Abstract
Metformin is the most commonly prescribed oral anti-diabetic agent worldwide. Surprisingly, about 35% of diabetic patients either lack or have a delayed response to metformin treatment, and many patients become less responsive to metformin over time. It remains unknown how metformin resistance or insensitivity occurs. Recently, we found that therapeutic metformin concentrations suppressed glucose production in primary hepatocytes through AMPK; activation of the cAMP-PKA pathway negatively regulates AMPK activity by phosphorylating AMPKα subunit at Ser-485, which in turn reduces AMPK activity. In this study, we find that metformin failed to suppress glucose production in primary hepatocytes with constitutively activated PKA and did not improve hyperglycemia in mice with hyperglucagonemia. Expression of the AMPKα1(S485A) mutant, which is unable to be phosphorylated by PKA, increased both AMPKα activation and the suppression of glucose production in primary hepatocytes treated with metformin. Intriguingly, salicylate/aspirin prevents the phosphorylation of AMPKα at Ser-485, blocks cAMP-PKA negative regulation of AMPK, and improves metformin resistance. We propose that aspirin/salicylate may augment metformin's hepatic action to suppress glucose production.
Keywords: AMP-activated kinase (AMPK), glucogenesis, liver, metformin, protein kinase A (PKA), aspirin
Introduction
Diabetes is the fastest-growing chronic disease worldwide, and type 2 diabetes (T2D)2 accounts for more than 90% of diabetes cases. Metformin has been used to treat T2D since the 1950s and works primarily by controlling fasting hyperglycemia (1). Now, over 150 million people worldwide take this medication; guidelines for the treatment of T2D published by the American Diabetes Association and the European Association for the Study of Diabetes in 2012 jointly recommended metformin as the initial drug for T2D treatment (2).
Surprisingly, about 35% of diabetic patients either lack or have a delayed response to metformin treatment (3–6), and many patients become less responsive to metformin over time (4). It remains unknown how metformin resistance/insensitivity occurs. Several genes have been reported to be associated with glycemic control by metformin. In particular, variants of the organic cation transporter and the metformin transporter, have been linked to a reduction in metformin action (7–9). Because of the high occurrence of metformin resistance in diabetic patients, genetic variants in known genes alone cannot explain this phenomenon; therefore, nongenetic factors may also contribute to the response to metformin.
Recently, we found that treatment with metformin prior to the addition of cAMP led to greater suppression of glucose production and AMPK activation when compared with simultaneous treatment with metformin and cAMP (10), suggesting that the cAMP-PKA pathway exerts a negative effect on AMPK activation. In both diabetic animal models and human subjects, serum glucagon levels and the ratios of glucagon to insulin are often elevated (11–14) and are responsible for increased hepatic glucose output and hyperglycemia in T2D (15). Elevated glucagon levels in type 2 diabetic patients are variable, with a ∼5-fold difference in serum glucagon levels among patients with T2D (16). Low metformin concentrations, typically found in the portal vein after a therapeutic dosage, suppress glucose production in primary hepatocytes through AMPK (10). Therefore, the hyperglucagonemia of diabetes can antagonize metformin efficacy by activating the cAMP-PKA pathway and cause metformin resistance. We and other investigators have found that the cAMP-PKA pathway negatively regulates AMPK activity through phosphorylation of the AMPKα subunit at Ser-485, which in turn reduces net phosphorylation at Thr-172 (10, 17–19). It is conceivable that the blockade of AMPKα phosphorylation at Ser-485 will augment AMPK activity and metformin efficacy. One potential drug that might improve metformin's effect is salicylate, which is known to activate AMPK by binding to the AMPKβ subunit (20). In the current study, we tested whether hyperglucagonemia of diabetes mellitus causes metformin resistance and whether blocking the phosphorylation of AMPKα at Ser-485 by salicylate can alleviate metformin resistance in a diabetic animal models with constitutively activated PKA and with hyperglucagonemia.
Experimental Procedures
Adenoviruses
To generate adenoviral expression vectors, mouse α1, β1, and γ1 genes were cloned into a pENTR2B vector (Invitrogen). AMPKα1S485A was created using site-directed mutagenesis (Stratagene). These genes were transferred into the pAd/CMV/V5-DEST vector (Invitrogen) by recombination to generate expression clones (10). Ad-CMV-Cre and Ad-CMV-GFP adenoviruses were purchased from the Viral Vector Core at the University of Iowa.
Glucose Production Assays
Mouse primary hepatocytes were cultured in William's medium E supplemented with ITS (BD Biosciences) and dexamethasone (10). After 16 h of planting, primary hepatocytes were subjected to 3 h serum starvation. Cells were washed twice with PBS, and then the medium was replaced with 1 ml of glucose production medium, consisting of glucose-free DMEM supplemented with 20 mm sodium lactate (Sigma) and 2 mm sodium pyruvate (Sigma) or with metformin (Alexis), salicylate (Sigma), and 0.2 mm Bt-cAMP (Sigma). In assays with 3 h salicylate pretreatment, salicylate was added to FBS-free DMEM during serum starvation and to the glucose production medium. After 3∼4 h incubation with glucose production medium, both medium and cells were collected. The medium was used to determine glucose concentrations with the EnzyChrom Glucose Assay Kit (21, 22).
Immunoprecipitation and Analysis of AMPK Phosphorylation in in Vitro Assays
Anti-FLAG M2 monoclonal antibody was used to immunoprecipitate FLAG-tagged AMPKα1, β1, and γ1 from cell lysates following the procedure recommended by the manufacturer (Sigma). To assess the effect of salicylate on the formation of the AMPK heterotrimer complex, purified FLAG-tagged AMPKα1 and 2-fold of β1 and γ1 were incubated with different concentrations of salicylate for 1 h at 4 °C, and then 1 μl of anti-AMPKα1 antibody (abcam) was added. The reaction was incubated at 4 °C for 1 h, followed by the addition of protein G beads (Active Motif) to pull down the AMPK heterotrimer complex. To test for the phosphorylation of AMPKα1, purified α1 was incubated with the indicated amounts of salicylate for 1 h at 4 °C in the presence of 2-fold of β1 and γ1 subunits. Then, 25 ng of LKB1-STRAD-MO25 (Millipore) was added, and the reactions were incubated at room temperature for 30 min (23).
AMPK Activity Assay
The FLAG-tagged proteins were immunoprecipitated from cell lysates using anti-FLAG M2 monoclonal antibody (Sigma) after incubation overnight at 4 °C. AMPK activity was determined by measuring the incorporation of 32P into the synthetic SAMS peptide (abcam), as described previously (10, 23). Briefly, immunoprecipitated FLAG-tagged AMPKα1 proteins were incubated at 30 °C for 20 min in the presence of [32P]ATP (1 μCi), AMP (200 μm), and either with or without SAMS peptide (200 μm). Aliquots of the reaction mixture were spotted on Whatman p81 phosphocellulose filter papers, which were dropped immediately in 1% phosphoric acid for 20 min with rotation. This wash was repeated twice, followed by a 5-min wash in acetone. Filters were air-dried, and radioactivity was counted in 3 ml of liquid scintillation fluid. The counts without the SAMS peptide were used as the background.
Animal Experiment
All animal protocols were approved by the Institutional Animal Care and Use Committee of Johns Hopkins University. C57BL/6 mice were purchased from the Jackson laboratory. After feeding on a high-fat diet (60% caloric intake) for 2 weeks, mice were given metformin (25 mg/kg) and aspirin (10 mg/kg) in drinking water, based on the calculation that each mouse drinks 4 ml of water per day. For the experiment using floxed PKAR1a mice (24), 3-month-old mice were injected with Ad-CMV-Cre and Ad-CMV-GFP adenoviruses through the jugular vein (3 × 109 pfu/mouse). A metformin tolerance test was conducted 8 days after viral injection (200 mg/kg body weight, intraperitoneal injection).
Glucagon Pump Implantation
Glucagon was dissolved in a cetrimide solution (Sigma) at a molar ratio of 6 mol of cetrimide to 1 mol of glucagon to maintain long-term solubility. Glucagon solutions were made to be delivered at 0.5 μg/h for 28 days. 12-week-old male C57BL6 mice, fed a high-fat diet (60% caloric intake) for 9 weeks were implanted with glucagon micro-osmotic pumps (Model 1004, DURECT Corporation) in the peritoneum (25). Control mice were implanted with the pump containing the same concentration of cetrimide. On the second day after the implantation, mice with glucagon infusion were divided into 4 groups and treated with vehicle, metformin (50 mg/kg/day), salicylate (28.6 mg/kg/day), or metformin plus salicylate through drinking water. After 3 weeks of drug treatment, mice were subjected to a 4 h fast before performance of a pyruvate tolerance test (2 g/kg) to assess glucose production.
Immunoblot
Immunoblots were conducted as previously described (26). Cellular lysates were passed 15 times through a syringe needle or were sonicated for 15 s three times sequentially. The lysates were immunoblotted to examine the target proteins with antibodies against AMPKα1, pAMPKα (Thr-172, Ser-485), CREB, pCREB (Ser-133), ACC, pACC (Ser-79) (Cell Signaling), Pck1, and AMPKα1, β1, γ1 (abcam) at the concentrations recommended by the manufacturers; secondary antibodies were used at concentrations around 1:5000.
Statistical Analyses
Statistical significance was calculated with a Student's t test and ANOVA test. Significance was accepted at the level of p < 0.05.
Results
The cAMP-PKA Pathway Negatively Regulates AMPK Activation
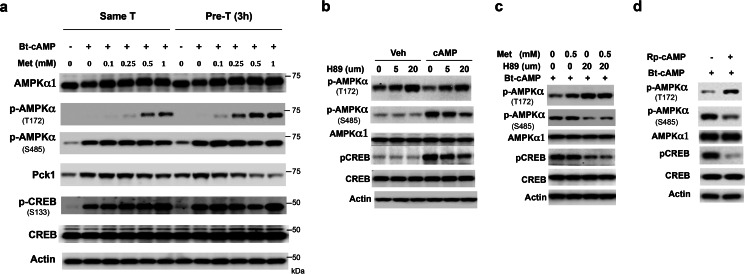
Previously, we found that pretreatment of primary hepatocytes with metformin led to greater suppression of glucose production (10). Since metformin suppresses hepatic glucose production through the activation of AMPK (10, 27), we examined AMPK activation by measuring the phosphorylation of the α-subunit at Thr-172, a crucial phosphorylation site in the activation of AMPK (28), in primary hepatocytes pre-treated with metformin. As shown in Fig. 1a, pretreatment of primary hepatocytes with metformin 3 h before the addition of the cAMP analog dibutyryl-cAMP (Bt-cAMP) (Pre-T) led to greater AMPK phosphorylation at Thr-172 and greater inhibition of Pck1 protein levels than simultaneous treatment with metformin and Bt-cAMP (Same-T). Since phosphorylation of AMPKα subunit at Ser-485 by PKA reduces both AMPK phosphorylation at Thr-172 and AMPK activity (10, 17–19), we determined the degree of phosphorylation of AMPKα subunit at Ser-485 and found that Bt-cAMP increased AMPKα subunit phosphorylation at Ser-485 as well as the phosphorylation of CREB at Ser-133 to a comparable extent in both the metformin pretreatment and simultaneous treatment groups (Fig. 1a). To test further that the cAMP-PKA pathway exerts a negative effect on AMPK activation, we used the PKA inhibitor-H89 to block the cAMP-PKA pathway in Hepa1–6 cells, a mouse hepatocyte cell line. Treatment with H89 decreased CREB phosphorylation at Ser-133, indicating blockade of the cAMP-PKA pathway; and we found that H89 reduced AMPKα subunit phosphorylation at Ser-485 and increased AMPKα subunit phosphorylation at Thr-172, which occurred in a concentration-dependent manner (Fig. 1b). We previously observed that Bt-cAMP treatment reduced metformin-mediated AMPKα subunit phosphorylation at Thr-172 (10). Therefore, we tested whether the PKA inhibitor-H89 could reverse the negative effect of Bt-cAMP on Thr-172 phosphorylation of AMPKα subunit. Treatment with H89 prior to the addition of Bt-cAMP indeed eliminated the negative effect of Bt-cAMP on metformin-mediated AMPKα subunit phosphorylation at Thr-172 in Hepa1–6 cells (Fig. 1c). To prove further that the cAMP-PKA pathway negatively regulates AMPK activation, we treated Hepa1–6 cells with a PKA competitive inhibitor Rp-cAMP. We found that administration of Rp-cAMP 1 h before the addition of 0.2 mm Bt-cAMP increased AMPKα subunit phosphorylation at Thr-172 (Fig. 1d). The above data demonstrate that the cAMP-PKA pathway negatively regulates activation of AMPK.
FIGURE 1.
The cAMP-PKA pathway negatively regulates AMPK activation. a, primary hepatocytes were treated with the indicated amount of metformin (Pre-T) or vehicle (Same-T) for 3 h during serum starvation, then washed with PBS, followed by incubation of primary hepatocytes from both groups with 0.2 mm Bt-cAMP and indicated amounts of metformin for 4 h before harvest. b, inhibition of PKA by H89 attenuated phosphorylation of AMPKα at Ser-485 and CREB at Ser-133, while the phosphorylation of AMPKα at Thr-172 was elevated in primary hepatocytes. Hepatocytes were pretreated with H89 for 6 h before the addition of Bt-cAMP. c, Hepa1–6 cells were treated with 0.5 mm metformin or vehicle for 2 h, then 20 μm H89 was added. After 1-h incubation, 0.2 mm Bt-cAMP was added. d, the PKA competitive inhibitor Rp-cAMP (100 μm) was administrated 1 h before the addition of 0.2 mm Bt-cAMP in Hepa1–6 cells.
Constitutively Activated PKA Decreases Metformin Efficacy in Suppression of Glucose Production
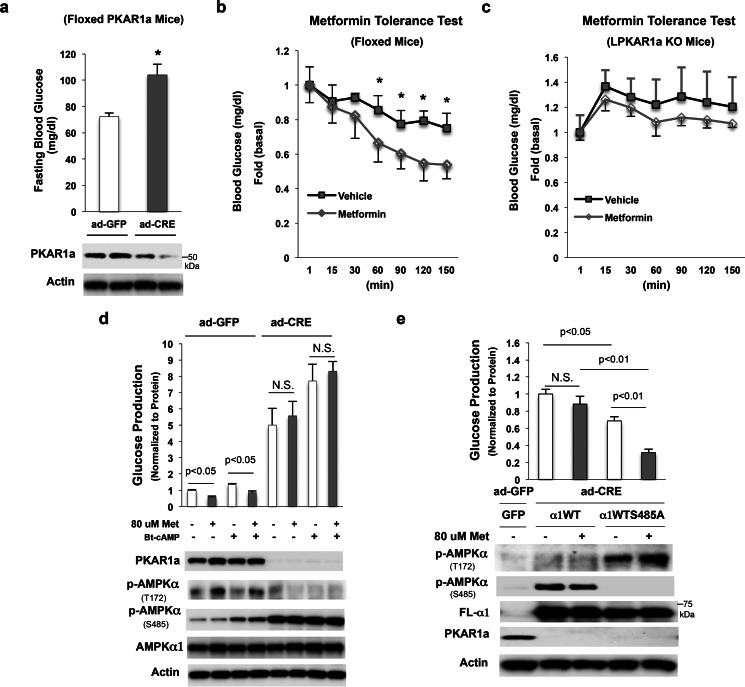
Given that low metformin concentrations typically found in the portal vein suppress hepatic glucose production through the activation of AMPK (10), and that the cAMP-PKA pathway negatively modulates AMPK activity (Fig. 1), we tested whether activation of the cAMP-PKA pathway would decrease metformin efficacy in the suppression of hepatic glucose production. As the loss of PKA regulatory subunit 1a (PKAR1a) leads to the constitutive activation of PKA (24), we employed Ad-CMV-Cre to floxed PKAR1a mice through jugular vein injection and generated liver-specific PKAR1a knock-out mice (Fig. 2a, bottom panel), and then tested metformin efficacy in vivo. In agreement with a previous report (29), PKAR1a knock-out led to a significant elevation in fasting blood glucose levels (Fig. 2a). Next, we conducted a metformin tolerance test on floxed PKAR1a mice and PKAR1a knock-out mice. In these experiments, metformin significantly decreased blood glucose levels in floxed PKAR1a mice (Fig. 2b); however, metformin administration did not significantly affect blood glucose levels in the PKAR1a knock-out mice (Fig. 2c). These data clearly demonstrate that activation of the cAMP-PKA pathway antagonizes metformin efficacy.
FIGURE 2.
Activation of the cAMP-PKA pathway causes metformin resistance. a, 10 days after adenovirus injection, fasting blood glucose levels were measured (15h fasting, n = 4). b and c, metformin tolerance test in 4-h fasted floxed PKAR1a mice (b) and liver-specific PKAR1a knock-out mice (c) (n = 4). d, 8 h after the planting of primary hepatocytes from floxed PKAR1a mouse, ad-GFP, and ad-CRE were added; 48 h later, cells were treated with 80 mm metformin for 21 h, followed by 3 h of serum starvation and the supplementing of medium with 80 μm metformin; cells were then washed with PBS, and 0.2 mm Bt-cAMP and 80 μm metformin were added in 1 ml of glucose production medium for 4 h. e, 8 h after the planting of primary hepatocytes isolated from floxed PKAR1a mice with ad-CRE injection, ad-AMPKα1WT and ad-AMPKα1S485A were added to cells. 24 h later, cells were treated with 80 μm metformin for 21 h followed by 3 h of serum starvation and the medium being supplemented with 80 μm metformin. Then, cells were washed with PBS, and 80 μm metformin was added in 1 ml of glucose production medium for 4 h.
We further assessed the effect of PKA activation on metformin's suppression of glucose production in primary hepatocytes. In primary hepatocytes isolated from floxed PKAR1a mice, Ad-CMV-Cre adenovirus-mediated depletion of PKAR1a (Fig. 2d, bottom panel) resulted in a significant increase in glucose production without Bt-cAMP stimulation (Fig. 2d, upper panel). Metformin significantly suppressed glucose production in the basal and after Bt-cAMP treatment in primary hepatocytes transduced with control Ad-CMV-GFP adenovirus (Fig. 2d). In contrast, metformin's effect on the suppression of glucose production was abolished in primary hepatocytes with the loss of PKAR1a and was associated with elevated phosphorylation of AMPKα at Ser-485 (Fig. 2d). Since inhibitor H89 or Rp-cAMP increased AMPKα subunit phosphorylation at Thr-172 along with a reduction in AMPKα subunit phosphorylation at Ser-485 (Fig. 1, b–d), and PKA-mediated phosphorylation of AMPKα at Ser-485 negatively affects AMPK activity (10, 17–19), we examined whether blocking AMPKα subunit phosphorylation at Ser-485 would have an effect on glucose production in primary hepatocytes with constitutively activated PKA. Using adenoviral vectors needed to express similar amounts of the wild type (WT) and S485A mutant of AMPKα1 proteins were used to primary hepatocytes isolated from floxed PKAR1a mice injected with Ad-CMV-Cre. As shown in Fig. 2e (bottom panel), PKAR1a protein was completely depleted, and AMPKα1 subunit phosphorylation at Thr-172 was only observed in primary hepatocytes with AMPKα1S485A overexpression, not in primary hepatocytes with AMPKα1WT overexpression. These results suggest that PKA-mediated phosphorylation of AMPKα at Ser-485 reduces phosphorylation of AMPKα at Thr-172. Importantly, hepatocytes with AMPKα1S485A overexpression produced significantly less glucose compared with hepatocytes with AMPKα1WT overexpression (Fig. 2e, upper panel). Metformin treatment did not significantly suppress glucose production in primary hepatocytes with AMPKα1WT overexpression; in contrast, metformin decreased glucose production by 54% in primary hepatocytes with AMPKα1S485A overexpression. The above results suggest that PKA-mediated phosphorylation of AMPKα at Ser-485 indeed negatively affects metformin's suppression of glucose production.
Salicylate Blocks the Phosphorylation of AMPKα at Ser-485
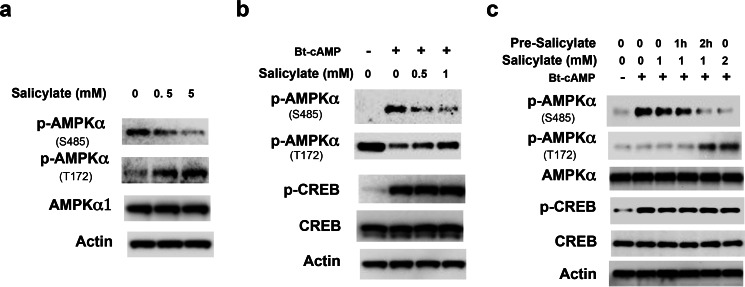
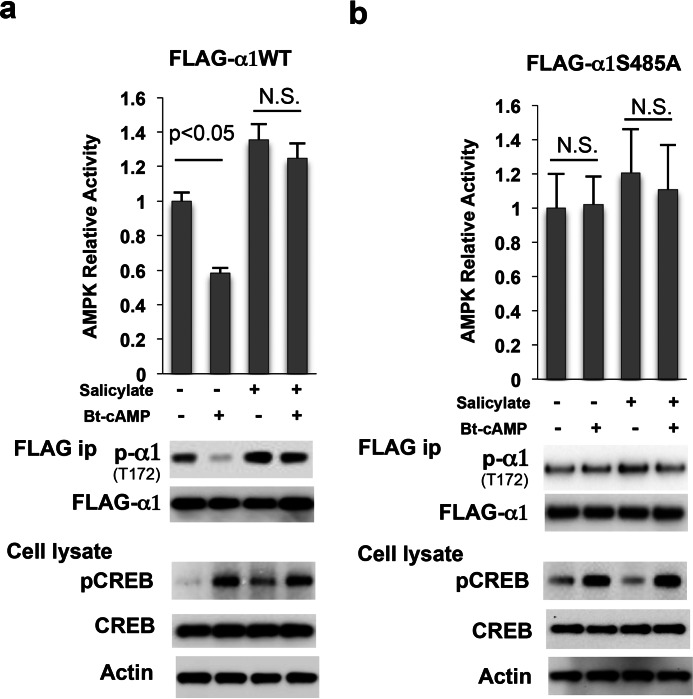
Salicylate and its synthetic derivative, aspirin, have been reported to activate AMPK and increase the phosphorylation of AMPKα at Thr-172 (20). We reasoned that salicylate might affect the phosphorylation of AMPKα at Ser-485 and therefore augment AMPK activity; to test this hypothesis, Hepa1–6 cells were treated with different concentrations of salicylate and we found that salicylate decreased the basal phosphorylation of AMPKα subunit at Ser-485, aligning with an increase in AMPKα subunit phosphorylation at Thr-172 (Fig. 3a). Furthermore, salicylate also blocked Bt-cAMP-stimulated AMPKα subunit phosphorylation at Ser-485 without affecting the CREB phosphorylation at Ser-133 by Bt-cAMP (Fig. 3b). The blockade of Bt-cAMP-stimulated AMPKα subunit phosphorylation at Ser-485 by salicylate is both time- and concentration-dependent (Fig. 3c). The above data suggest that salicylate blocks AMPKα phosphorylation at Ser-485 and therefore increases AMPKα phosphorylation at Thr-172. In light of this finding, we determined the effect of Bt-cAMP on the phosphorylation and enzymatic activity of FLAG-tagged-AMPKα1WT and FLAG-tagged-AMPKα1S485A mutant. Bt-cAMP treatment significantly decreased AMPKα phosphorylation at Thr-172 and AMPK activity in FLAG-tagged-AMPKα1WT, and salicylate blocked the Bt-cAMP effect (Fig. 4a). In contrast, Bt-cAMP and/or salicylate treatment had no effect on phosphorylation at Thr-172 and AMPK activity of the FLAG-tagged AMPKα1S485A mutant (Fig. 4b).
FIGURE 3.
Salicylate affects AMPKα phosphorylation. a, Hepa1–6 cells were grown in DMEM with 5% FBS and were treated with the indicated amounts of salicylate for 4 h. b, 24 h after the planting of primary hepatocytes, cells were subjected to serum starvation for 3 h, and medium was supplemented with the indicated amounts of salicylate. Then, the cells were washed with PBS, and 0.2 mm Bt-cAMP and salicylate were added in 1 ml of glucose production medium for 3 h. c, Hepa1–6 cells were treated with the indicated amounts of salicylate for 0, 1, and 2 h, and then 0.2 mm Bt-cAMP was added for 2 h.
FIGURE 4.
Salicylate blocks the negative effects of cAMP on AMPK activity. a and b, 48 h after the addition of adenoviral FLAG-tagged AMPKα1 (a) and its S485A mutant (b), Hepa1–6 cells were treated with 2 mm salicylate and/or 0.2 mm Bt-cAMP for 24 h. N.S., not significant.
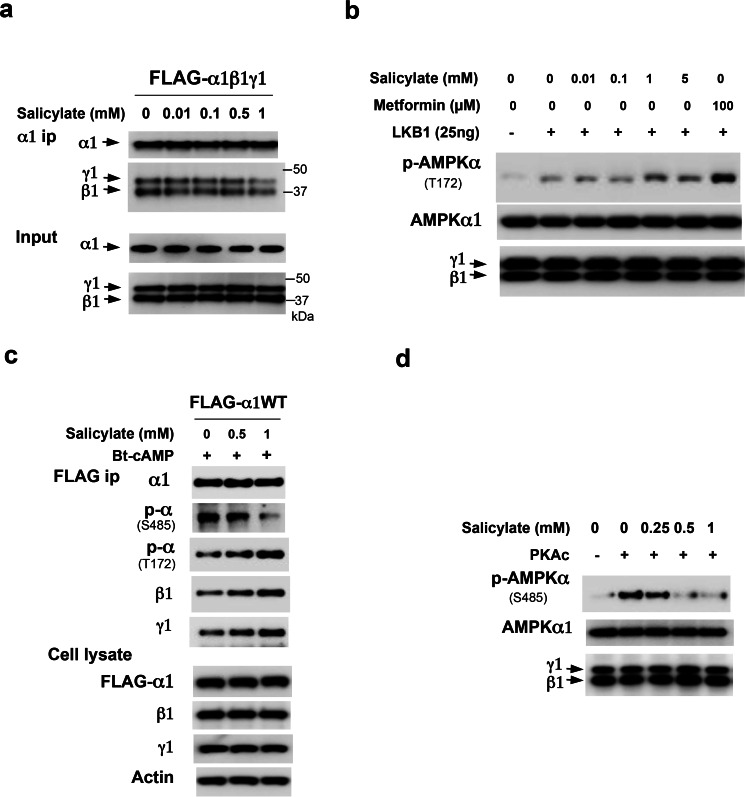
We previously reported that low concentrations of metformin promote the formation of the AMPKαβγ heterotrimeric complex (23). To test whether salicylate is able to directly affect the formation of the AMPKαβγ heterotrimeric complex like metformin does, we conducted an in vitro assembly assay. Purified FLAG-tagged AMPKα1 and 2-fold greater concentrations of purified β1 and γ1 were incubated with different concentrations (0, 0.01, 0.1, 0.5, and 1 mm) of salicylate for 1 h, then AMPKα1 subunits was immunoprecipitated using anti-AMPKα1 subunit specific antibody. Unlike metformin, salicylate had no effect on promoting the formation of the AMPKαβγ heterotrimeric complex in vitro (Fig. 5a). Next, we tested whether salicylate could affect AMPKα1 subunit phosphorylation at Thr-172 by upstream kinase LKB1. We conducted an in vitro phosphorylation assay using LKB1-STRAD-MO25 (referred to as LKB1 kinase), and found that salicylate had a minimal effect on LKB1-mediated AMPKα1 phosphorylation at Thr-172 (Fig. 5b). Interestingly, however, salicylate blocked Bt-cAMP-mediated phosphorylation of AMPKα subunit at Ser-485, increased phosphorylation of AMPKα subunit at Thr-172, and promoted AMPKα1 association with β1 and γ1 subunits in Hepa1–6 cells (Fig. 5c). We further examined whether salicylate could directly inhibit the phosphorylation of AMPKα1 subunit at Ser-485 by PKA. Because S485 in AMPKα1 subunit is a direct target of PKA (10), we therefore performed an in vitro phosphorylation assay. As shown in Fig. 5d, salicylate significantly decreased phosphorylation of AMPKα at Ser-485 by the PKA catalytic subunit (Fig. 5d). Since salicylate did not alter the phosphorylation of CREB at Ser-133 (Figs. 3, b and c and 4), this suggests that PKA is unlikely a direct target of salicylate. However, salicylate is able to bind to an AMPK subunit (20), it is possible that salicylate binding masks the phosphorylation of AMPKα at Ser-485, consequently increasing phosphorylation at Thr-172 and the formation of the AMPK heterotrimeric complex.
FIGURE 5.
Salicylate blocks the phosphorylation of AMPKα at Ser-485. a, purified FLAG-tagged AMPK α1, β1, and γ1 were combined and incubated with different concentrations of salicylate for 1 h before the addition of an α1-specific antibody to pull-down the complex. Anti-FLAG M2 antibody was used to examine the FLAG-tagged AMPK subunits. b, purified α1, β1, and γ1 were incubated with the indicated different amounts of salicylate or metformin for 1 h at 4 °C before the addition of 25 ng LKB1 in an in vitro assay. c, 48 h after the addition of adenoviral FLAG-tagged AMPK α1, Hepa1–6 cells were treated with salicylate for 3 h, and then 0.2 mm Bt-cAMP was added. d, purified FLAG-tagged α1, β1, and γ1 were incubated with different amounts of salicylate for 16 h at 4 °C before the addition of the PKA catalytic subunit (50 ng).
Salicylate/Aspirin Improves Metformin Efficacy on AMPK Activation and Suppression of Hepatic Glucose Production
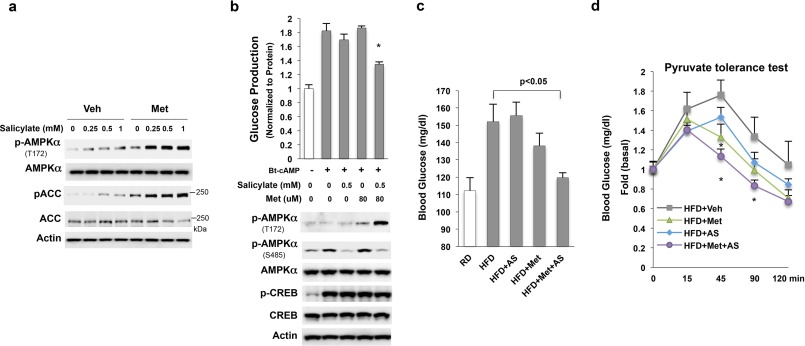
Because the AMPKα1S485A mutation augmented AMPKα subunit phosphorylation at Thr-172 and abolished the negative effect of Bt-cAMP on metformin-mediated AMPKα phosphorylation at Thr-172 (Fig. 2e), we tested whether the blockade of AMPKα Ser-485 phosphorylation by salicylate would increase AMPKα phosphorylation at Thr-172 by metformin. Hepa1–6 cells were treated with metformin and/or salicylate, we found indeed treatment with salicylate and metformin led to a dramatic increase in AMPKα phosphorylation at Thr-172 as well as the phosphorylation of ACC, the downstream target of AMPK (Fig. 6a) compared with treatment with either agent alone. In our previous study, we found that simultaneous treatment with Bt-cAMP negated the suppression effect of low metformin concentrations on glucose production in primary hepatocytes due to the inhibition of metformin-stimulated AMPK activation (10). We reasoned that salicylate might recover the ability of low concentrations of metformin's inhibition of glucose production in primary hepatocytes treated simultaneously with Bt-cAMP. To test whether salicylate and metformin have a synergistic effect on the suppression of glucose production, we conducted a glucose production assay in primary hepatocytes. Either metformin or salicylate alone at the tested concentrations did not significantly suppress Bt-cAMP-stimulated glucose production, consistent with previous data (10). However, we observed dramatically increased levels of AMPKα phosphorylation at Thr-172 together with decreased levels of AMPKα phosphorylation at Ser-485 when primary hepatocytes were treated with 0.5 mm salicylate and 80 μm metformin simultaneously (Fig. 6b). This treatment also led to significantly suppressed Bt-cAMP-stimulated glucose production. Again, treatment with salicylate and metformin did not affect CREB phosphorylation.
FIGURE 6.
Synergistic activation of AMPK by metformin and salicylate. a, only the treatment with both salicylate and metformin (0.25 mm) for 24 h greatly increased the endogenous AMPKα phosphorylation at Thr-172 in Hepa 1–6 cells. b, 18 h after cell seeding, primary hepatocytes were subjected to serum starvation in DMEM (1 g/liter glucose), and then washed twice with PBS; 1 ml glucose production was added along with 0.2 mm Bt-cAMP and the indicated amount of salicylate and metformin simultaneously and incubated for 3 h. c, blood glucose levels (6 h fast) in mice treated with 25 mg/kg metformin and/or 10 mg/kg aspirin for 3 weeks (The y-axis has been broken and begins at 80 mg/dL). d, pyruvate tolerance test (1.5 g/kg pyruvate) in fasted mice (6 h). An asterisk (*) signifies that groups with the same treatment are significantly different (p < 0.05).
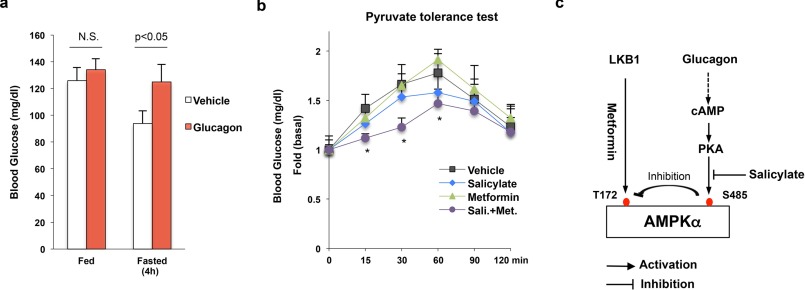
Next, we conducted an in vivo animal experiment in which mice were treated with metformin and/or aspirin. This experiment was designed to test the combined effect of metformin and aspirin treatment, using therapeutic doses of metformin. We found that fasting glucose levels (6 h fasting) were significantly lower in high-fat-diet-fed mice treated with both metformin (25 mg/kg) and aspirin (10 mg/kg) compared with high-fat-diet-fed control mice (Fig. 6c). Although we observed decreased fasting blood glucose levels in metformin-treated mice, these data were not significantly different from control high-fat-diet-fed mice. We further conducted a pyruvate tolerance test to examine hepatic glucose production in vivo. Combined metformin and aspirin administration significantly decreased blood glucose levels to a greater extent than either agent alone (Fig. 6d). Finally, we tested whether salicylate could improve metformin's effect on suppression of hepatic glucose production in mice with hyperglucagonemia. Mice were implanted with glucagon micro-osmotic pumps in the peritoneum (25). Continuous infusion of exogenous glucagon resulted in fasting hyperglycemia without affecting blood glucose levels in the fed state (Fig. 7a). In addition, these mice were fed on a high-fat diet and treated with both metformin (50 mg/kg) and/or salicylate (28.6 mg/kg) for 3 weeks. Then, we conducted a pyruvate tolerance test to examine hepatic glucose production. As shown in Fig. 7b, metformin's effect on suppression of hepatic glucose production was completely lost in these mice, suggesting that glucagon exerts a negative effect on metformin suppression of hepatic glucose production. In addition, salicylate alone did not change blood glucose levels significantly. However, combined metformin and salicylate administration led to a significant decrease in blood glucose levels (Fig. 7b).
FIGURE 7.
Salicylate improves metformin action in mice with continuous infusion of exogenous glucagon. a, blood glucose levels in mice with continuous infusion of glucagon (7 days). b, mice with glucagon infusion were treated with vehicle, metformin (50 mg/kg/day), salicylate (28.6 mg/kg/day), or metformin plus salicylate for 3 weeks. Then, a pyruvate tolerance test (2 g/kg) was conducted in these mice after 4 h of fasting (n = 4). c, proposed model for the activation of AMPK by metformin and salicylate.
Discussion
Our previous study showed that low metformin concentrations (<80 μm), found in the portal vein after therapeutic dosage, suppress hepatic glucose production through AMPK, as evidenced by the fact that low metformin concentrations are able to suppress glucose production in primary hepatocytes and that this metformin effect is lost in primary hepatocytes with AMPKα1/2 depletion (10). Moreover, low metformin concentrations suppressed glucose production without decreasing cellular ATP levels or increasing the AMP/ATP ratio. On the contrary, we observed an increase in ATP levels after metformin treatment. These data are consistent with the findings that long-term metformin administration in animals actually augmented mitochondrial complex 1 activity (30), suggesting that alteration of cellular energy levels is not required for metformin action. Of note, in primary hepatocytes, AMPK is activated by metformin even at 10 μm, corresponding to metformin's concentration in the circulation (28). After we found that low metformin concentrations suppress hepatic glucose production through AMPK, we proceeded to investigate the mechanism of AMPK activation by metformin. Having seen the unequal levels of AMPK subunits in different tissues, we reasoned that metformin might affect the formation of a functional AMPK heterotrimeric complex by augmenting the phosphorylation of the α subunit at Thr-172 by upstream kinase LKB1. In in vitro assays, low metformin concentrations did indeed increase the assembly of the AMPK heterotrimeric complex and the phosphorylation of the α subunit at Thr-172 by LKB1(23). This effect was lost when the γ1 subunit was absent in the reaction, suggesting that the AMPKγ subunit plays an essential role in metformin-stimulated phosphorylation of the α1 subunit at Thr-172 by LKB1(23). This result is in good agreement with a previous study shown that cells expressing a mutant AMPKγ subunit (R531G) were resistant to metformin-mediated AMPK activation (31). This evidence also indicates that AMPK subunits are metformin's direct targets and that metformin binding to an AMPK subunit promotes the formation of the AMPK heterotrimeric complex, making the phosphorylation site of the α subunit at Thr-172 more accessible to LKB1.
In the clinic, over one-third of diabetic patients exhibit either a lack of response or a delayed response to metformin treatment (3–6) and become less sensitive to metformin over time (4). We found that PKA is able to directly phosphorylate AMPKα subunit at Ser-485 (10), and several studies have documented that phosphorylation of AMPK by PKA reduces α subunit phosphorylation at Thr-172 and decreases AMPK enzymatic activity (17–19). Therefore, we hypothesized the elevated serum glucagon levels or glucagon to insulin ratios often observed in diabetes would lead to the increase in PKA-mediated AMPKα subunit phosphorylation at Ser-485, and therefore, cause metformin resistance/insensitivity (11–14). Indeed, in a pyruvate tolerance test, mice with constitutively activated PKA exhibited metformin resistance when compared with WT mice (Fig. 2, b and c). In addition, mice with continuous infusion of glucagon to mimic hyperglucagonemia also resisted metformin's effect on suppression of hepatic glucose production (Fig. 7b). Metformin was unable to suppress glucose production in primary hepatocytes with constitutively activated PKA, which occurred along with a drastic increase in the phosphorylation of the AMPKα subunit at Ser-485 (Fig. 2d). However, overexpression of the AMPKα1S485A mutant in primary hepatocytes with constitutively activated PKA restored metformin's effect on the suppression of glucose production and AMPKα phosphorylation at Thr-172 (Fig. 2e).
As activation of the cAMP-PKA pathway leads to metformin resistance/insensitivity through the phosphorylation of AMPKα subunit at Ser-485, blocking the phosphorylation of AMPKα subunit at Ser-485 should improve metformin's effect. Salicylate/aspirin can activate AMPK and increase AMPKα subunit phosphorylation at Thr-172 (20) and also exhibits an anti-hyperglycemic effect in animal models and diabetic patients (32, 33). We tested the effect of salicylate on AMPKα subunit phosphorylation at Ser-485 at therapeutic concentrations and found that salicylate blocked the basal- and Bt-cAMP-stimulated AMPKα subunit phosphorylation at Ser-485 (Fig. 3). AMPKα subunit phosphorylation at Ser-485 produces a negative regulatory effect on the activation of AMPK, therefore, blocking this phosphorylation event by salicylate can improve metformin's effect on suppression of glucose production, because these two drugs can activate AMPK through different pathways (Fig. 7c). Our results demonstrate that treatment of primary hepatocytes with both salicylate and metformin at therapeutic concentrations greatly increases AMPKα subunit phosphorylation at Thr-172 and suppression of glucose production versus administration of either agent alone (Figs. 6 and 7). Given that diabetic patients often have hyperglucagonemia, the combination of salicylate and metformin in a low dose might enhance AMPK activation and subsequent treatment efficacy without producing unwanted toxicity associated with high doses of these drugs. Of note, activation of AMPK by metformin and salicylate increases ACC phosphorylation (Fig. 6a), and this should augment fatty acid oxidation and subsequently lead to the improvement of insulin sensitivity (34) and ameliorate high-fat diet induced hyperglycemia (35).
Author Contributions
L. H. designed the experiments. L. H., E. V., J. P., and H. A. conducted experiments. C. A. S. generated floxed PKAR1a mice. S. M. helped the husbandry of the floxed PKAR1a mice. L. H., S. R., and F. E. W. analyzed the data. L. H. and F. E. W. wrote the manuscript.
This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health: R00DK085142 (to L. H.), R01DK107641 (to L. H.), and R01DK063349 (to F. E. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- T2D
- type 2 diabetes
- AMPK
- AMP-activated kinase
- PKA
- protein kinase A
- Bt-cAMP
- dibutyryl-cAMP.
References
- 1. Witters L. A. (2001) The blooming of the French lilac. J. Clin. Invest. 108, 1105–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi S. E., Bergenstal R. M., Buse J. B., Diamant M., Ferrannini E., Nauck M., Peters A. L., Tsapas A., Wender R., and Matthews D. R. (2012) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35, 1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hermann L. S. (1990) Biguanides and sulfonylureas as combination therapy in NIDDM. Diabetes Care 13 Suppl 3, 37–41 [DOI] [PubMed] [Google Scholar]
- 4. Bailey C. J., and Turner R. C. (1996) Metformin. Metformin. N. Engl. J. Med. 334, 574–579 [DOI] [PubMed] [Google Scholar]
- 5. Kahn S. E., Haffner S. M., Heise M. A., Herman W. H., Holman R. R., Jones N. P., Kravitz B. G., Lachin J. M., O'Neill M. C., Zinman B., and Viberti G. (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443 [DOI] [PubMed] [Google Scholar]
- 6. Cook M. N., Girman C. J., Stein P. P., and Alexander C. M. (2007) Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with Type 2 diabetes in UK primary care. Diabet. Med. 24, 350–358 [DOI] [PubMed] [Google Scholar]
- 7. Shu Y., Sheardown S. A., Brown C., Owen R. P., Zhang S., Castro R. A., Ianculescu A. G., Yue L., Lo J. C., Burchard E. G., Brett C. M., and Giacomini K. M. (2007) Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Invest. 117, 1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shu Y., Brown C., Castro R. A., Shi R. J., Lin E. T., Owen R. P., Sheardown S. A., Yue L., Burchard E. G., Brett C. M., and Giacomini K. M. (2008) Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin. Pharmacol. Ther. 83, 273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pawlyk A. C., Giacomini K. M., McKeon C., Shuldiner A. R., and Florez J. C. (2014) Metformin pharmacogenomics: current status and future directions. Diabetes 63, 2590–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao J., Meng S., Chang E., Beckwith-Fickas K., Xiong L., Cole R. N., Radovick S., Wondisford F. E., and He L. (2014) Low concentrations of metformin suppress glucose production in hepatocytes through AMP-activated protein kinase (AMPK). J. Biol. Chem. 289, 20435–20446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burcelin R., Katz E. B., and Charron M. J. (1996) Molecular and cellular aspects of the glucagon receptor: role in diabetes and metabolism. Diabetes Metab. 22, 373–396 [PubMed] [Google Scholar]
- 12. Unger R. H. (1985) Glucagon physiology and pathophysiology in the light of new advances. Diabetologia 28, 574–578 [DOI] [PubMed] [Google Scholar]
- 13. Baron A. D., Schaeffer L., Shragg P., and Kolterman O. G. (1987) Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, 274–283 [DOI] [PubMed] [Google Scholar]
- 14. Reaven G. M., Chen Y. D., Golay A., Swislocki A. L., and Jaspan J. B. (1987) Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 64, 106–110 [DOI] [PubMed] [Google Scholar]
- 15. Consoli A. (1992) Role of liver in pathophysiology of NIDDM. Diabetes Care 15, 430–441 [DOI] [PubMed] [Google Scholar]
- 16. Unger R. H., Aguilar-Parada E., Müller W. A., and Eisentraut A. M. (1970) Studies of pancreatic alpha cell function in normal and diabetic subjects. J. Clin. Invest. 49, 837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Djouder N., Tuerk R. D., Suter M., Salvioni P., Thali R. F., Scholz R., Vaahtomeri K., Auchli Y., Rechsteiner H., Brunisholz R. A., Viollet B., Mäkelä T. P., Wallimann T., Neumann D., and Krek W. (2010) PKA phosphorylates and inactivates AMPKα to promote efficient lipolysis. EMBO J. 29, 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hurley R. L., Barré L. K., Wood S. D., Anderson K. A., Kemp B. E., Means A. R., and Witters L. A. (2006) Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J. Biol. Chem. 281, 36662–36672 [DOI] [PubMed] [Google Scholar]
- 19. Ning J., Xi G., and Clemmons D. R. (2011) Suppression of AMPK activation via S485 phosphorylation by IGF-I during hyperglycemia is mediated by AKT activation in vascular smooth muscle cells. Endocrinology 152, 3143–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hawley S. A., Fullerton M. D., Ross F. A., Schertzer J. D., Chevtzoff C., Walker K. J., Peggie M. W., Zibrova D., Green K. A., Mustard K. J., Kemp B. E., Sakamoto K., Steinberg G. R., and Hardie D. G. (2012) The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336, 918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He L., Naik K., Meng S., Cao J., Sidhaye A. R., Ma A., Radovick S., and Wondisford F. E. (2012) Transcriptional co-activator p300 maintains basal hepatic gluconeogenesis. J. Biol. Chem. 287, 32069–32077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He L., Meng S., Germain-Lee E. L., Radovick S., and Wondisford F. E. (2014) Potential biomarker of metformin action. J. Endocrinol. 221, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meng S., Cao J., He Q., Xiong L., Chang E., Radovick S., Wondisford F. E., and He L. (2015) Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J. Biol. Chem. 290, 3793–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirschner L. S., Kusewitt D. F., Matyakhina L., Towns W. H. 2nd, Carney J. A., Westphal H., and Stratakis C. A. (2005) A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 65, 4506–4514 [DOI] [PubMed] [Google Scholar]
- 25. Webb G. C., Akbar M. S., Zhao C., Swift H. H., and Steiner D. F. (2002) Glucagon replacement via micro-osmotic pump corrects hypoglycemia and alpha-cell hyperplasia in prohormone convertase 2 knockout mice. Diabetes 51, 398–405 [DOI] [PubMed] [Google Scholar]
- 26. Wondisford A. R., Xiong L., Chang E., Meng S., Meyers D. J., Li M., Cole P. A., and He L. (2014) Control of Foxo1 gene expression by co-activator P300. J. Biol. Chem. 289, 4326–4333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M. A., Radovick S., and Wondisford F. E. (2009) Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137, 635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., and Moller D. E. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song W. J., Seshadri M., Ashraf U., Mdluli T., Mondal P., Keil M., Azevedo M., Kirschner L. S., Stratakis C. A., and Hussain M. A. (2011) Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab. 13, 308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin-Montalvo A., Mercken E. M., Mitchell S. J., Palacios H. H., Mote P. L., Scheibye-Knudsen M., Gomes A. P., Ward T. M., Minor R. K., Blouin M. J., Schwab M., Pollak M., Zhang Y., Yu Y., Becker K. G., Bohr V. A., Ingram D. K., Sinclair D. A., Wolf N. S., Spindler S. R., Bernier M., and de Cabo R. (2013) Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hawley S. A., Ross F. A., Chevtzoff C., Green K. A., Evans A., Fogarty S., Towler M. C., Brown L. J., Ogunbayo O. A., Evans A. M., and Hardie D. G. (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 11, 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller J. D., Ganguli S., and Sperling M. A. (1983) Prostaglandin synthesis inhibitors impair hepatic glucose production in response to glucagon and epinephrine stimulation. Diabetes 32, 439–444 [DOI] [PubMed] [Google Scholar]
- 33. Hundal R. S., Petersen K. F., Mayerson A. B., Randhawa P. S., Inzucchi S., Shoelson S. E., and Shulman G. I. (2002) Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Invest. 109, 1321–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ford R. J., Fullerton M. D., Pinkosky S. L., Day E. A., Scott J. W., Oakhill J. S., Bujak A. L., Smith B. K., Crane J. D., Blümer R. M., Marcinko K., Kemp B. E., Gerstein H. C., and Steinberg G. R. (2015) Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem. J. 468, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. An H., and He L. (2016) Current understanding of metformin effect on the control of hyperglycemia in diabetes. J. Endocrinol. 228, R97–R106 [DOI] [PMC free article] [PubMed] [Google Scholar]