients received the initially scheduled cisplatin. To reduce the bias by risk factors, which are known to increase the risk for cisplatin-induced ototoxicity, 67 patients were further excluded from the analysis because of age <5 years, renal insufficiency at diagnosis, cranial irradiation or pre-existing hearing loss [1, 3, 5, 13–17]. Finally, 49 patients had to be excluded from the final comparison because of nonevaluable audiograms, missing baseline audiograms or missing audiograms after completion of therapy (Figure 1). Based on the results of their audiological examination the remaining 64 patients were classified into group N (no ototoxicity) and O (ototoxicity) according to the Muenster classification [11]. Group O comprised 36 patients who experienced hearing impairment ≥1 during cisplatin treatment and group N consisted of 28 patients with no signs of ototoxicity (Table 1).
Figure 1. Consort flow diagram.
Table 1.
Patients’ characteristics.
| Group N | Group O | p-value | ||
|---|---|---|---|---|
| Age at therapy (years) | (Mean, range) | 14.0 (5–20) | 11.8 (5–22) | 0.042† |
| Sex | (Male/female) | 18/10 | 20/16 | 0.481 |
| Diagnosis | Osteosarcoma | 17 | 24 | 0.182‡ |
| Neuroblastoma | 1 | 3 | ||
| Brain tumor | 4 | 8 | ||
| Germ cell tumor | 6 | 1 | ||
| Cumulative cisplatin dose (mg/m2) |
(Mean, range) | 420 (161–560) | 412 (120–644) | 0.766† |
| Infusion rate (mg cisplatin/min) |
(Median, 25th– 75th percentile) |
0.0278 (0.0278–0.333) | 0.0278 (0.0278–0.1940) | 0.346§ |
| Treatment duration (days)¶ |
(Median, 25th– 75th percentile) |
143 (130–160) | 155 (90.5–201) | 0.511§ |
t-test.
Fisher’s exact test.
Mann–Whitney U test.
Time interval between first and last cisplatin administration of therapy.
Table 1 summarizes the clinical data of patients in groups N and O. The mean age of all evaluable patients at diagnosis was 12.8 years. The patients with ototoxicity (group O) were significantly younger compared with patients without ototoxicity (group N; p = 0.037, Student’s t-test). This difference was attributed to the higher number of patients with brain tumors and neuroblastomas in group O (31% in group O vs 18% in group N), who were younger compared with the patients with germ cell tumors and osteosarcomas. Cisplatin treatment varied according to tumor type as well as protocol. However, the distribution of diagnoses, cumulative cisplatin doses, cisplatin infusion rates and treatment duration were not statistically different between both groups (Table 1).
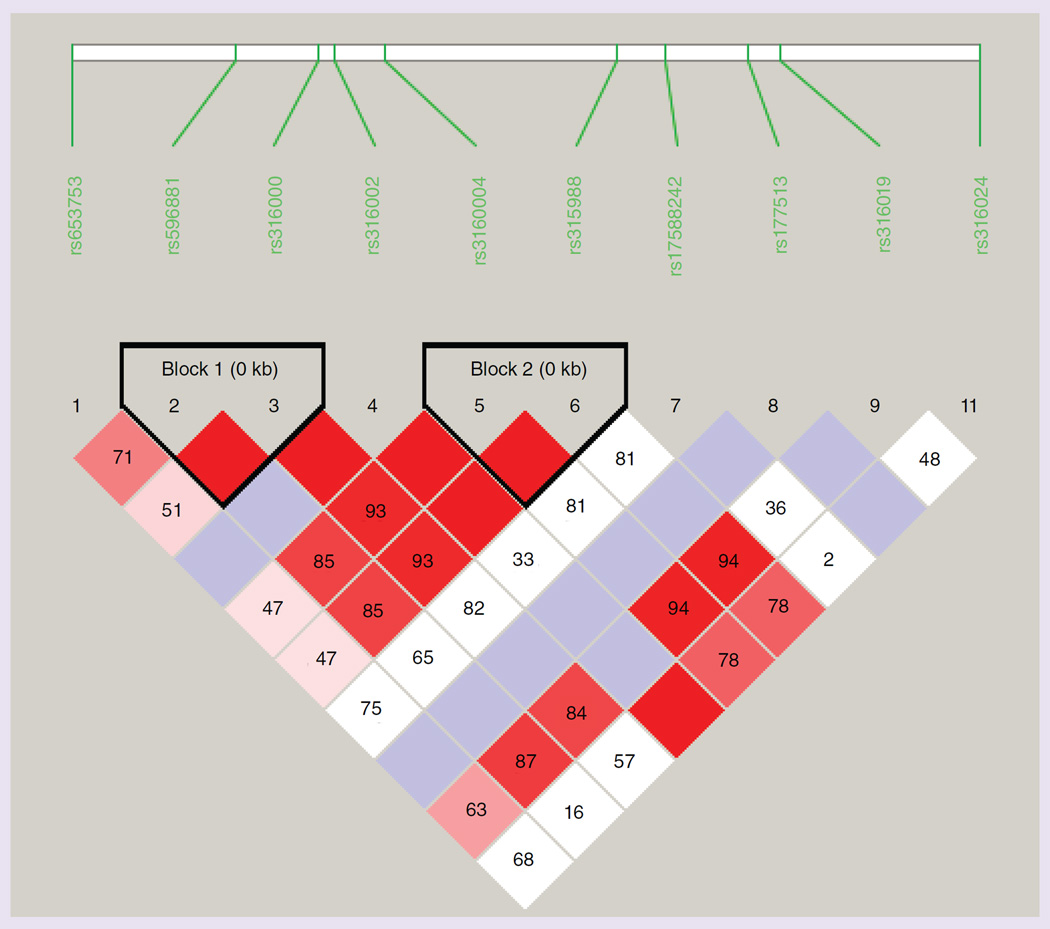
The results for the distribution of alleles and genotypes of the SNPs of the SLC22A2 gene and the SNP rs10981694 of the SLC31A1 gene are listed in the Supplementary Table 2. All SNPs showed no deviation from the Hardy–Weinberg equilibrium. Altogether, for all SNPs no significant differences in allele distribution were observed between patients and reference group [36]. The allele distribution of the SLC31A1 SNP rs10981694 was not different between group N and O, and out of the 11 SNPs of the SLC22A2 gene only the allele distribution of the nonsynonymous SNP rs316019 was significantly different between patients who experienced ototoxicity and those who did not. The frequency of the T allele of rs316019 was significantly higher in group N (0.161) than group O (0.042), the group of all patients (0.095) and the reference group (0.097). This represented a significant intergroup difference (N vs O: χ2 = 5.595; p < 0.05; Table 2). In contrast to the HapMap CEU reference group we detected no T/T genotype of the SNP rs316019 in all 194 patients. We also detected lower frequencies for the T allele of rs315988, the A allele of rs596881, the G allele of rs316000 and the T allele of rs316004 in group O compared with group N. However, these differences did not reach statistical significance. This association also becomes apparent by graphical visualization of the haplotype structure of the analyzed SLC22A2 SNPs, which showed high D′-values for rs316019, rs316000, rs316004, rs315988 and rs596881 (Figure 2). The SNP rs8177509 was not included in this analysis because of an observed allele frequency below 1%.
Table 2.
Results of the association analysis perfomed by Haploview.
| SNP-ID | Associated allele |
Ratio counts |
Frequencies |
χ2 | p-value | ||
|---|---|---|---|---|---|---|---|
| Group O | Group N | Group O | Group N | ||||
| rs653753 | C>G | 12:60 | 5:49 | 0.167 | 0.093 | 1.45 | 0.228 |
| rs596881 | G>A | 4:68 | 7:47 | 0.056 | 0.130 | 2.12 | 0.145 |
| rs316000 | A>G | 10:62 | 11:43 | 0.139 | 0.204 | 0.93 | 0.334 |
| rs316002 | C>T | 5:67 | 8:46 | 0.069 | 0.148 | 2.07 | 0.151 |
| rs316004 | G>T | 10:62 | 11:43 | 0.139 | 0.204 | 0.93 | 0.334 |
| rs315988 | C>T | 11:61 | 13:41 | 0.153 | 0.241 | 1.55 | 0.213 |
| rs17588242 | T>C | 20:52 | 13:41 | 0.278 | 0.241 | 0.22 | 0.640 |
| rs8177513 | C>G | 0:72 | 1:53 | 0.000 | 0.019 | 1.34 | 0.255 |
| rs316019 | G>T | 3:69 | 9:45 | 0.042 | 0.167 | 5.60 | 0.018 |
| rs316024 | G>A | 18:54 | 16:38 | 0.250 | 0.296 | 0.34 | 0.562 |
| rs10981694 | T>G | 7:65 | 8:46 | 0.097 | 0.148 | 0.76 | 0.382 |
χ2 test used throughout [36].
Figure 2. Haplotype structure overview of the SLC22A2 SNPs analyzed.
The normalized D′ values are given in the respective boxes.
Regarding the fact that despite of the exclusion of children < 5 years, the mean age of patients in group N was significantly higher compared with group O, we applied a multiple logistic regression model to analyze probability of ototoxicity accounting for age at treatment start. Again we could confirm our findings with an odds ratio of 0.12 (95% CI [0.02–0.58], p = 0.009) for G/T genotype in rs316019 compared with G/G genotype. The odds ratio for age in this model was 0.84 (95% CI [0.73–0.97], p = 0.02), denoting a decrease of chance of ototoxicity of 16% comparing two patients with age difference of 1 year at treatment start. These results were confirmed when repeating the analyses after inclusion of patients <5 years (results not shown).
A cohort of 66 adult patients (32 male and 34 female) with solid tumors treated with cisplatin-based chemotherapy was used to independently re-analyze significant associations observed in the pediatric cohort. Ototoxicity associated with cisplatin treatment of any grade ≥1 according to CTCAE criteria (group O) was observed in 18 patients (27%), and was absent (group N) in 48 patients (73%). Gender distribution did not differ between group N and O. Unlike the pediatric cohort no significant difference with respect to age was observed between group N and O. Similar to findings observed in the pediatric cohort, we found that the frequency of the T allele of rs316019 was significantly higher for group N (0.094) than group O (0.00), and the group of all patients (0.068). This represented a significant intergroup difference (N vs O: p = 0.048, Fisher’s exact test) in the adult population.
Discussion
Susceptibility to cisplatin-induced ototoxicity varies between individuals and the reasons for these variations have not yet been fully elucidated. However, uptake of cisplatin by transporters selectively expressed in certain organs might contribute to the distinct pattern of organ toxicities induced by cisplatin [27–32]. So far, various groups confirmed cisplatin transport by SLC22A2 [29–32]. Moreover, apart from the kidney SLC22A2 is expressed in the cochlea of mice, and SLC22A1/2 deficient mice were less prone to cisplatin-induced ototoxicity [30]. We addressed the role of SLC22A2 for cisplatin-induced ototoxicity in humans and analyzed retrospectively in an explorative manner the allele frequencies of 11 SNPs in the SLC22A2 gene in a cohort 194 children and adolescents who either themselves and/or whose guardians agreed to participate in a study aiming at the identification of pharmacogenetic risk factors predicting cisplatin-induced ototoxicity.
We included three nonsynonymous SNPs in the SLC22A2 gene, namely rs8177513, rs316019 and rs8177509, because the resulting amino acid exchanges are supposed to affect transport functions of SLC22A2. The SNPs rs316019 and rs8177509 are located in the transmembrane domain of the SLC22A2 protein, while rs8177513 is located in the cytoplasmatic domain [37]. The nonsynonymous SNPs were primarily chosen as tag SNPs because of their reported allele frequencies in order to increase the probability to detect differently distributed allele frequencies between group N and O. The observed minor allele frequencies of the nonsynonymous SNPs rs8177513 and rs8177509 were found to be below 1%, and only one G allele of rs8177513 was detected in one out of 194 patients screened. We noted a statistically significant association between the nonsynonymous SNP rs316019 and cisplatin-induced ototoxicity, in that the T allele occurred more frequently in patients who experienced no ototoxicity from cisplatin treatment.
Grading of ototoxicity is crucial for the evaluation of risk factors and for the comparison of results from different studies addressing these issues. Compared with the NCI-CTCAE scale [6], the Brock [8] and the SIOP Boston [1] ototoxicity scales, which were primarily developed as outcome measures to report the incidence and severity of acquired hearing loss in children after completion of platinum-based chemotherapies, the Muenster classification was optimized for the early detection of ototoxicity during cisplatin treatment. Considering tinnitus and minimal hearing loss (>10 to ≤20 dB HL) as grade 1 the Muenster classification consequently identified more patients with hearing impairment than the NCI-CTCAE scale and the Brock scale [1, 8, 11]. Despite of using the more sensitive Muenster classification for phenotyping of the pediatric cohort, we were also able to confirm significant findings for rs316019 in the adult cohort graded for ototoxicity according to the less sensitive NCI-CTCAE criteria. The fact that in contrast to the pediatric cohort no T allele carriers were observed in the group of adults with ototoxicity might be explained by the use of the less sensitive NCI-CTCAE criteria.
Apart from ototoxicity grading the definition of the study population is critical for the evaluation of genetic risk factors for cisplatin-induced ototoxicity. Because irrespective of tumor type and treatment protocol all children treated with cisplatin are at risk to experience ototoxicity, we decided to admit all patients who were supposed to receive cisplatin to our study. Due to different diagnoses and treatment protocols cisplatin administration varied with respect to dose and infusion rates, which are both significantly associated with patients’ risk to suffer from cisplatin-induced ototoxicity. Though cumulative cisplatin doses ranged from 120 to 644 mg/m2 and infusion rates from 0.028 to 1.33 mg/min, neither the cumulative cisplatin doses administered nor infusion rates differed between group N and group O (Table 1). In addition, the infusion rates and cisplatin dose were not significantly different between patients, who carried the 808G>T variant and those who did not (p > 0.05, Mann–Whitney U test). Thus, patients carrying the 808G>T variant were not protected because of lower cisplatin-doses or lower cisplatin infusion rates. To exclude confounding factors we defined a number of eligibility criteria with the result that only one third of the 194 genotyped patients was eligible for final comparison. Thirty five percent of patients were considered ineligible because they were already at risk to experience ototoxicity because of cranial irradiation, pre-existing renal impairment or an age <5 years. Because preexisting hearing impairment was reported to increase the risk for cisplatin-induced ototoxicity [5], we decided to require a complete and reliable baseline evaluation before onset of therapy in order to identify and exclude those patients. In addition, an evaluable audiogram within 6 month after completion of therapy was also required to confirm existence or continued absence of ototoxicity. Due to the lower noise thresholds used by the Muenster classification all audiograms had to be performed at the Department of Phoniatrics and Pedaudiology at the University Hospital of Muenster to guarantee that all examinations were carried out and evaluated by accordingly experienced staff and to reduce artifacts (e.g., by earphone placement) and inter-rater variation. Implementing these criteria another 49 patients (approximately 25% of the initial cohort) had to be excluded from final analysis, which is comparable to drop outs reported from other studies addressing cisplatin-induced ototoxicity in children [16]. The excluded patients might well have compromised the final study results. However, the allele distribution in the group of excluded patients did not differ from the group of all patients (n = 194) and the patients included in the final comparison (n = 64). This indicates that no imbalance with respect to allele distribution was introduced by the exclusion of patients not meeting the inclusion criteria for this study (Supplementary Table 2). Moreover, the replication of the pediatric study results in a cohort of adult patients did not suggest an exclusion bias.
Though we tried to eliminate age as confounder by exclusion of patients <5 years [14], the patients in the group O were significantly younger compared with the group N and logistic regression analysis identified a significant association between children’s age and their risk to experience ototoxicity by cisplatin treatment. However, the multiple logistic regression model confirmed a significant association between cisplatin ototoxicity and rs316019 as well as age. In the adult cohort no significant differences between group N and O were observed with respect to age. This is in line with previous studies in a cohort of 400 adult cancer patients, which observed no influence of patients’ demographics such as age, tumor type, concurrent chemotherapy (taxol or VP16), serum chemistry parameters and environmental variables such as smoking, alcohol on cisplatin ototoxicity in adult patients [38].
In addition our data are in line with previous observations that T allele carriers of rs316019 were less prone to cisplatin-induced nephrotoxicity [31, 32]. However, in our study nephrotoxicity was not ascertained along with ototoxicity, because the vigorous hydration regimens prescribed in the pediatric treatment protocols virtually eliminated cisplatin nephrotoxicity [39].
Reduced Km and Vmax values along with reduced uptake of SLC22A2 substrates like 1-methy-4-phenylpyridinium, dopamine, norepinephrine and propranolol were reported by the SLC22A2 variant 808G>T [37, 40–42]. Supposing that in humans, like in mice, SLC22A2 is expressed in the cochlea, and that the SLC22A2 variant 808G>T has a reduced affinity for cisplatin as well, one might speculate that a reduced accumulation of cisplatin in the inner ear protects this organ from the deleterious effects of cisplatin and, thus prevents from cisplatin-induced ototoxicity [29, 30]. However, because the effects of the SLC22A2 variant 808G>T on cisplatin transport as well as the expression of SLC22A2 in the human cochlea still need to be determined, the exact mechanism underlining the observed phenomenon of less ototoxicity in T allele carriers of rs316019 remains unknown.
We also analyzed the SNP rs10981694 in the SLC31A1 gene. But, in contrast to a pharmacogenetic study in adults with non-small-cell lung cancer, we failed to find an association between the SNP rs10981694 of the SLC31A1 gene and cisplatin-induced ototoxicity [26]. The observation that genetic deletion of SLC22A1 and SLC22A2 or treatment with the SLC22A2 inhibitor cimetidine were sufficient to protect mice from cisplatin-induced ototoxicity, suggests that SLC31A1, though expressed in the inner ear and involved in cisplatin transport, is probably less important for cisplatin-induced ototoxicity [29, 30]. On the other hand replication failures occur and need careful reflection. A high-throughput screening approach identified variants of the TPMT and the COMT gene to be significantly associated with an increased risk for cisplatin-induced ototoxicty. Though validated and replicated in large cohorts of pediatric cancer patients with various tumors, it could not be replicated in a large cohort of medulloblastoma patients [19, 20, 43]. The composition of the study population by patient numbers, demographics and ethnicities, the definition of inclusion criteria and phenotype classification affect the identification of risk factors and can lead to contradictory results. Moreover, if complex networks of interacting pathways are involved, variations in multiple genes might affect the phenotype to different degrees, thereby complicating associations between geno- and phenotype. This and different ethnicities (Asian and Caucasian) may be responsible for the different results regarding the role of the SLC31A1 SNP rs10981694 for cisplatin-induced ototoxicity in the study of Xu et al. and our studies [26].
In this respect, the replication of the significant findings for rs316019 in the pediatric cohort in an entirely different cohort has to be acknowledged accordingly. But, before these findings can be considered for clinical decision-making independent confirmation by prospective studies in children and adults are warranted.
Conclusion
In conclusion, we were able to link the nonsynonymous SNP rs316019 of the SLC22A2 gene, which adversely affects transport of SLC22A2 substrates, to patients’ individual risk to experience cisplatin-induced totoxicity. Taking into account that in mice reduced expression of SLC22A 1 and 2 protects from cisplatin-induced ototoxicity, our results point to a critical role of the SLC22A2 transporter in the in vivo handling of cisplatin and its contribution to the organ specific side effects of this drug.
Future perspective
We identified a novel, plausible pharmacogenomic marker, which should be considered in further pharmacogenomics studies aiming at the definition of algorithms for the individual risk of patients to experience cisplatin-induced ototoxicity. Furthermore, our results point to the critical role of SLC22A2 for cisplatin-induced ototoxicity in humans. This provides a direct rationale for the development of novel targeted approaches to mitigate cisplatin-induced ototoxicity through the use of SLC22A2 inhibitors provided that the inhibitors do not interfere with the antitumor effect of cisplatin.
Supplementary Material
Executive summary.
Background
Assuming that genetic variants of the SLC22A2 gene affect patients’ interindividual susceptibility to cisplatin-induced ototoxicity, we analyzed the distribution of 11 SLC22A2 variants and the SLC31A1 SNP rs10981694 in pediatric and adult cancer patients who experienced cisplatin-induced ototoxicity and those who did not.
Results
The SLC22A2 SNP rs316019, which adversely affects SLC22A2 function, protected from cisplatin-induced ototoxicity in the initial cohort of pediatric patients as well as in the independent replication cohort of adult patients.
Conclusion
Apart from the identification of rs316019 as potential pharmacogenomic marker for protection from cisplatin-induced ototoxicity our results indicate that interference with SLC22A2 function effects cisplatin-induced ototoxicity in humans and, thus, stimulate further efforts to prevent cisplatin-induced ototoxicity by selective inhibition of SLC22A2 function.
Acknowledgments
This study was supported by the Deutsche Krebshilfe Grant 108539 (to G Ciarimboli, AG am Zehnhoff-Dinnesen and H Jürgens), the Interdisciplinary Center for Clinical Research (IZKF) Münster Grant Cia2/013/13 (to G Ciarimboli, AG am Zehnhoff-Dinnesen and E Schlatter), the American Lebanese Syrian Associated Charities (ALSAC), USPHS Cancer Center Support Grant P30CA021765 and NCI Grant 5R01CA151633 (to A Sparreboom).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1. Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J. Clin. Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110.. • Comprehensive review about clinical relevance, supposed mechanisms and grading of cisplatin-induced ototoxicity
- 2.Knijnenburg SL, Mulder RL, Schouten-Van Meeteren AY, et al. Early and late renal adverse effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst. Rev. 2013;10:CD008944. doi: 10.1002/14651858.CD008944.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J. Clin. Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 4.Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early changes in auditory function as a result of platinum chemotherapy: use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J. Clin. Oncol. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 5.Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br. J. Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallegos-Castorena S, Martínez-Avalos A, Mohar-Betancourt A, Guerrero-Avendaño G, Zapata-Tarrés M, Medina-Sansón A. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatr. Hematol. Oncol. 2007;24:403–408. doi: 10.1080/08880010701451244. [DOI] [PubMed] [Google Scholar]
- 7.Fisher MJ, Lange BJ, Needle MN, et al. Amifostine for children with medulloblastoma treated with cisplatin-based chemotherapy. Pediatr. Blood Cancer. 2004;43:780–784. doi: 10.1002/pbc.20132. [DOI] [PubMed] [Google Scholar]
- 8.Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Med. Pediatr. Oncol. 1991;19:295–300. doi: 10.1002/mpo.2950190415. [DOI] [PubMed] [Google Scholar]
- 9.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J. Clin. Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 10.Khan AB, D’Souza BJ, Wharam MD, et al. Cisplatin therapy in recurrent childhood brain tumors. Cancer Treat. Rep. 1982;66:2013–2020. [PubMed] [Google Scholar]
- 11.Schmidt CM, Bartholomäus E, Deuster D, Heinecke A, Dinnesen AG. The “Muenster classification” of high frequency hearing loss following cisplatin chemotherapy. HNO. 2007;55:299–306. doi: 10.1007/s00106-005-1368-1. [DOI] [PubMed] [Google Scholar]
- 12.Lafay-Cousin L1, Purdy E, Huang A, et al. Early cisplatin induced ototoxicity profile may predict the need for hearing support in children with medulloblastoma. Pediatr. Blood Cancer. 2013;60:287–292. doi: 10.1002/pbc.24307. [DOI] [PubMed] [Google Scholar]
- 13.Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin-associated ototoxicity in pediatric oncology patients. Pediatr. Blood Cancer. 2012;59:144–148. doi: 10.1002/pbc.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. Eur. J. Cancer. 2004;40:2445–2451. doi: 10.1016/j.ejca.2003.08.009.. • Sound study on risk factors for cisplatin-induced ototoxicity in children.
- 15.Lewis MJ, DuBois SG, Fligor B, Li X, Goorin A, Grier HE. Ototoxicity in children treated for osteosarcoma. Pediatr. Blood Cancer. 2009;52:387–391. doi: 10.1002/pbc.21875. [DOI] [PubMed] [Google Scholar]
- 16.Landier W, Knight K, Wong FL, et al. Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales – a report from the Children’s Oncology Group. J. Clin. Oncol. 2014;32:527–534. doi: 10.1200/JCO.2013.51.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuur CL, Simis YJ, Lansdaal PE, et al. Risk factors of ototoxicity after cisplatin-based chemo-irradiation in patients with locally advanced head-and-neck cancer: a multivariate analysis. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1320–1325. doi: 10.1016/j.ijrobp.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 18.Skinner R, Pearson AD, Amineddine HA, Mathias DB, Craft AW. Ototoxicity of cisplatinum in children and adolescents. Br. J. ancer. 1990;61:927–931. doi: 10.1038/bjc.1990.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross CJ, Katzov-Eckert H, Dubé MP, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat. Genet. 2009;41:1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 20.Pussegoda K, Ross CJ, Visscher H, et al. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children. Clin. Pharmacol. Ther. 2013;94:243–251. doi: 10.1038/clpt.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters U, Preisler-Adams S, Hebeisen A, et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs. 2000;11:639–643. doi: 10.1097/00001813-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione S-transferase genotypes in testicular cancer survivors. J. Clin. Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 23.Caronia D, Patiño-García A, Milne RL, et al. Common variations in ERCC2 are associated with response to cisplatin chemotherapy and clinical outcome in osteosarcoma patients. Pharmacogenomics J. 2009;9:347–353. doi: 10.1038/tpj.2009.19. [DOI] [PubMed] [Google Scholar]
- 24.Riedemann L, Lanvers C, Deuster D, et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8:23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- 25.Choeyprasert W, Sawangpanich R, Lertsukprasert K, et al. Cisplatin-induced ototoxicity in pediatric solid tumors: the role of glutathione S-transferases and megalin genetic polymorphisms. J. Pediatr. Hematol. Oncol. 2013;35:e138–e143. doi: 10.1097/MPH.0b013e3182707fc5. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Ren H, Zhou B, et al. Prediction of copper transport protein 1 (CTR1) genotype on severe cisplatin induced toxicity in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2012;77:438–442. doi: 10.1016/j.lungcan.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Ivy KD, Kaplan JH. A re-evaluation of the role of hCTR1, the human high-affinity copper transporter, in platinum-drug entry into human cells. Mol. Pharmacol. 2013;83:1237–1246. doi: 10.1124/mol.113.085068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katano K, Kondo A, Safaei R, et al. Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res. 2002;62:6559–6565. [PubMed] [Google Scholar]
- 29.More SS, Akil O, Ianculescu AG, Geier EG, Lustig LR, Giacomini KM. Role of the copper transporter, CTR1, in platinum-induced ototoxicity. J. Neurosci. 2010;30:9500–9509. doi: 10.1523/JNEUROSCI.1544-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ciarimboli G, Deuster D, Knief A, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am. J. Pathol. 2010;176:1169–1180. doi: 10.2353/ajpath.2010.090610.. •• Preclinical evidence for the role of SLC22A2 in cisplatin transport and protection from cisplatin ototoxicity by downregulation of SLC22A1/2 expression and SLC22A2 inhibition.
- 31.Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin. Pharmacol. Ther. 2009;86:396–402. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filipski KK, Loos WJ, Verweij J, Sparreboom A. Interaction of cisplatin with the human organic cation transporter 2. Clin. Cancer Res. 2008;14:3875–3880. doi: 10.1158/1078-0432.CCR-07-4793. [DOI] [PubMed] [Google Scholar]
- 33.US FDA Drug Interactions & Labeling. www.fda.gov.
- 34.Loos WJ, de Jongh FE, Sparreboom A, et al. Evaluation of an alternate dosing strategy for cisplatin in patients with extreme body surface area values. J. Clin. Oncol. 2006;24:1499–1506. doi: 10.1200/JCO.2005.03.0056. [DOI] [PubMed] [Google Scholar]
- 35.Haploview. www.broadinstitute.org/scientificcommunity/science/programs/medical-and-population-genetics.
- 36.International HapMap Project. www.hapmap.org.
- 37.Leabman MK, Huang CC, Kawamoto M, et al. Pharmacogenetics of Membrane Transporters Investigators. Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function. Pharmacogenetics. 2002;12:395–405. doi: 10.1097/00008571-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 38.de Jongh FE, van Veen RN, Veltman SJ, et al. Weekly high-dose cisplatin is a feasible treatment option: analysis on prognostic factors for toxicity in 400 patients. Br. J. Cancer. 2003;88:1199–1206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanvers-Kaminsky C, Krefeld B, Dinnesen AG, et al. Continuous or repeated prolonged cisplatin infusions in children: a prospective study on ototoxicity, platinum concentrations, and standard serum parameters. Pediatr. Blood Cancer. 2006;47:183–193. doi: 10.1002/pbc.20673. [DOI] [PubMed] [Google Scholar]
- 40. Zolk O, Solbach TF, König J, Fromm MF. Functional characterization of the human organic cation transporter 2 variant p.270Ala>Ser. Drug Metab. Dispos. 2009;37:1312–1318. doi: 10.1124/dmd.108.023762.. • Describes altered substrate affinity of the SLC22A2 rs316019 variant compared with wild-type SLC22A2.
- 41.Kang HJ, Song IS, Shin HJ, et al. Identification and functional characterization of genetic variants of human organic cation transporters in a Korean population. Drug Metab. Dispos. 2007;35:667–675. doi: 10.1124/dmd.106.013581. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Zhou W. Ameliorative effects of SLC22A2 gene polymorphism 808 G/T and cimetidine on cisplatin-induced nephrotoxicity in Chinese cancer patients. Food Chem. Toxicol. 2012;50:2289–2293. doi: 10.1016/j.fct.2012.03.077. [DOI] [PubMed] [Google Scholar]
- 43.Yang JJ, Lim JY, Huang J, et al. The role of inherited TPMT and COMT genetic variation in cisplatin-induced ototoxicity in children with cancer. Clin. Pharmacol. Ther. 2013;94:252–259. doi: 10.1038/clpt.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.