Abstract
Objective
Surgical hindlimb ischemia (HLI) in mice has become a valuable preclinical model to study peripheral arterial disease (PAD). We previously identified that the different phenotypical outcomes following HLI across inbred mouse strains is related a region on the short arm of mouse chromosome 7. The gene coding the interleukin-21 receptor (IL-21R) lies at the peak of association in this region.
Approach and Results
With quantitative RT-PCR, we found that a mouse strain with a greater ability to up-regulate IL-21R following HLI had better perfusion recovery than a strain with no up-regulation after HLI. Immunofluorescent staining of ischemic hind-limb tissue showed IL-21R expression on endothelial cells (EC) from these C57BL/6 mice. An EC-enriched fraction isolated from ischemic hind-limb muscle showed higher Il-21R levels than an EC-enriched fraction from non-ischemic limbs. In-vitro, human umbilical vein EC (HUVEC) showed elevated IL-21R expression after hypoxia and serum starvation. Under these conditions, IL-21 treatment increased cell viability, decreased cell apoptosis, and augmented tube formation. In-vivo, either knockout Il21r or blocking IL-21 signaling by treating with IL-21R-Fc (fusion protein that blocks IL-21 binding to its receptor) in C57BL/6 mice resulted in less perfusion recovery after HLI. Both in-vitro and in-vivo modulation of the IL-21/IL-21R axis under hypoxic conditions resulted in increasedSTAT3 phosphorylation and a subsequent increase in the BCL-2/BAX ratio.
Conclusion
Our data indicate that IL-21R up-regulation and ligand activation in hypoxic endothelial cells may help perfusion recovery by limiting/preventing apoptosis and/or favoring cell survival and angiogenesis through the STAT3 pathway.
Keywords: Interleukin 21 receptor, Peripheral arterial diseases, Angiogenesis, STAT3 pathway
Introduction
While rates of death from ischemic heart disease in the United States have fallen over the past decades, the prevalence of peripheral arterial disease (PAD) has actually increased and PAD will become an even greater health care problem in the years ahead as the major drivers for PAD are advancing age, smoking, and diabetes1–5. The primary problem in PAD is reduced blood flow to the leg and since total occlusions in one or more of the major inflow arteries to the leg(s) is common in patients with PAD, the magnitude of distal blood flow becomes dependent on the number and extent of collateral blood vessels that connect to the distal microvasculature6, 7. Current medical therapies used to treat PAD include anti-platelet agents, statins to lower cholesterol, anti-hypertensive therapy with angiotensin converting enzyme inhibitors or receptor blocker and/or beta-blockers, blood glucose control and smoking cessation8, 9. However, there are no agents that have shown the ability to improve leg blood flow in patients with PAD and thus no medical therapies are available to directly treat the primary problem of reduced blood flow. New treatment paradigms are needed for PAD6.
The response that follows surgically induced hind-limb ischemia (HLI) in mice has been widely used to test agents to treat PAD10, 11. Genetic factors modify the clinical outcomes when PAD is present12, 13. Our lab and others have shown that C57BL/6 mice recover from HLI much better than BALB/c mice11, 14. We previously showed that a quantitative trait locus that spans a 31 million base pair region on the short arm of mouse chromosome 7 (termed LSq-1) was sufficient to determine the extent of perfusion recovery and tissue loss after HLI and using the outcome additional inbred mouse strains gene blocks within this region were identified11. The interleukin-21 receptor (IL-21R) whose modulation is being clinically studied in a variety of disease15–17, lies at or/near the peak of the strength of association across this region based on locations are based on the NCBI37/mm10 genome (Supplemental Figure. 1).
IL-21 is a type I cytokine whose receptor is one of six receptors that forms a heterodimeric receptor complex with the common γ chain (γc)15, 18 IL-21 has pleiotropic actions and regulates the differentiation and function of lymphoid and myeloid cells19, 20. Due to its ability to regulate immune responses, enhancing or inhibiting the action of IL-21 or IL-21R has been demonstrated to have therapeutic effects on a wide range of diseases21. To the best of our knowledge there is no information for a role of IL-21R in PAD, but phase I and II clinical trials investigating the effects of administering IL-21 as an anti-cancer agent or blocking IL-21R in autoimmune disease are underway16, 17. Here, we present data suggesting that IL-21R upregulation and activation induces a protective effect in hypoxic endothelium, and modulation of IL-21R needs to be studied in PAD.
Material and Methods
Materials and Methods are available in the online-only Supplement.
Results
1. IL-21 receptor mRNA level varies greatly between C57BL/6 and BALB/c in ischemic muscle tissue
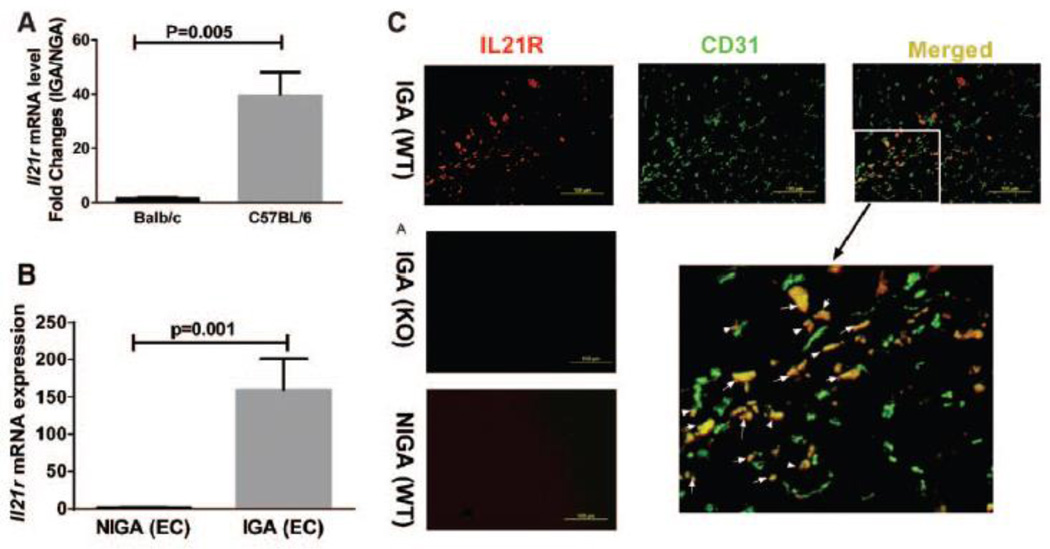
Although C57BL/6 and BALB/c mice have different outcomes following HLI11, 22, the degree of perfusion recovery was comparable at an early (day 3) time point following HLI11. We first studied 5 mice from each strain at day 3 post-HLI by qPCR, Il21r mRNA level was much more highly up-regulated in ischemic hindlimb muscles from C57BL/6 mice (~39-fold) than from BALB/c (~1.7-fold) mice (Figure 1A) when. Furthermore, the Il21r up-regulation after ischemia in C57BL/6 mice was also found in earlier (day 1 post-HLI) and later (days 7 and 21, post-HLI) time points (Supplemental Figure II).
Figure 1. Interleukin-21 receptor (IL-21R) levels vary greatly between C57BL/6 and BALB/c mice after hindlimb ischemia (HLI).
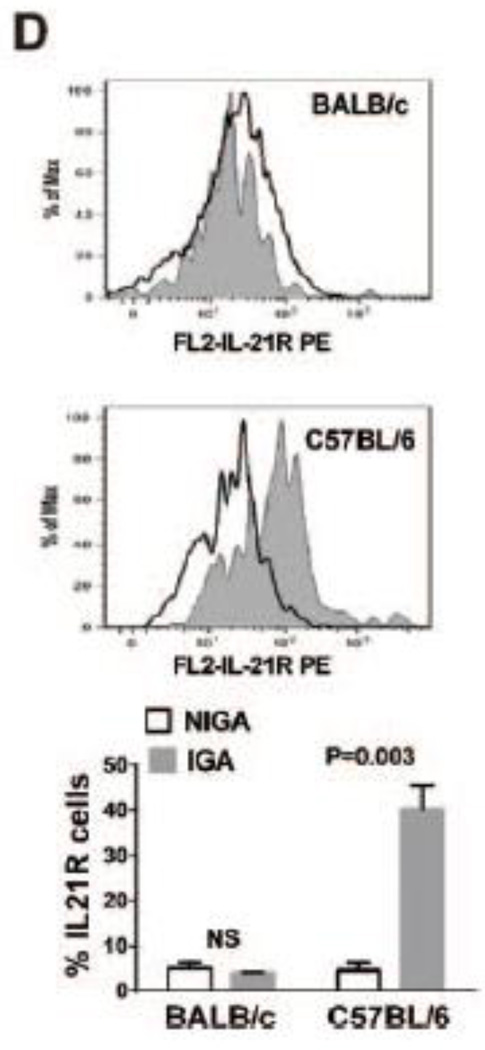
(A) Three days after HLI, ischemic gastrocnemius muscle from C57BL/6 mice showed upregulation (~39-fold) of Il21r mRNA level compared to non-ischemic gastrocnemius muscle. BALB/c mice also showed Il21r mRNA level upregulation (~1.7-fold) after HLI. However, the fold changes in BALB/c mice were significantly smaller than C57BL/6 mice (p<0.001, n = 5/group). (B) Il21r mRNA level of CD31+ (CD31 is an endothelial cell marker) cell isolated from C57BL/6 mouse hindlimb muscle tissue is significantly up-regulated ~160-fold) 3 days after HLI when compared to endothelial cells from non-ischemic hindlimb. (p<0.001, n = 4~5/group). (C) Immunofluorescence of ischemic and non-ischemic gastrocnemius muscle IL-21R (red), CD31 (green) and merged from WT or Il21r−/− C57BL/6 mice; co-staining of CD31 and IL21R can be visualized in the ischemic muscle from the WT mice. IL-21R was not detected either in muscle tissue from non-ischemic limb or in the ischemic muscle tissue from Il21r−/− mice. (D) FACS analysis in CD31+ cell fraction isolated from IGA and NGA, IL-21R level in CD31 cells showed a significant increase in IGA compared to NGA from C57BL/6 mouse, but did not show statistical difference from BALB/c mouse. IGA indicates ischemic gastrocnemius muscle, NGA indicates non-ischemic gastrocnemius muscle. WT, wild type. KO, Il21r knockout.
We sought to determine whether endothelial cells contribute to the IL21R elevation after HLI, CD31+ cells were isolated from C57BL/6 hindlimb23, and showed a higher IL-21R mRNA expression (~159-fold, Figure 1B) in CD31+ cells from the ischemic side. Immunofluorescence of the ischemic muscle from C57BL/6 mice at day 3 post-HLI showed numerous examples of co-staining of IL-21R with CD31 (Figure 1C). Moreover, flow cytometry showed that CD31+ fractions from ischemic hindlimb muscle had higher IL-21R protein level than the CD31+ fraction from nonischemic hindlimb muscle in C57BL/6 mice, but CD31+ fraction from BALB/c mice did not show a difference of IL-21R between ischemic and nonischemic muscle (Figure 1D). We also examined the levels of IL-21 protein by western blotting using lysates from ischemic muscle from BALB/c and C57BL/6 mice, but did not find any difference (Supplemental Figure III).Thus differences between C57BL/6 and BALB/c was at the level of the IL-21 receptor, not its ligand.
2. IL-21R activation has pro-survival effects in endothelial cells under hypoxia serum starvation (HSS) conditions
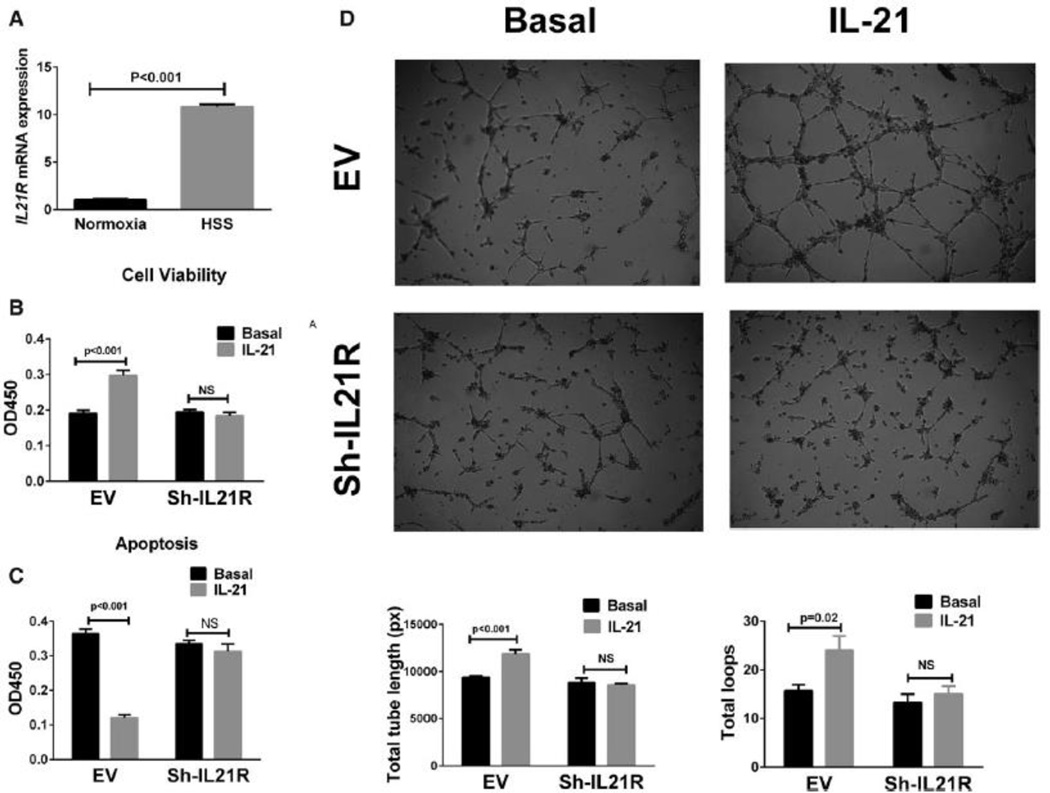
The association of better outcomes after HLI in C57BL/6 mice compared to BALB/c mice and greater IL-21R expression in EC isolated from C57BL/6 ischemic muscle led us to look for an in-vitro correlate. Using methods similar to those previously described10, in-vitro endothelial cells (HUVECs) showed ~10 fold IL-21R expression (p<0.05) when exposed to HSS (Figure 2A). Under HSS conditions that induced IL-21 receptor up-regulation, treatment of HUVEC with IL-21 (50 ng/mL) increased cell viability (Figure 2B), reduced cell apoptosis (Figure 2C) and enhanced endothelial tube formation in Matrigel models (Figure 2D). The specificity of these effects induced by IL-21 was demonstrated by the inhibition of survival benefit when using human IL21R shRNA (Figure 2 B–D, Supplemental Figure IV) and IL-21R-Fc fusion protein respectively24. Collectively, these data indicate a pro-angiogenic/anti-apoptotic effect from activation of the IL-21/IL-21R axis in-vitro in the setting of HSS. However, under normoxic conditions, IL-21 treatment did not change the survival of HUVECs (Supplemental Figure V).
Figure 2. IL-21R expression and the effect of IL-21 treatment in human umbilical vein endothelial cells (HUVECs).
(A) Following 24 hours of hypoxia and serum starvation (HSS), qPCR showed ~10-fold increase in il21R mRNA expression when compared to HUVECs cultured under normoxia (normoxia indicates cells cultured under normal condition). (B) More viable cells were detected with 24h after IL-21 (50 ng/mL) treatment for in HSS conditions. Cell viability assay is based on the cleavage of the tetrazolium salt to formazan by cellular mitochondrial dehydrogenase. (C) In situ terminal dUTP nick end-labeling (TUNEL) showed that IL-21 (50 ng/mL) treatment for 24h decreases HUVEC cell apoptosis in HSS conditions. (D) HUVECs were plated on matrigel with reduced growth factor and incubated for 10 hours in HSS conditions with basal or treatment medium, IL-21 treated HUVECs showed enhanced tube formation, which was quantified as total length of the cords per visual filed, and total loops per visual field as represented by the bar graph. The lL-21 effects on HUVECs viability, apoptosis and tube formation (B-D) were inhibited when IL-21R expression was knocked down by using shRNA. All the above data are representative of 2–3 separate batches of HUVECs, n = 8–12 samples/group.
Vascular smooth muscle cells (VSMC) and myocytes are also important cell types for the recovery from HLI. However, in a cultured, immortalized myocyte cell line (C2C12), IL-21R was expressed at a low level and did not show any difference in expression under hypoxic conditions (Supplemental Figure VI). Similarly, in cultured VSMC, IL-21R was not detectable either in normoxic or hypoxic conditions. By immunofluorescence of ischemic gastrocnemius muscle IL-21R and α-smooth muscle actin (α-SMA) showed little overlap (Supplemental Figure VII).
3. IL-21 treatment regulates the STAT3 pathway in endothelial cells under HSS
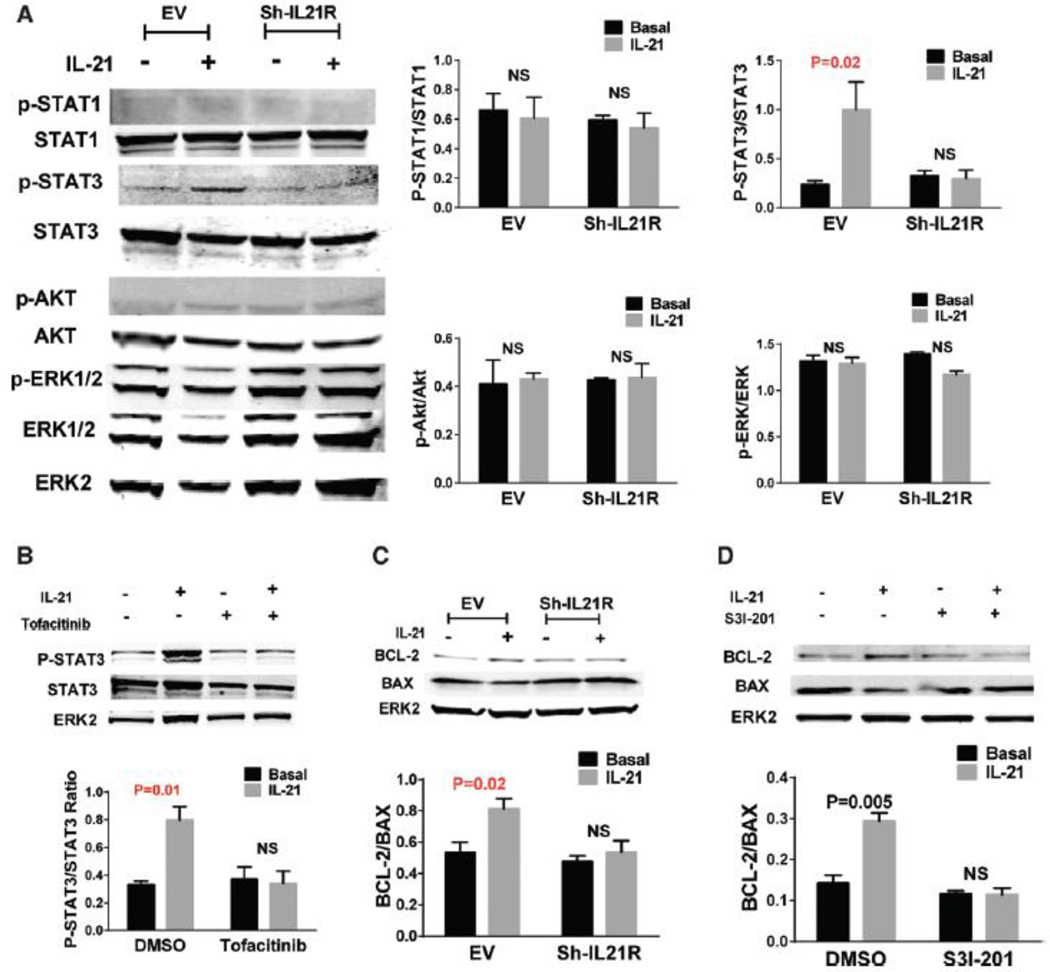
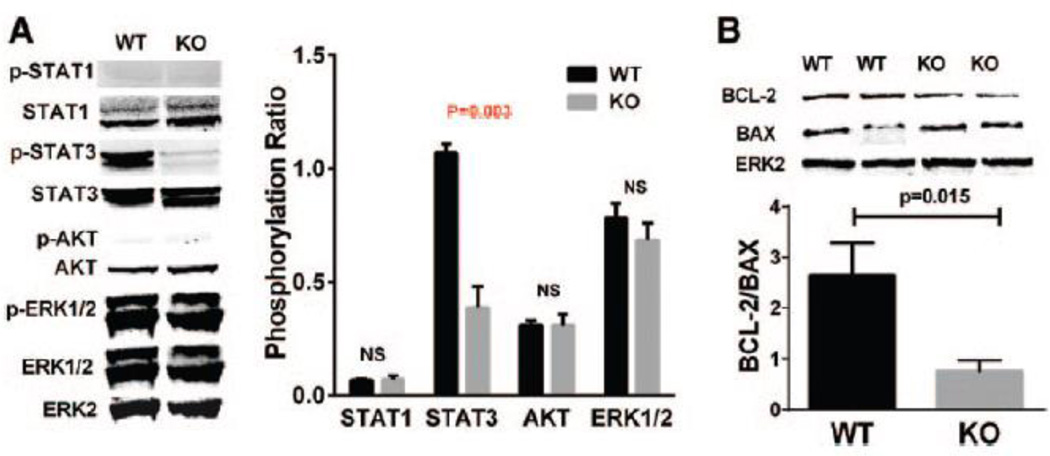
Signal transducer and activator of transcription (STAT) 1, STAT3, AKT and extracellular-signal-regulated kinases1/2 (ERK1/2) pathways are activated by IL-21 in various cell types under different conditions19, 25. To test whether these signaling pathways were activated by IL-21 treatment under HSS conditions, we performed western blot analysis on protein lysates from HUVECs. After 24h exposure to HSS conditions, which induces IL-21R expression, HUVECs were treated with 50 ng/mL IL-21 for a series of time points. IL-21 induced peak increase of STAT3 phosphorylation 15 minutes after treatment, but did not show significant changes in STAT1, AKT1 or ERK1/2 phosphorylation at any time point (Supplemental Figure VIII). The IL-21 induced STAT3 phosphorylation was abolished by either IL-21R ShRNA or Janus kinase 3 (JAK3) inhibitor (tofacitinib, 5µM) (Figure 3 A & B), which indicated that IL-21 activates STAT3 pathways via IL-21R and JAK3 in hypoxic endothelial cells.
Figure 3. IL-21 increases STAT3 phosphorylation and BCL-2/BAX ratio in HUVECs under HSS conditions.
(A) After cultured under HSS conditions for 24h, HUVECs were treated with 50 ng/mL IL-21 or saline (control) for 15 minutes and then harvested for western blotting. IL-21 treatment induced higher STAT3 phosphorylation, but did not significantly change STAT1, AKT or ERK1/2 phosphorylation. The lL-21 effects on STAT3 phosphorylation were inhibited when IL-21R expression was knocked down by using shRNA. (B)Phosphorylation of STAT3 is not changed with IL-21 treatment when Janus kinase 3 (JAK3) were inhibited by tofacitinib. (C) Western blotting of HUVEC 24h after IL-21 treatment under HSS conditions showed higher BCL-2/BAX ratio compared to saline treated. The lL-21 effects on BCL-2/BAX increase were blunted when IL-21R were knocked down by shRNA. (D) BCL-2/BAX ratio is not changed with IL-21 treatment when STAT3 were inhibited by S3I-201. The above data are representative of 2 separate batches of HUVECs, n = 4/group.
The ratio of B-cell lymphoma leukemia-2 (BCL-2)/BCL-2–associated X protein (BAX) expression has been reported to be regulated by STAT3 phosphorylation26. To investigate whether BCL-2 or BAX expression were modulated in our experiment, HUVECs were treated with IL-21 under HSS conditions, which showed increased ratio of BCL-2/BAX 24h after treatment compared to vehicle treatment by Western blot analysis (Figure 3C). The specificity of the IL-21 effects was demonstrated by blunted BCL-2/BAX changes when using IL-21R ShRNA (Figure 3 B–D).
We next investigate whether STAT3 phosphorylation induced by IL-21 signaling is required for the survival effects of IL-21 treatment in hypoxic endothelial cells. And found that STAT3 inhibition blunted IL-21 induced cell viability, tube formation (Supplemental Figure IX) and BCL-2/BAX increase (Figure 3D). To determine whether the increase of BCL-2 level induced by IL-21 signaling is required for the protective effects in endothelial cells under HSS, we reduced BCL-2 level by using siRNA (Supplemental Figure X). With BCL-2 knock-down, HUVECs incubated in IL-21 did not show significant change in cell viability and tube formation (Supplemental Figure IX). These data show that IL-21-mediated STAT3 phosphorylation and BCL-2 increase contribute to cell survival and tube formation in hypoxic endothelial cells. Immunofluorescence staining shows that STAT3 and Caspase 3 were expressed in the endothelial cells in the ischemic muscle tissue from mouse HLI model (Supplemental Figure XI).
4. Removal of IL-21 ligand or knockout of IL-21R impairs perfusion recovery and increases tissue loss, in-vivo, following HLI
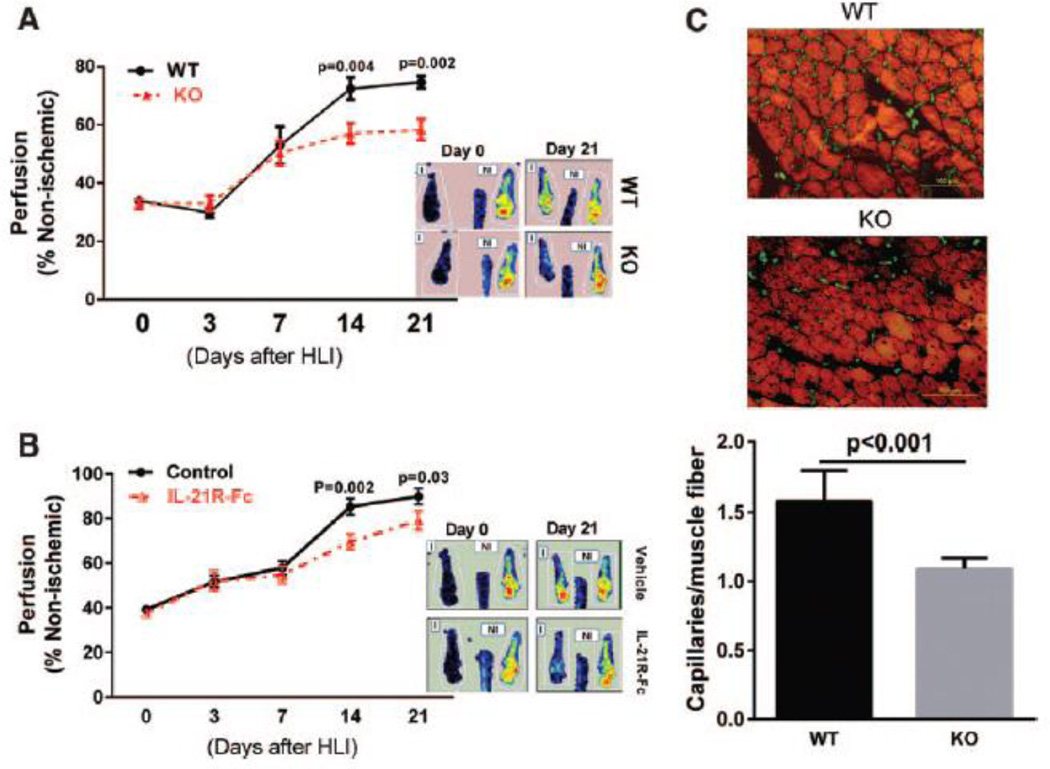
We monitored perfusion recovery after HLI in Il21r−/− mice compared to age-matched and gender-matched WT littermates and found that Il21r−/− mice showed attenuated perfusion recovery starting at day 14 and continued through day 21 after HLI (Figure 4A). Consistent with poorer perfusion recovery, Il21r−/− mice tended to show higher, though not statistically different, rates of tissue loss (Table 1). Ischemic muscle tissue from Il21r−/− mice, 21 days after HLI, showed a lower capillary density than WT littermates (Figure 4C).
Figure 4. Loss of IL-21R signaling results in impaired perfusion recovery following HLI.
(A) Laser Doppler imaging showed significantly impaired perfusion recovery in Il21r−/− mice in C57BL/6 background compared to gender-matched and age matched wide type (WT) littermates at day14 and 21 after HLI (n=9–12 per group). (B) WT C57BL/6 mice that received 6 intraperitoneal (i.p) doses of IL-21R-Fc fusion protein (200 mg/mouse at days 0, 1, 3, 7, 9 and 11 after HLI) showed significantly lower perfusion recovery when compared with vehicle treated mice (control) at days 14 and 21 after HLI (n = 11–12/group). (C) At day 21 after HLI, ischemic gastrocnemius muscle from Il21r−/− mice showed significant lower capillary density than WT littermates (n = 9/group). Average capillaries per muscle fiber: 1.6 ± 0.1 vs 1.1 ± 0.1, p<0.001. Data = mean ± SEM. WT, wild type. KO, Il21r knockout.
Table 1.
Severity of Necrosis in Mice after Hindlimb Ischemia (HLI)
| Genotype | n | Mice with any necrosis |
Necrosis Grade | ||
|---|---|---|---|---|---|
| I | II | III | |||
| Il21r (+/+) | 9 | 2/9 (22) | 2/9 (22) | None | None |
| Il21r (−/−) | 12 | 5/12 (42) | 3/12 (25) | 1/12 (8.3) | 1/12 (8.3) |
Necrosis grade is from 0 through 4, as described in Methods. Values are expressed as number of mice affected in each category/total number of mice in the category (%). Distribution is to the right. IL21R knockout mice showed a higher necrosis rate compared to the wide type littermates after HLI though not statistical different.
To determine whether IL-21R in leukocytes plays a role in the perfusion recovery after HLI, we measured the levels of several cytokines produced by leukocyte in the ischemic muscle tissue. Compared to WT mice, Il21r−/− mice do not show significant difference in the levels of monocyte chemotactic protein-1 (MCP-1, protein level, 8.9 ± 2.6 vs. 8.6 ± 1.1 pg/µg total protein), interleukin-6 (IL-6, protein level, 1.2 ± 0.4 vs. 0.8 ± 0.2, n=5 pg/µg total protein) and vascular endothelial growth factor-A (VEGF-A, mRNA level by qPCR, P=0.53). These may suggest that IL-21R knockout does not affect leukocyte secretion of certain cytokines.
The above data demonstrated that the IL-21R plays a role in perfusion recovery after HLI. To determine whether IL-21 ligand is also required, neutralizing IL-21R-Fc fusion protein was injected into wild type C57BL/6 mice27. Similar to the results seen with Il21r−/− mice, neutralization of IL-21 resulted in impaired perfusion recovery compared to vehicle treated mice (Figure 4B).
We then sought to determine if the IL-21 pathways found in HUVEC under HSS are present in muscle tissue in-vivo. Consistent with the in-vitro results, knockout of IL-21R decreased STAT3 phosphorylation one day after HLI when compared with tissue from WT mice (Figure 5A). However, Il21r−/− mice did not show any statistical difference in the phosphorylation of the other 3 proteins. Furthermore, on day 7 after HLI, as assessed by western blotting, Il21r−/− mice showed a lower BCL-2/BAX ratio in the ischemic muscle tissue than did WT littermates (Figure 5B).
Figure 5. Il21r knockout mice had decreased STAT3 phosphorylation and BCL-2/BAX ratio after HLI.
(A) Consistent with in vitro data shown in Figure 3, Il21r−/− mice showed less STAT3 phosphorylation in ischemic gastrocnemius muscle, but did not show any difference of STAT1, AKT or ERK1/2 phosphorylation 1 day after HLI when compared to ischemic gastrocnemius muscle from the WT mice. (B) In gastrocnemius muscle at day 7 after HLI, l21r KO had a decreased BCL-2/BAX ratio. N=4–5/group, Data = mean ± SEM. WT, wild type. KO, Il21r knockout.
Discussion
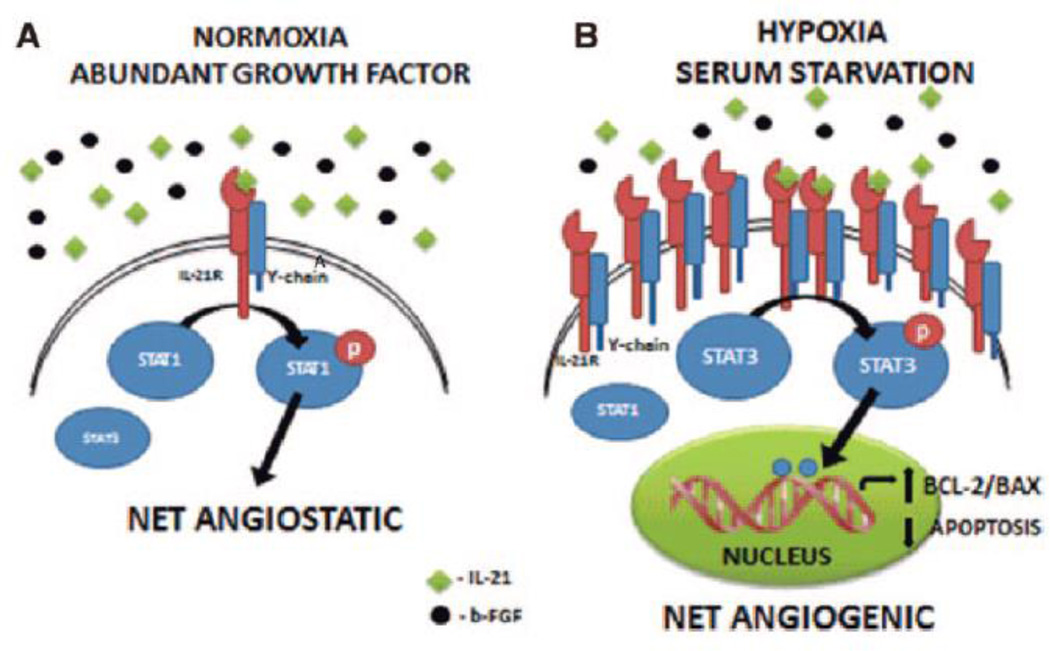
Our study describes a new role for the IL-21 receptor-ligand axis in modulating perfusion recovery after HLI via receptor activation in hypoxic endothelial cells after HLI. IL-21 is known to play a key role in innate and adaptive immunity and signals via a heterodimeric receptor formed by IL-21R and γc and is known to regulate at least 4 pathways19, 25. Three of these pathways (STAT3, ERK1/2 and AKT-1) are known to enhance cell survival and angiogenesis28–30. However, STAT1 activation can induce endothelial cell apoptosis and inhibit proliferation31. We began this study with the knowledge that the Il21r was possibly implicated as influencing the extent of recovery after HLI based on prior quantitative trait locus analysis, but Castermans et al32 described that IL-21 ligand mediated receptor activation inhibited basic fibroblast growth factor (b-FGF) induced proliferation of cultured mouse endothelial cells and decreased microvessel density within the tumors from a EG7 tumor-bearing mouse model, with the angiostatic properties being mediated through increased phosphorylation of STAT1 and decreased phosphorylation of STAT3. In totality, our study demonstrates that the effect of IL-21 on angiogenesis are likely to be context- and condition- dependent, with angiogstatic effects being dominant when normal oxygen tensions are present and cytokines are abundant versus angiogenesis occurring when endothelial cells are hypoxic and the receptor is up-regulated and activated (Figure 6). Previous studies have shown that IL-21 receptor activation has pro-apoptotic effects on B cells when B cell receptor signaling is absent; however, in the presence of B cell receptor signaling, IL-21 induces cell proliferation and differentiation33. Thus, there is precedent for differential effects by IL-21, depending on the context.
Figure 6. A schematic of proposed IL-21/IL-21R signal in endothelial cells.
(A) From Castermans et al32, under normoxic conditions with the presence of basic fibroblastic growth factor (b-FGF), IL-21 activates the STAT1 pathway, which results in angiostatic effects. (B) From our study, under HSS conditions, IL-21R is upregulated; IL-21 promotes STAT3 signaling, leading to increased BCL-2/BAX ratio, and results in increased endothelial cell survival and angiogenesis.
STAT3 is a transcription factor that is activated by a number of cytokines and growth factors. Upon activation, STAT3 becomes phosphorylated on tyrosine residue (Y705) and forms homodimers that translocate to the cell nucleus, where they modulate the transcription of target genes28. STAT3 activation is found in ischemic tissue from a spectrum of ischemic diseases, including stroke and myocardial infarction, and functions as a protective factor to improve the recovery of these diseases34, 35. Interestingly, increased STAT3 phosphorylation was also found in the ischemic hindlimb muscle tissue when compared to muscle tissue from the healthy leg in our study (Supplemental Figure 5). Recent studies demonstrate that STAT3 activation improves tissue recovery from ischemia by increasing the expression of the anti-apoptotic gene BCL-2 and by decreasing expression of pro-apoptotic gene BAX26, 36. A higher BCL-2/BAX ratio is related to better perfusion recovery from HLI37, 38. Consistent with these findings, our data suggest that loss of IL-21/IL-21R signaling results in impaired perfusion recovery after hindlimb ischemia correlated with reduction of BCL-2/BAX ratio and inhibition of STAT3 activation (Figure 6).
Endothelial cells are a major cell type involved in the perfusion recovery following HLI. Our data showed an elevated level of IL-21R in endothelial cell fractions from the ischemic hindlimb tissue when compared to the endothelial cells from non-ischemic hindlimb tissue, in the mouse strain with good perfusion recovery. Consistent with this, elevated IL-21R expression was also found in cultured endothelial cells when cultured in HSS conditions, which mimic the in-vivo ischemic condition. IL-21 treatment of endothelial cells in the conditions with IL-21R elevation results in improved cell survival and tube formation, and reduced cell apoptosis. The protective effects on endothelial cells in HSS conditions are related to IL-21-induced BCL-2/BAX increase via STAT3 activation. For myocytes and VSMC in-vitro, IL-21R was expressed in low to undetectable levels and did not show a change with hypoxia. These data suggest that elevated IL-21R levels in endothelial cell in ischemic muscle are adaptive, and IL-21R activation in endothelial cells contributes to the perfusion recovery after HLI.
Vascular endothelial growth factor (VEGF) is a potent stimulant for the induction of endothelial cell migration, proliferation, repair, and survival via the AKT or ERK1/2 pathways39, 40. However, the use of VEGF in human trials has been largely unsuccessful in the treatment of PAD6, 41. This suggests that modulation of the AKT and ERK1/2 pathways may not be beneficial in PAD treatment. We have shown that IL-21 induced angiogenesis requires STAT3 rather than AKT or ERK1/2, and exploring this pathway may be promising as an approach to identify new treatment options for PAD treatment provided the receptor is present and accessible to ligand.
Our data has other potential clinical implications. The role of IL-21 in autoimmune disease is broad, contributing to development of type1 diabetes, systemic lupus erythematosus, and experimental allergic uveitis in animal models15, 18, 21, 33, suggesting both humoral and cellular immunological contributions to autoimmune disease but none of these would exclude the treatment of the majority of patients with PAD. Interestingly, antagonists of IL-21 are also being evaluated for treating autoimmune diseases or following organ transplantation. The data from our study may prove to be important to understand potential safety issues if the patients treated in these trials have, or develop, PAD.
Currently, listings at http://www.clinicaltrials.gov show that recombinant IL-21 is currently being tested in at least 12 different clinical trials mostly being used in various malignancies17. Further studies of IL-21 treatment for HLI mouse model may provide the opportunity to allow for the rapid assessment of IL-21 to treat PAD.
Collectively our data indicate that IL-21R upregulation in response to hindlimb ischemia may well be adaptive, and that IL-21 signaling in endothelial cells may contribute to improved perfusion recovery after HLI by activating the STAT3 pathway and increasing the BCL-2/BAX ratio. Thus, IL-21R is a potential target for pharmacological modulation to in PAD.
Significance
Peripheral arterial disease (PAD), caused by atherosclerosis that impairs blood flow to the lower extremities, is a major health problem. Currently there are no medical therapies for PAD that have the ability to increase perfusion and correct the problem of impaired blood flow. In this study, we have shown that interleukin (IL)-21 receptor (IL-21R) expression is adaptively up-regulated after HLI. Under ischemic conditions, either IL-21 ligand or receptor inhibition contributes to impaired perfusion recovery following experimental hind-limb ischemia (HLI, widely used as preclinical PAD model) through STAT3 pathway. Due to its potent effects in modulating immune system, IL-21 has been used for human diseases in at least 12 clinical studies, and blocking IL-21R has also been tested in several other phase I clinical study. Our study is the first one to elucidate the role of IL-21/IL-21R in PAD, and may establish novel therapeutic targets for PAD therapy.
Supplementary Material
Acknowledgments
None
Source of funding
W.J.L. and R.S. are supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, NIH. This work was supported by RO1HL16455 and RO1HL121635 to B.H.A.
Abbreviation
- α-SMA
α-smooth muscle actin
- BCL-2
B-cell lymphoma leukemia-2
- BAX
BCL-2–associated X protein
- b-FGF
basic fibroblast growth factor
- EC
Endothelial cell
- ERK1/2
Extracellular-signal-regulated kinases1/2
- HLI
Hindlimb ischemia
- HSS
Hypoxia and serum starvation
- HUVEC
Human umbilical vein endothelial cell
- IL-21
Interleukin 21
- IL-21R
Interleukin 21 receptor
- PAD
Peripheral arterial disease
- qPCR
Quantitative RT-PCR
- STAT
Signal transducer and activator of transcription
- TUNEL
terminal dUTP nick end-labeling
- VEGF
Vascular endothelial growth factor
- VSMC
Vascular smooth muscle cell
Footnotes
Disclosure
W.J.L. and R.S. are inventors on NIH patents related to IL-21.
Reference
- 1.Wennberg PW. Approach to the Patient With Peripheral Arterial Disease. Circulation. 2013;128:2241–2250. doi: 10.1161/CIRCULATIONAHA.113.000502. [DOI] [PubMed] [Google Scholar]
- 2.Owens CD, Conte MS. Medical Management of Peripheral Arterial Disease Bridging the "Gap"? Circulation. 2012;126:1319–1321. doi: 10.1161/CIRCULATIONAHA.112.129692. [DOI] [PubMed] [Google Scholar]
- 3.Barrett PM, Wall CAM, Stack AG. Peripheral Artery Disease Prevalence and Mortality Trends of United States Dialysis Population: 1995–2005. Irish J Med Sci. 2010;179:S409–S410. [Google Scholar]
- 4.Peacock JM, Keo HH, Yu XH, Oldenburg N, Duval S, Henry TD, Jaff MR, Baumgartner I, Hirsch AT. The Incidence and Health Economic Burden of Critical Limb Ischemia and Ischemic Amputation in Minnesota: 2005–2007. Circulation. 2009;120:S1148–S1148. [PMC free article] [PubMed] [Google Scholar]
- 5.White C. Intermittent claudication. New Engl J Med. 2007;356:1241–1250. doi: 10.1056/NEJMcp064483. [DOI] [PubMed] [Google Scholar]
- 6.Annex BH. Therapeutic angiogenesis for critical limb ischaemia. Nat Rev Cardiol. 2013;10:387–396. doi: 10.1038/nrcardio.2013.70. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly R, Yeung JMC. Therapeutic angiogenesis: a step forward in intermittent claudication. Lancet. 2002;359:2048–2050. doi: 10.1016/S0140-6736(02)08946-8. [DOI] [PubMed] [Google Scholar]
- 8.Jones MR, Apelberg BJ, Samet JM, Navas-Acien A. Smoking, Menthol Cigarettes, and Peripheral Artery Disease in U.S. Adults. Nicotine Tob Res. 2013;15:1183–1189. doi: 10.1093/ntr/nts253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch AT. Treatment of peripheral arterial disease--extending "intervention" to "therapeutic choice". N Engl J Med. 2006;354:1944–1947. doi: 10.1056/NEJMe068037. [DOI] [PubMed] [Google Scholar]
- 10.Hazarika S, Farber CR, Dokun AO, Pitsillides AN, Wang T, Lye RJ, Annex BH. MicroRNA-93 Controls Perfusion Recovery After Hindlimb Ischemia by Modulating Expression of Multiple Genes in the Cell Cycle Pathway. Circulation. 2013;127:1818–1828. doi: 10.1161/CIRCULATIONAHA.112.000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Md AOD, Keum S, Hazarika S, Li Y, Lamonte GM, Wheeler F, Marchuk DA, Annex BH. A quantitative trait locus (LSq-1) on mouse chromosome 7 is linked to the absence of tissue loss after surgical Hindlimb ischemia. Circulation. 2008;117:1207–1215. doi: 10.1161/CIRCULATIONAHA.107.736447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chi YW, Jaff MR. Peripheral Artery Disease and Genetics: Is There a Cause-and-Effect Relationship? Postgrad Med. 2010;122:170–176. doi: 10.3810/pgm.2010.07.2183. [DOI] [PubMed] [Google Scholar]
- 13.Murabito JM, Guo CY, Fox CS, D'Agostino RB. Genetic contributions to peripheral arterial disease: Heritability of the ankle-brachial blood pressure index in the Framingham Heart Study. Circulation. 2006;113:E325–E325. [Google Scholar]
- 14.Leeper NJ, Kullo IJ, Cooke JP. Genetics of Peripheral Artery Disease. Circulation. 2012;125:3220–3228. doi: 10.1161/CIRCULATIONAHA.111.033878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andorsky DJ, Timmerman JM. Interleukin-21: biology and application to cancer therapy. Expert Opin Biol Ther. 2008;8:1295–1307. doi: 10.1517/14712598.8.9.1295. [DOI] [PubMed] [Google Scholar]
- 16.Hashmi MH, Van Veldhuizen PJ. Interleukin-21: updated review of Phase I and II clinical trials in metastatic renal cell carcinoma, metastatic melanoma and relapsed/refractory indolent non-Hodgkin's lymphoma. Expert Opin Biol Ther. 2013;10:807–817. doi: 10.1517/14712598.2010.480971. [DOI] [PubMed] [Google Scholar]
- 17.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nature Reviews Drug Discovery. 2014;13:381–393. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 18.Monteleone G, Pallone F, Macdonald TT. Interleukin-21 as a new therapeutic target for immune-mediated diseases. Trends Pharmacol Sci. 2009;30:441–447. doi: 10.1016/j.tips.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Leonard WJ. The Yin and Yang of Interleukin-21 in Allergy, Autoimmunity and Cancer. Blood. 2011;118:1809–1810. doi: 10.1016/j.coi.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhave NS, Carson WE., 3rd Immune modulation with interleukin-21. Ann N Y Acad Sci. 2009;1182:39–46. doi: 10.1111/j.1749-6632.2009.05071.x. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger R, Kuchen S, Lipsky PE. Interleukin 21 as a target of intervention in autoimmune disease. Annals of the Rheumatic Diseases. 2008;67:83–86. doi: 10.1136/ard.2008.098400. [DOI] [PubMed] [Google Scholar]
- 22.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive Hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34:775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 23.Imoukhuede PI, Dokun AO, Annex BH, Popel AS. Endothelial cell-by-cell profiling reveals the temporal dynamics of VEGFR1 and VEGFR2 membrane localization after murine hindlimb ischemia. Am J Physiol-Heart C. 2013;304:H1085–H1093. doi: 10.1152/ajpheart.00514.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T, Cunningham A, Dokun AO, Hazarika S, Chen L, Lye RJ, Leonard WJ, Annex B. Angiogenic Properties of Interleukin-21 under Hypoxic Conditions. [e-Letter] Blood. 2014 [Google Scholar]
- 25.Monteleone G, Pallone F, Macdonald TT. Interleukin-21 (IL-21)-mediated pathways in T cell-mediated disease. Cytokine Growth F R. 2009;20:185–191. doi: 10.1016/j.cytogfr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Tian YK, Zhang WJ, Xia DC, Modi P, Liang DG, Wei MX. Postconditioning inhibits myocardial apoptosis during prolonged reperfusion via a JAK2-STAT3-Bcl-2 pathway. Journal of Biomedical Science. 2011;18:53. doi: 10.1186/1423-0127-18-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spolski R, Wang L, Wan CK, Bonville CA, Domachowske JB, Kim HP, Yu ZX, Leonard WJ. IL-21 Promotes the Pathologic Immune Response to Pneumovirus Infection. J Immunol. 2012;188:1924–1932. doi: 10.4049/jimmunol.1100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Han ZC. STAT3: A critical transcription activator in angiogenesis. Med Res Rev. 2008;28:185–200. doi: 10.1002/med.20101. [DOI] [PubMed] [Google Scholar]
- 29.Schweighofer B, Schultes J, Pomyje J, Hofer E. Signals and genes induced by angiogenic growth factors in comparison to inflammatory cytokines in endothelial cells. Clin Hemorheol Micro. 2007;37:57–62. [PMC free article] [PubMed] [Google Scholar]
- 30.Somanath PR, Razorenova OV, Chen JH, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5:512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang SY, Bucana CD, Van Arsdall M, Fidler IJ. Stat1 negatively regulates angiogenesis, tumorigenicity and metastasis of tumor cells. Oncogene. 2002;21:2504–2512. doi: 10.1038/sj.onc.1205341. [DOI] [PubMed] [Google Scholar]
- 32.Castermans K, Tabruyn SP, Zeng R, van Beijnum JR, Eppolito C, Leonard WJ, Shrikant PA, Griffioen AW. Angiostatic activity of the antitumor cytokine interleukin-21. Blood. 2008;112:4940–4947. doi: 10.1182/blood-2007-09-113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 34.Bolli R, Stein AB, Guo YR, Wang OL, Rokosh G, Dawn B, Molkentin JD, Sanganalmath SK, Zhu YQ, Xuan YT. A murine model of inducible, cardiac-specific deletion of STAT3: Its use to determine the role of STAT3 in the upregulation of cardioprotective proteins by ischemic preconditioning. J Mol Cell Cardiol. 2011;50:589–597. doi: 10.1016/j.yjmcc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Sakata H, Narasimhan P, Niizuma K, Maier CM, Wakai T, Chan PH. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 2012;135:3298–3310. doi: 10.1093/brain/aws259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada M, Qin YJ, Takano H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 37.Yang YG, Guan H, Liu CW, Li YJ. Myoglobin over-expression attenuates angiogenic response in hindlimb ischemia in mice. Chin Med J (Engl) 2009;122:1056–1060. [PubMed] [Google Scholar]
- 38.Hu Z, Zhang F, Yang Z, Yang N, Zhang D, Zhang J, Cao K. Combination of simvastatin administration and EPC transplantation enhances angiogenesis and protects against apoptosis for hindlimb ischemia. J Biomed Sci. 2008;15:509–517. doi: 10.1007/s11373-008-9243-1. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 40.Tsubokawa T, Nakanishi C, Tagawa S, Takabatake S, Fujioka K, Yagi K, Ino H, Ishibashi-Ueda H, Yamagishi M. Impact of PI3/Akt Pathway on Production of Vascular Endothelial Growth Factor and Associated Myocardial Salvage by Mesenchymal Stem Cell wtih Transient Overexpression of Heme Oxygenase-1. Circulation. 2009;120:S592–S592. [Google Scholar]
- 41.Rajagopalan S, Mohler ER, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease - A phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.