Abstract
Nicotinamide adenine dinucleotide (NAD+) participates in redox reactions and NAD+-dependent signaling pathways. Although the redox reactions are critical for efficient mitochondrial metabolism, they are not accompanied by any net consumption of the nucleotide. On the contrary, NAD+-dependent signaling processes lead to its degradation. Three distinct families of enzymes consume NAD+ as substrate: poly(ADP-ribose) polymerases, ADP-ribosyl cyclases (CD38 and CD157), and sirtuins (SIRT1–7). Because all of the above enzymes generate nicotinamide as a byproduct, mammalian cells have evolved an NAD+ salvage pathway capable of resynthesizing NAD+ from nicotinamide. Overexpression of the rate-limiting enzyme in this pathway, nicotinamide phosphoribosyltransferase, increases total and mitochondrial NAD+ levels in astrocytes. Moreover, targeting nicotinamide phosphoribosyltransferase to the mitochondria also enhances NAD+ salvage pathway in astrocytes. Supplementation with the NAD+ precursors nicotinamide mononucleotide and nicotinamide riboside also increases NAD+ levels in astrocytes. Amyotrophic lateral sclerosis (ALS) is caused by the progressive degeneration of motor neurons in the spinal cord, brain stem, and motor cortex. Superoxide dismutase 1 (SOD1) mutations account for up to 20% of familial ALS and 1–2% of apparently sporadic ALS cases. Primary astrocytes isolated from mutant human superoxide dismutase 1-overexpressing mice as well as human post-mortem ALS spinal cord-derived astrocytes induce motor neuron death in co-culture. Increasing total and mitochondrial NAD+ content in ALS astrocytes increases oxidative stress resistance and reverts their toxicity toward co-cultured motor neurons. Taken together, our results suggest that enhancing the NAD+ salvage pathway in astrocytes could be a potential therapeutic target to prevent astrocyte-mediated motor neuron death in ALS.
Keywords: amyotrophic lateral sclerosis (ALS) (Lou Gehrig disease), astrocyte, mitochondria, NAD biosynthesis, NAMPT, oxidative stress
Introduction
Nicotinamide adenine dinucleotide (NAD+) is an essential redox molecule and a key player in several signaling pathways that govern fundamental biological processes (1, 2). In the redox reactions, a hydride equivalent is reversibly transferred at the nicotinamide moiety, resulting in a switch between oxidized (NAD+) and reduced (NADH) forms of the nucleotide. Although the redox reactions are critical for efficient mitochondrial metabolism, they are not accompanied by any net consumption of the nucleotide. On the contrary, NAD+-dependent signaling processes lead to its degradation.
Three distinct families of enzymes consume NAD+ as substrate: poly(ADP-ribose) polymerases (PARPs),2 ADP-ribosyl cyclases (CD38 and CD157), and sirtuins (SIRT1–7) (3–5). PARPs hydrolyze NAD+ and transfer the ADP-ribose moiety of NAD+ to a receptor amino acid, building poly(ADP-ribose) polymers. PARPs regulate DNA damage repair, tumorigenesis, cell differentiation, and metabolism (3, 6, 7). CD38 is a ubiquitously expressed multifunctional enzyme that catalyzes the production of second messengers (like cyclic ADP-ribose) (8), which act as potent intracellular calcium-mobilizing agents to control cell cycle, insulin signaling, and microglial activation (8–10). Sirtuins are a highly conserved family of proteins capable of catalyzing NAD+-dependent deacylation and mono(ADP-ribosyl)ation reactions (11). Sirtuin activation has been shown to modulate mitochondrial biogenesis and all major mitochondrial processes, including the tricarboxylic acid cycle, fatty acid metabolism, oxidative phosphorylation, and antioxidant response (4, 12–15). Because all of the above NAD+-consuming enzymes generate nicotinamide (NAM) as a byproduct, mammalian cells have evolved an NAD+ salvage pathway capable of resynthesizing NAD+ from NAM (16).
Although NAD+ synthesis can occur from l-tryptophan (kynurenine pathway), nicotinic acid (Preiss-Handler pathway), or nicotinamide riboside (NR) (17–19), the salvage pathway appears to account for the majority of NAD+ synthesis in mammalian cells. The enzyme nicotinamide phosphoribosyltransferase (NAMPT) catalyzes the conversion of NAM and 5′-phosphoribosyl 1-pyrophosphate to nicotinamide mononucleotide (NMN); subsequently nicotinamide mononucleotide adenylyltransferases (NMNATs) transfer adenine from ATP to NMN to generate NAD+ (20, 21). NAMPT is the rate-limiting enzyme in this pathway. Accordingly, overexpression of NAMPT, but not NMNATs, increases NAD levels (22–24).
All the different types of NAD+-consuming reactions have been described in the mitochondria, but in general NAMPT appears to be absent from the mitochondrial compartment (22, 24–26), and the origin of the mitochondrial NAD+ pool in mammalian cells is not yet fully characterized. Treatment with intermediate precursors like NMN and NR has been shown to increase mitochondrial NAD+ levels in culture (24, 27). NR is likely converted to NMN in the cytosol, and it has been proposed that NMN is transported into the mitochondria for NAD+ synthesis (24, 28). Although the identity of the mitochondrial NMNAT remains to be established, the existence of a mitochondrial enzymatic activity capable of synthesizing NAD+ starting from NMN and ATP is widely accepted (2, 28–30).
Amyotrophic lateral sclerosis (ALS) is caused by the progressive degeneration of motor neurons in the spinal cord, brain stem, and motor cortex. Motor neuron death leads to muscle weakness and paralysis, causing death in 1–5 years from the time of symptom onset. Most ALS cases are sporadic (SALS), and exposure to yet unidentified environmental toxicants might be responsible for SALS (31). About 5–10% of the cases are inherited (familial ALS (FALS)), and the first ALS-linked gene identified was superoxide dismutase 1 (SOD1) (32). Mutations in SOD1 account for up to 20% of FALS and 1–2% of apparently SALS cases. Mutations in several other genes have now been identified in many FALS pedigrees (31, 33, 34). Each mutated gene has its own genetic and molecular signature, but FALS and SALS are phenotypically indistinguishable, and a significant share of our understanding comes from the study of rodents overexpressing ALS-linked mutant SOD1 that develop an ALS-like phenotype (35). The molecular mechanism underlying the toxic gain of function of mutant hSOD1s remains uncertain. However, several lines of evidence suggest that toxicity to motor neurons requires dysfunction of non-neuronal cells (36–38). In line with this observation, primary astrocytes isolated from mutant hSOD1-overexpressing mice induce motor neuron death in co-culture (39–41). The non-cell-autonomous component for other ALS-linked mutations remains under investigation, but it has been demonstrated that astrocytes differentiated from human post-mortem ALS spinal cord-derived progenitor cells and astrocytes obtained from the transdifferentiation of fibroblasts from FALS and SALS patients are also toxic for motor neurons in co-culture (42, 43).
Here, we determined the effect of NAD+ precursors and NAMPT overexpression on total and mitochondrial NAD+ content in non-transgenic and mutant hSOD1-expressing astrocytes. In addition, we investigated the effect of a mitochondrially targeted NAMPT on mitochondrial NAD+ recycling in astrocytes. Increasing total and mitochondrial NAD+ content in ALS astrocytes increases oxidative stress resistance and reverts their toxicity toward co-cultured motor neurons. Taken together, our results suggest that enhancing the NAD+ salvage pathway in astrocytes could be a potential therapeutic target for ALS.
Experimental Procedures
Reagents
All chemical and reagents were from Sigma-Aldrich unless otherwise specified. Culture media and serum were obtained from Life Technologies. Primers and siRNAs were obtained from Integrated DNA Technologies. NR was obtained from BOC Sciences.
Animals and Primary Cultures
B6.Cg-Tg(SOD1*G93A)1Gur/J (35) mice were obtained from The Jackson Laboratory. hSOD1H46R/H48Q mice were provided by Dr. David Borchelt (44). Primary astrocyte cultures were prepared from cortex and spinal cord of 1-day-old mice as described previously (39). Cortical astrocyte cultures have a significantly higher yield than spinal cord astrocyte cultures. Thus, to minimize the number of animals needed, some experiments were performed with cortical astrocytes, whereas the spinal cord astrocytes were reserved for critical experiments (e.g. the effect of mitochondrially targeted NAMPT, mitochondrial function, and co-cultures). Motor neuron cultures were prepared from 12.5-embryonic-day mouse spinal cords as described previously (45). For co-culture experiments, motor neurons were plated on mouse astrocyte monolayers at a density of 300 cells/cm2 and maintained in supplemented L15 medium (39). Motor neurons were identified by immunostaining with anti-neurofilament (Sigma-Aldrich), and survival was determined by counting all cells displaying intact neurites longer than four cells in diameter. Counts were performed over an area of 0.90 cm2 in 24-well plates.
NAD+/NADH Quantification
NAD+ and NADH levels were determined based on the differential sensitivity of the nucleotides to pH and temperature (46). Samples were divided in two: one part was homogenized in 0.5 m perchloric acid and maintained on ice (NAD+ fraction), and the other part was homogenized in 50 mm NaOH plus 1 mm EDTA and incubated at 60 °C for 30 min (NADH faction). Following neutralization, NAD+ and NADH levels were determined by an enzymatic cycling assay (47) and corrected by protein concentration determined with a BCA protein assay (Thermo Scientific). NADP+/NADPH quantification was performed with a fluorometric NADP+/NADPH Ratio Assay kit (AAT Bioquest).
Expression Vectors and Adenovirus Packaging
A mouse Myc-DDK-tagged NAMPT cDNA clone was obtained from OriGene Technologies (MR207867). To generate a mitochondrially targeted NAMPT, the mitochondrial targeting sequence from the human cytochrome c oxidase subunit VIII (48) was inserted in front of the NAMPT ORF. Subcloning into an adenoviral plasmid and adenoviral packaging were performed by Vector Biolabs. The pDsRed2-Mito plasmid was obtained from Clontech. To provide in situ evidence of increased mitochondrial NAD+ levels, we used the formation of immunodetectable poly(ADP-ribose) polymers mediated by a mitochondrially targeted fusion protein consisting of EGFP and the catalytic domain of PARP1. The mitochondrially targeted PARP plasmid, the control mitochondrially targeted EGFP plasmid, and the assay have been described previously (49, 50). SIRT1-FLAG- (ID 13812 (51)) and SIRT3-FLAG (ID 33309 (52))-coding plasmids were obtained from Addgene.
Cell Treatment, Transfections, and Mitochondrial Isolation
Confluent astrocyte monolayers were treated with 5 mm NMN, 5 mm NR, or vehicle control 24 h before motor neuron plating or subsequent analysis. Plasmid transfections were performed as described previously (53). Adenovirus-mediated transfections were performed at 50 multiplicity of infection, and astrocytes were used 48 h post-transfection. H2O2 was diluted in Dulbecco's PBS and applied to astrocyte monolayers at the indicated final concentrations. Survival was assayed 24 h later by determining the release of lactate dehydrogenase using the CytoTox Non-Radioactive Cytotoxicity Assay kit (Promega). siRNA transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Astrocytes were transfected with 25 nm Sirt1 siRNA (5′-CAACAGCAUCUUGCCUGAUUUGUAA-3′), Sirt3 siRNA (5′-GGGAACGUGGCAAGCUGGAUGGACA), or negative control (NC1) siRNA 24 h before motor neuron plating or 48 h before sample collection for analysis. Mitochondrial isolation was performed by differential centrifugation in a buffer containing 10 mm Tris, 1 mm EDTA, 0.32 m sucrose, and 0.2 mg/ml digitonin. This preparation routinely yields mitochondrial fractions with less than 1.5% of the total cellular lactate dehydrogenase activity.
Mitochondrial Reactive Oxygen Species (ROS)
Confluent astrocyte monolayers were treated for 2 h with 300 μm H2O2 or vehicle. Following treatments, duplicate sets of cells were incubated for 30 min in Hanks' balanced salt solution with 4 μm MitoSox (Life Technologies) or 0.2 μm MitoTracker Green (Life Technologies). Mitochondrial reactive oxygen species production (MitoSox; excitation/emission, 530/590 nm) was corrected by mitochondrial content (MitoTracker; excitation/emission, 485/530 nm).
Western Blotting, Immunoprecipitation, Real Time PCR, and Immunofluorescence
Western blotting and immunoprecipitation were performed as described previously (45, 54). Membranes were incubated overnight with one of the following antibodies: anti-NAMPT (Abcam, ab45890, lot GR71423–1), anti-Actin (Sigma, A5441, lot 061M4808), anti-VDAC1 (Cell Signaling Technology, 4661S, lot 4), anti-poly(ADP-ribose) (PAR) polymers (Abcam, ab119484, lot GR84202–2), anti-SIRT1 (Cell Signaling Technology, 9475P, lot 1), anti-SIRT3 (Cell Signaling Technology, 5490S, lot 3), anti-isocitrate dehydrogenase 2 (IDH2) (Cell Signaling Technology, 12652S, lot 1), and anti-acetylated lysine (Cell Signaling Technology, 9814S, lot 5). Image acquisition was performed in a chemiluminescence Western blot scanner (LI-COR Biosciences) or exposed on Eastman Kodak Co. BioMax Light film. Quantifications were performed using Image Studio Software (LI-COR Biosciences) or ImageJ software (National Institutes of Health). RNA extraction, RNA retrotranscription, and real time PCR were performed as described previously (45). Specific primers were as follows: Nampt/5′, 5′-GCCACCTTATCTTAGAGTCATTCA-3′; Nampt/3′, 5′-GAGACATTCTCGATACTCCACTTC-3′; Nmnat1/5′, 5′-GAGCTGGCCAAGGACTATATG-3′; Nmnat1/3′, 5′-GGATGAGCCCTTTCTTCTTGTA-3′; Nmnat2/5′, 5′-GGAGGTGGGAAGACTTTATAGC-3′; Nmnat2/3′, 5′-CAGAGTACACCCATCCTCATTAC-3′; Nmnat3/5′, 5′-CACCAGGGTTCCCAATATCC-3′; Nmnat3/3′, 5′-CAAACAAGCAGGCAGTCATAAA-3′; Parp1/5′, 5′-GGAGACCCGATTGGCTTAAT-3′; Parp1/3′, 5′-CCCTTGGGTAACTTGCTGATA-3′; Cd38/5′, 5′-GTGGCAGGATTAGAAGGTATGG-3′; Cd38/3′, 5′-TGAAGGCTGTTAGTGGAATAGTG-3′; Sirt3/5′, 5′-ATCCCGTACCCTGAAGCCATCTTT-3′; Sirt3/3′, 5′-TCAAGCCCGTCGATGTTCTGTGTA-3′; Sirt1/5′, 5′-AGCAACATCTCATGATTGGCACCG-3′; Sirt1/3′, 5′-TCTGCCACAGCGTCATATCATCCA-3′; Actin/5′, 5′-TGTGATGGTGGGAATGGGTCAGAA-3′; and Actin/3′, 5′-TGTGGTGCCAGATCTTCTCCATGT-3′. Immunofluorescence was performed as described previously (39) using an anti-FLAG antibody (Sigma, F1804, lot SLBK1346V).
Respirometry Assay, Mitochondrial Biogenesis, and ATP Measurement
Mitochondrial oxygen consumption rate measurements were performed using a Seahorse Bioscience XF-96 instrument (Seahorse Bioscience) as described previously (55). Briefly, average basal rates are the averages of the third and fourth basal rate measurements. At the end of the experiment, cells were fixed with 4% paraformaldehyde and stained with DAPI. The number of cells per well was determined in an IN Cell analyzer (GE Healthcare) and used to normalize the raw oxygen consumption rate data. DNA extraction from astrocytes was performed with a QIAamp DNA Mini kit (Qiagen). Relative mitochondrial and nuclear DNA copy determinations were performed by real time PCR as described previously (56). ATP determination was performed using a commercial kit (BioVision) and corrected by protein concentration.
Statistical Analysis
Each experiment was repeated at least in three independent primary culture preparations. Multiple group comparison was performed by one-way analysis of variance with Tukey's post-test. When comparing the effect of genotype and treatments, two-way analysis of variance was used followed by Tukey's post-test. Differences were declared statistically significant if p was ≤0.05. All statistical computations were performed using Prism 6.0 (GraphPad Software).
Results
NAD+ Precursors Increase Total and Mitochondrial NAD+ Content in Astrocytes
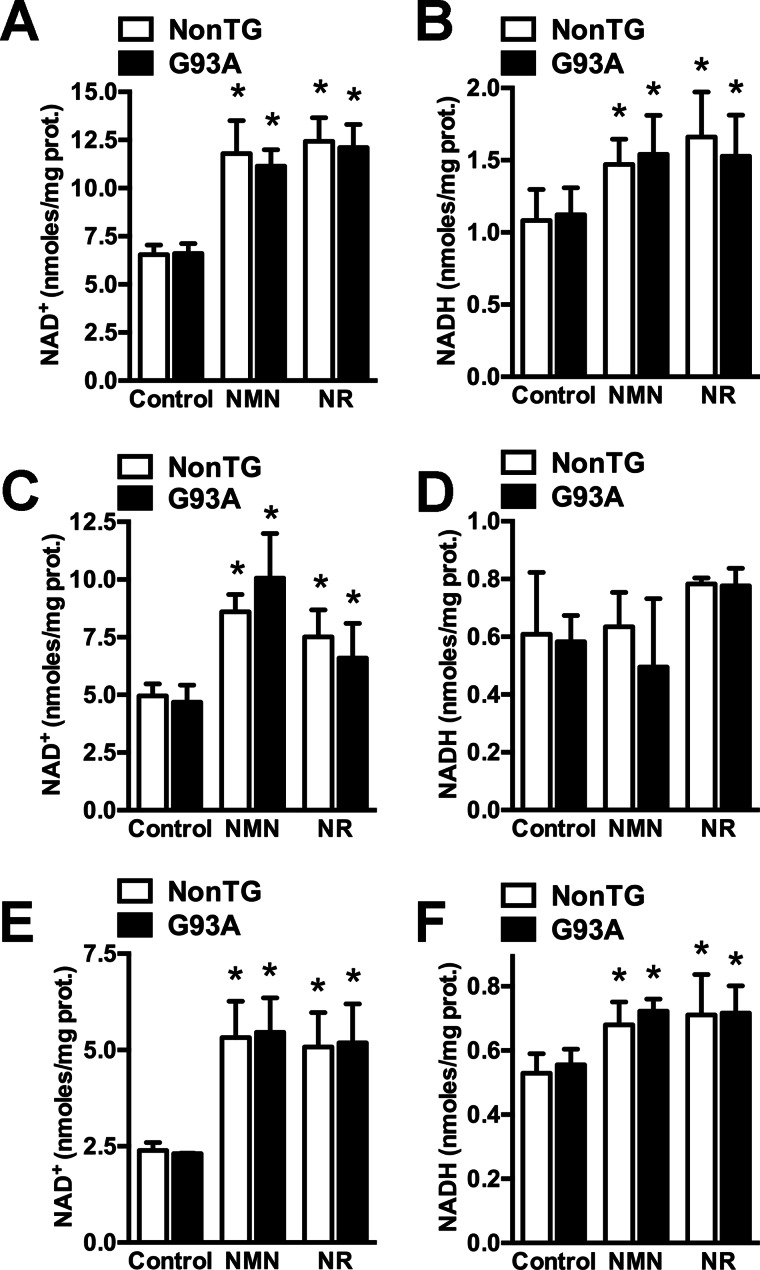
NMN and NR supplementation significantly increase total NAD+ in primary cortical astrocytes obtained from non-transgenic and hSOD1G93A-overexpressing mice (Fig. 1). Because total NAD+ increases more than total NADH levels (about 100 and 35% change, respectively), treatments also modify the NAD+/NADH ratio (Fig. 1, A and B). Both precursors also significantly increase mitochondrial NAD+ content, but no significant change was observed in mitochondrial NADH (Fig. 1, C and D). In spinal cord astrocytes, treatments induce a similar -fold change in total NAD+ and NADH as in cortical astrocytes (about 100 and 30% change, respectively; Fig. 1, E and F), indicating that astrocytes from these different regions of the CNS respond in a similar way to the treatments. Untreated astrocytes from both genotypes do not display significant differences in NAD+ or NADH content.
FIGURE 1.
NAD+ precursors increase total and mitochondrial NAD+ content in astrocytes. Primary confluent cortical astrocytes obtained from non-transgenic (NonTG) and hSOD1G93A (G93A) mice were treated with vehicle (control), 5 mm NMN, or 5 mm NR. 24 h later total NAD+ (A), total NADH (B), mitochondrial NAD+ (C), and mitochondrial NADH (D) were determined as described under “Experimental Procedures” and corrected by protein (prot.) content. Primary confluent spinal cord astrocytes from non-transgenic and G93A mice were treated as above, and 24 h later total NAD+ (E) and total NADH (F) were determined and corrected by protein content. Each data bar represents the mean ± S.D. (error bars) of at least three independent experiments. *, significantly different from its respective control (p < 0.05).
A Mitochondrially Targeted NAMPT Increases Total and Mitochondrial NAD+ Content in Astrocytes
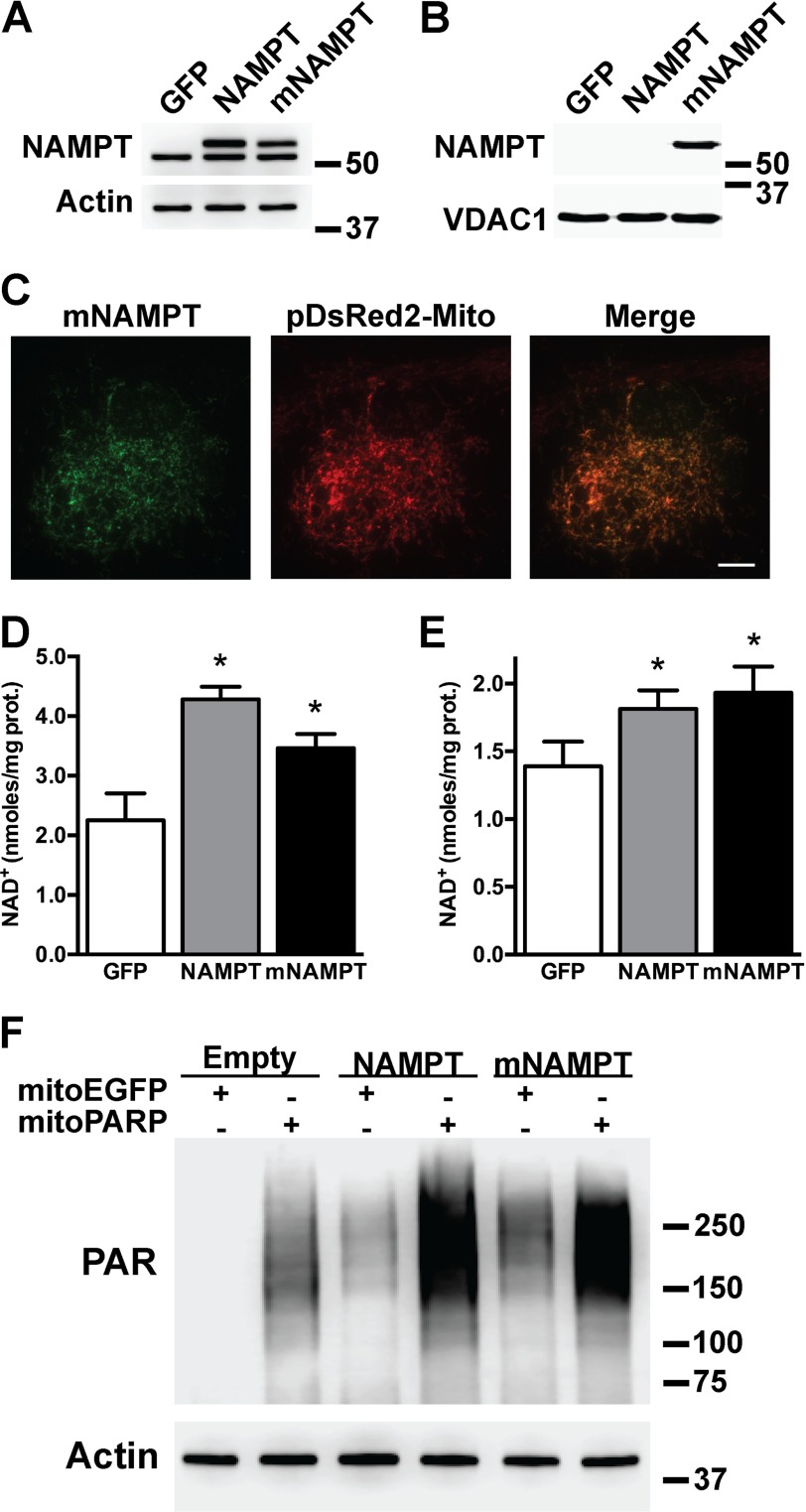
To investigate the effect of enhancing NAD+ salvage in astrocytes, we overexpressed NAMPT or a mitochondrially targeted version of NAMPT (mNAMPT). Overexpression was confirmed by Western blotting of total cell lysates using antibodies against NAMPT (Fig. 2A). The presence of the Myc-DDK tag increases the size of the exogenous proteins and allows the distinction from the endogenous NAMPT. NAMPT is absent from astrocytic mitochondrial fractions even when overexpressed (Fig. 2B). However, when fused to the leading peptide of human cytochrome c oxidase subunit VIII, NAMPT is directed to the mitochondria as evidenced by subcellular fractionation and immunofluorescence (Fig. 2, B and C). Overexpression of NAMPT and mNAMPT in spinal cord astrocytes increases total NAD+ about 90 and 55%, respectively. Mitochondrial NAD+ content increases about 30% with both approaches (Fig. 2, D and E). Because mitochondrial NAD+ synthesis is not yet fully characterized and to demonstrate that the effect of the mNAMPT is not only restricted to astrocytes, we used the formation of immunodetectable PAR polymers in the mitochondria as a reporter for mitochondrial NAD+ content in HEK293 cells. Targeted expression of the catalytic domain of PARP1 into the mitochondrial matrix (mitochondrially targeted PARP1 catalytic domain (mitoPARP)) leads to the constitutive presence of PAR polymers in the mitochondria (49, 50). Because changes in the mitochondrial NAD+ pool result in changes in the amount of the immunodetectable PAR produced by the mitoPARP, this system serves as a reporter for mitochondrial NAD+ content. Fig. 2F provides in situ evidence of increased mitochondrial NAD+ content following overexpression of NAMPT or mNAMPT in HEK293 cells stably overexpressing mitoPARP.
FIGURE 2.
A mitochondrially targeted NAMPT increases total and mitochondrial NAD+ content in astrocytes. Primary confluent spinal cord astrocytes were transfected with adenovirus expressing GFP, NAMPT, or mNAMPT. 48 h post-transfection NAMPT protein levels were determined by Western blotting in whole (A) or purified mitochondrial fractions (B). Actin or VDAC1 levels were used as loading controls. C, micrographs showing co-localization of mNAMPT (anti-DDK antibody; green) and a red fluorescent protein targeted to the mitochondria (pDsRed2-Mito; red) in spinal cord astrocytes co-transfected with vectors coding for mNAMPT and pDsRed2-Mito. Scale bar, 8 μm. Total (D) and mitochondrial (E) NAD+ content in spinal cord astrocyte cultures 48 h after transfection with adenovirus expressing GFP, NAMPT, or mNAMPT was determined. NAD+ was determined as described under “Experimental Procedures” and corrected by protein (prot.) content. Each data bar represents the mean ± S.D. (error bars) of at least three independent experiments. *, significantly different from GFP (p < 0.05). F, increased mitochondrial NAD+ content in HEK293 cells evidenced by PAR polymer accumulation mediated by a mitochondrially targeted PARP1 catalytic domain following co-expression of NAMPT and mNAMPT. HEK293 cells stably transfected with a mitochondrially targeted EGFP (mitoEGFP) or mitoPARP were transient transfected with NAMPT, mNAMPT, or empty plasmids. 24 h post-transfection PAR polymer accumulation was determined by Western blotting. Actin levels were used as a loading control.
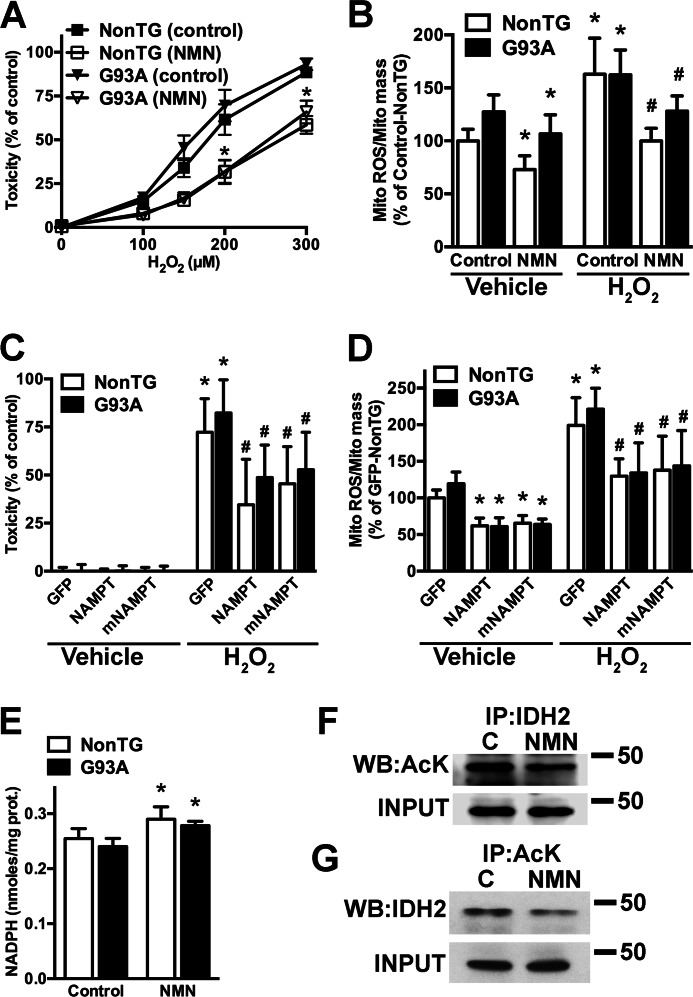
Increasing NAD+ Content Confers Resistance against Peroxide Toxicity and Decreases Mitochondrial Reactive Oxygen Species
NMN treatment confers astrocyte resistance to oxidative stress as reflected by reduced vulnerability to H2O2 treatment (Fig. 3A). To determine whether decreased mitochondrial oxidative stress contributes to the protection observed, the fluorescent probe MitoSox was used. MitoSox is selectively accumulated in mitochondria and oxidized as a function of ROS generation. MitoTracker Green was used in parallel to quantify total mitochondrial mass. Treatment of control astrocyte cultures with H2O2 induced a significant increase in mitochondrial ROS, which was prevented by NMN pretreatment (Fig. 3B). Likewise overexpression of NAMPT or mNAMPT also provides protection and reduces mitochondrial ROS production in response to H2O2 (Fig. 3, C and D). Moreover, NMN treatment and overexpression of NAMPT or mNAMPT also appear to decrease mitochondrial ROS levels under basal conditions (Fig. 3, B and D, vehicle bars). The mitochondrial IDH2 plays a key role in mitochondrial antioxidant defenses through its ability to generate NADPH, an essential reducing equivalent for mitochondrial antioxidant defenses. An increase in mitochondrial NAD+ availability could lead to SIRT3-mediated deacetylation and consequent activation of IDH2 (57, 58). NMN treatment induces about a 15% increase in NADPH content in astrocytes (Fig. 3E). Fig. 3, F and G, show a decrease in IDH2 acetylation following NMN treatment. This suggests that, at least in part, an increase in IDH2 activity could explain the observed increased resistance against peroxide toxicity and decreased mitochondrial ROS generation.
FIGURE 3.
Increasing NAD+ levels confers resistance against hydrogen peroxide toxicity and decreases mitochondrial reactive oxygen species. A, confluent cortical non-transgenic (NonTG) and hSOD1G93A (G93A) astrocyte monolayers were incubated with 5 mm NMN and 24 h later treated with the indicated concentrations of H2O2. Toxicity was assessed by lactate dehydrogenase release 24 h after peroxide treatment. Data are expressed as the percentage of the respective control. B, confluent non-transgenic and hSOD1G93A astrocyte monolayers were incubated with 5 mm NMN for 24 h. Following a change of medium, cultures were treated with H2O2 (300 μm) or vehicle, and 2 h later mitochondrial ROS (Mito ROS) production was determined. Mitochondrial reactive oxygen species was corrected by mitochondrial content (Mito mass). Data are expressed as the percentage of the non-transgenic control. C, confluent non-transgenic and hSOD1G93A astrocyte monolayers were transfected with adenovirus expressing GFP, NAMPT, or mNAMPT. 48 h post-transfection astrocytes were treated with vehicle or H2O2 (300 μm). Toxicity was assessed by lactate dehydrogenase release 24 h after peroxide treatment. Data are expressed as the percentage of their respective controls. D, confluent non-transgenic and hSOD1G93A astrocyte monolayers were transfected with adenovirus expressing GFP, NAMPT, or mNAMPT. 48 h post-transfection astrocytes were treated with H2O2 (300 μm) or vehicle. 2 h after treatment mitochondrial ROS production was determined as indicated above. Data are expressed as the percentage of non-transgenic GFP. For A, B, C, and D, each data point represents the mean ± S.D. (error bars) of at least three independent experiments. *, significantly different from vehicle-treated control/GFP for each respective genotype (p < 0.05). #, significantly different from H2O2-treated control/GFP for each respective genotype (p < 0.05). E, confluent non-transgenic and hSOD1G93A astrocytes were treated with 5 mm NMN, and 24 h later NADPH levels were determined as described under “Experimental Procedures” and corrected by protein (prot.) content. Each data bar represents the mean ± S.D. (error bars) of at least three independent experiments. *, significantly different from its respective control (p < 0.05). F, confluent astrocytes were treated with 5 mm NMN for 24 h. Following protein extraction, IDH2 was immunoprecipitated and analyzed by Western blotting using an antibody against acetylated lysine (AcK). G, lysine-acetylated proteins were immunoprecipitated (IP) and then analyzed by Western blotting using an antibody against IDH2 (WB: IDH2). For both panels, as input control (INPUT), 20 μg of whole protein extracts were analyzed by Western blotting using an antibody against IDH2.
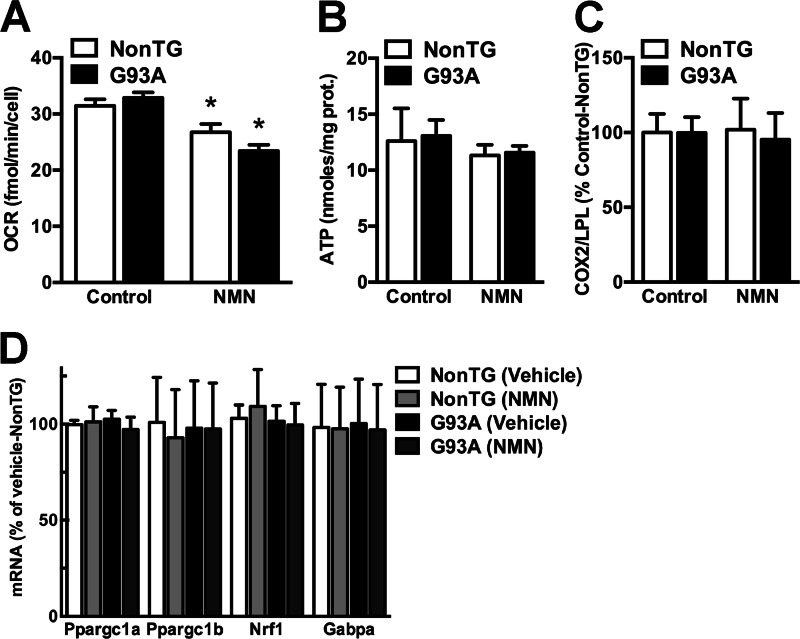
Although an increase in NAD+ levels has been shown to promote oxidative metabolism (27), we found that NMN treatment significantly decreases the basal oxygen consumption rate in non-transgenic and hSOD1G93A astrocytes (Fig. 4A). However, we did not observe changes in ATP content (Fig. 4B); mitochondrial content (Fig. 4C); transcription of the mitogenesis-related genes Ppargc1a, Ppargc1b, Nrf1, or Gabpa (Fig. 4D); or changes in mitochondrial membrane potential (data not shown).
FIGURE 4.
Increasing NAD+ levels decreases basal mitochondrial oxygen consumption rate in astrocytes. A, oxygen consumption rate (OCR) determined for basal conditions in confluent non-transgenic (NonTG) and hSOD1G93A (G93A) spinal cord astrocyte monolayers 24 h after treatment with vehicle (Control) or 5 mm NMN. B, ATP content in confluent non-transgenic and hSOD1G93A spinal cord astrocyte cultures 24 h after treatment with vehicle or 5 mm NMN. C, non-transgenic and hSOD1G93A spinal cord astrocyte cultures were treated with vehicle or 5 mm NMN, and 24 h later the relative mitochondrial to nuclear DNA ratio was estimated by real time PCR using primers specific for cytochrome c oxidase II (COX2) and lipoprotein lipase (LPL). D, relative expression of Ppargc1a, Ppargc1b, Nrf1, and Gabpa mRNA in spinal cord astrocyte cultures 24 h after treatment with 5 mm NMN. mRNA levels were determined by real time PCR and corrected by Actin mRNA levels. For all panels, each data point represents the mean ± S.D. (error bars) of at least three independent experiments. *, significantly different from vehicle treated non-transgenic astrocytes (p < 0.05). prot., protein.
Enhancing NAD+ Salvage Pathway Reverts the Toxicity of Primary Astrocytes Expressing ALS-linked Mutant SOD1
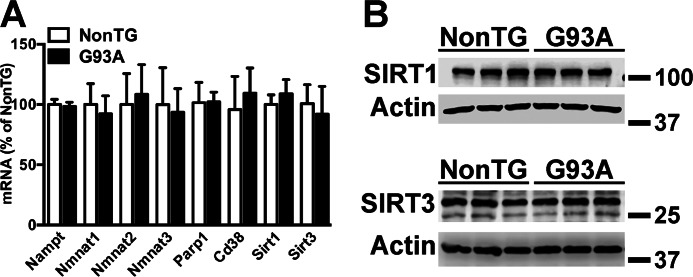
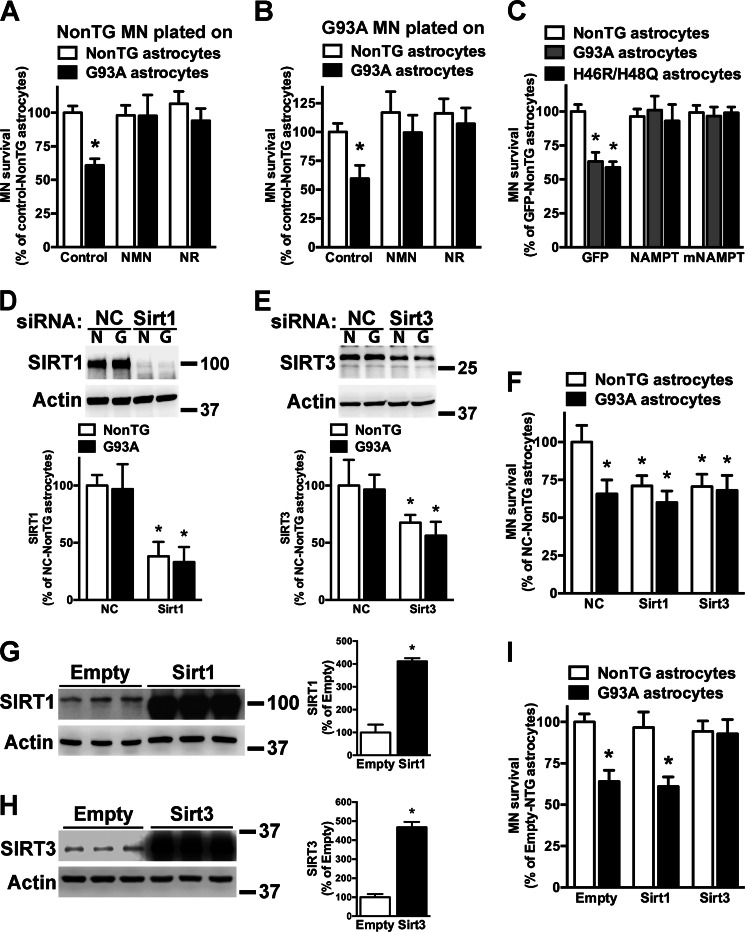
hSOD1G93A astrocytes do not display differences in NAD+ levels when compared with non-transgenic astrocytes (Fig. 1). In addition, we did not find evidence of decreased expression of NAD+ salvage enzymes or increased expression of NAD+-consuming enzymes, including SIRT1 and SIRT3, in hSOD1G93A astrocytes (Fig. 5). In contrast to the trophic support provided by non-transgenic astrocytes, astrocytes isolated from hSOD1G93A mice induce approximately a 40% decrease in the survival of co-cultured motor neurons (39, 40). Pretreatment with NMN or NR completely reverts the neurotoxic phenotype of hSOD1G93A astrocytes toward non-transgenic and hSOD1G93A motor neurons (Fig. 6, A and B). Accordingly, overexpression of NAMPT or mNAMPT in astrocytes also prevents motor neuron death in co-cultures (Fig. 6C). A similar protection was observed in co-cultures using astrocytes overexpressing the experimental hSOD1 mutation H46R/H48Q. Increased NAD+ availability has been associated with increased sirtuin activity (4); thus we wanted to test whether the reversion of the toxic phenotype induced by the increase in NAD+ levels was sirtuin-dependent. Because SIRT1 and SIRT3 have been previously implicated in models of ALS (59–61), we focused on these two enzymes. The ideal experimental approach would consist in decreasing the expression of the sirtuin before treatment with NMN to find whether the protection is lost. However, decreasing SIRT1 or SIRT3 expression in untreated non-transgenic astrocytes reduces per se the survival of co-cultured motor neurons to the levels observed in untreated hSOD1G93A astrocytes (Fig. 6, D, E, and F). Hence, because a mechanism different from the inherent toxicity of the hSOD1G93A astrocytes is also at play under these conditions, this experiment cannot provide a definite answer. Because a lack of a protective effect following an increase in NAD+ levels in the absence of SIRT1 or SIRT3 could not be accurately attributed to a sirtuin-dependent pathway, we did not pursue this approach. To partially circumvent this issue, we overexpressed SIRT1 and SIRT3 in astrocytes before co-culturing with motor neurons (Fig. 6, G and H). Fig. 6I shows that overexpression of SIRT3, but not SIRT1, reverts the toxic phenotype of hSOD1G93A astrocytes.
FIGURE 5.
The expression of NAD+ salvage enzymes and NAD+-consuming enzymes in hSOD1G93A astrocytes is not altered. A, mRNA levels of the indicated genes in confluent non-transgenic (NonTG) and hSOD1G93A (G93A) spinal cord astrocyte cultures. Each data bar represents the mean ± S.D. (error bars) of at least three independent experiments. B, SIRT1 and SIRT3 protein levels in non-transgenic and hSOD1G93A astrocytes. Actin levels were used as a loading control. Each lane represents an independent biological replicate. (SIRT1 quantification: non-transgenic, 100 ± 19; hSOD1G93A, 102 ± 11; SIRT3 quantification: non-transgenic: 100 ± 6; hSOD1G93A, 92 ± 7. No significant changes were found.)
FIGURE 6.
Enhancing NAD+ salvage pathway reverts the toxicity of astrocytes expressing ALS-linked mutant SOD1. A, confluent non-transgenic (NonTG) and hSOD1G93A (G93A) spinal cord astrocyte monolayers were treated with vehicle (Control), 5 mm NMN, or 5 mm NR. 24 h later purified motor neurons from non-transgenic E12.5 mice were plated on top of the astrocyte monolayer. B, the same experimental setup as in A was used, and purified motor neurons from hSOD1G93A E12.5 mice were plated on top of the astrocyte monolayer. For A and B, motor neuron survival was assessed 72 h later. *, significantly different from non-transgenic control (p < 0.05). C, confluent spinal cord astrocyte monolayers obtained from non-transgenic, hSOD1G93A, and hSOD1H46R/H48Q (H46R/H48Q) mice were transfected with adenovirus expressing GFP, NAMPT, or mNAMPT. 24 h later purified motor neurons from non-transgenic E12.5 mice were plated on top of the astrocyte monolayer. Motor neuron survival was assessed 72 h later. *, significantly different from non-transgenic GFP (p < 0.05). D and E, confluent non-transgenic (N) and hSOD1G93A (G) astrocytes were transfected with a negative control (NC), Sirt1, or Sirt3 siRNA, and 48 h later protein levels were determined by Western blotting. Actin levels were used as a loading control. A representative image is presented, and the bottom panels show the quantification of at least three experiments for each siRNA. *, significantly different from non-transgenic negative control (p < 0.05). F, confluent non-transgenic and hSOD1G93A spinal cord astrocytes monolayers were treated as in D and E, and 24 h later purified motor neurons from non-transgenic E12.5 mice were plated on top of the astrocyte monolayer. Motor neuron survival was assessed 72 h later. *, significantly different from non-transgenic negative control (p < 0.05). G and H, confluent hSOD1G93A astrocytes were transfected with plasmids coding for Sirt1, Sirt3, or an empty control plasmid, and 48 h later protein levels were determined by Western blotting. Actin levels were used as a loading control. The right panels show the quantification. To detect endogenous sirtuin expression (empty vector samples), the levels on the overexpressing samples had to be overexposed. Thus, the -fold change is likely underestimated. *, significantly different from empty control (p < 0.05). I, confluent non-transgenic and hSOD1G93A spinal cord astrocyte monolayers were transfected with plasmids coding for Sirt1, Sirt3, or an empty control plasmid, and 24 h later purified motor neurons from non-transgenic E12.5 mice were plated on top of the astrocyte monolayer. *, significantly different from non-transgenic empty (p < 0.05). For all panels, each data bar represents the mean ± S.D. (error bars) of at least three independent experiments.
Discussion
Although the mechanism responsible for the toxicity of ALS astrocytes toward motor neurons remains under investigation, we have shown before that decreasing oxidative stress in astrocytes prevents astrocyte-mediated toxicity (45, 55). Here we show that increasing NAD+ levels leads to decreased mitochondrial ROS production and reverts this neurotoxic phenotype. The protective effect was linked to an increase in NAD+ availability above normal levels. hSOD1G93A astrocytes do not display decreased NAD+ levels or altered expression of enzymes involved in NAD+ degradation or the NAD+ salvage pathway. Overexpression of a mitochondrially targeted NAMPT reverts the toxicity toward co-cultured motor neurons to the same extent as NAMPT overexpression. These data suggest that increasing the recycling of NAM to NAD+ in the mitochondria of ALS astrocytes is sufficient to revert the toxic phenotype and point to the critical role of the mitochondrial NAD+ pool.
Increasing cellular NAD+ availability can influence mitochondrial redox reactions as well as the activity of NAD+-consuming enzymes. Compartmentalization of NAD+ biosynthesis and utilization remains to be fully characterized, but it is known that the mitochondrial NAD+ pool can be relatively uncoupled from the cytosolic pool (26). Because NMN is the product of the rate-limiting enzyme in NAD+ salvage and can generate NAD+ following a single enzymatic reaction, NMN seems the most obvious choice for NAD+ precursor supplementation. However, an NMN transporter has not been identified in eukaryotic cells, and it appears that the nucleotide is degraded to the nucleoside (NR) to enter the cell (24). Once in the cytosol, NR can be phosphorylated by NR kinases to produce NMN, which is taken up by the mitochondria to serve as substrate for NAD+ synthesis. Regardless of the exact mechanism of transport and uptake, we show here that mitochondrial NAD+ levels parallel total NAD+ increases following NMN and NR treatments in astrocytes. In astrocytes, we observed that mitochondrial NAD+ accounts for ∼70% of total NAD+ in control conditions or following treatments with NMN and NR. Although there was an increase in total NADH, we did not detect an increase in mitochondrial NADH. This observation could be explained, at least in part, by the intrinsic difficulty of accurately quantifying NADH following the mitochondrial isolation process. However, it could also reflect that NADH levels are regulated by other mechanisms in addition to NAD+ availability (62).
NAD+-consuming enzymes generate NAM as a by-product, and continuous resynthesis of NAD+ by NAMPT is essential for mammalian cells (16). Because all NAD+-consuming reactions have been described in the mitochondria, we investigated the effect of increasing NAM recycling in the mitochondria by targeting NAMPT to the organelle. Our results show that a mitochondrially targeted NAMPT effectively increases total and mitochondrial NAD+ in astrocytes. This observation has several important implications. First, mitochondrial NAD+ degradation accounts for a significant portion of the NAD+ turnover in the cell. Thus, it is possible to modulate NAD+ levels by enhancing NAD+ salvage in a compartment-specific manner. Second, overexpression of NAMPT causes a larger increase in total NAD+ than overexpression of the mitochondrially targeted NAMPT, which is likely due to the fact that the latter form of the enzyme will not have access to the NAM generated by NAD+-consuming enzymes in the nucleus and the cytoplasm. However, both forms of the enzyme increase mitochondrial NAD+ levels in a similar fraction, suggesting that cytoplasmic NAMPT activity limits mitochondrial NAD+ levels in astrocytes. Finally, the data provide additional evidence for the existence of a mitochondrial enzymatic activity capable of using NMN to synthesize NAD+. Although NMNAT3 was originally identified as the mitochondrial adenylyltransferase responsible for this activity, this notion has been recently challenged (30). However, isolated mitochondria can synthesize NAD+ from NMN (29), and although the NMN generated by the mitochondrially targeted NAMPT could be exported to the cytoplasm to be converted to NAD+ and then imported back to the mitochondria, this appears to be a less likely explanation. To the best of our knowledge, this is the first work to report the effect of a mitochondrially targeted NAMPT in the NAD+ salvage pathway.
In cell cultures, hydrogen peroxide toxicity is in part due to NAD+ depletion caused by excessive PARP1 activation that could lead to glycolytic inhibition and mitochondrial failure (63, 64). Thus, higher NAD+ levels result in protection against peroxide treatment in astrocytes, and in particular, increasing specifically the mitochondrial NAD+ salvage pathway seems to confer significant protection. NMN treatment and overexpression of both forms of NAMPT decrease basal and peroxide-induced ROS production in the mitochondria. In general, an increase in the activity of NAD+-dependent sirtuins enhances metabolic efficiency and oxidative stress resistance (4) and could be partially responsible for the protective effect and decrease in mitochondrial ROS levels observed in astrocyte cultures. For example, an increase in NAD+ levels could promote SIRT1-mediated mitochondrial biogenesis (65), whereas an increase in mitochondrial NAD+ levels could promote SIRT3-mediated deacetylation and activation of superoxide dismutase 2 and isocitrate dehydrogenase 2 (58, 66). However, we did not observe an increase in basal oxidative metabolism or mitochondrial biogenesis that could indicate a SIRT1-mediated response. We did observe a decrease in IDH2 acetylation that can be partially responsible for the increased antioxidant defenses. The key role of IDH2 activity regulating mitochondrial antioxidant defenses has been clearly linked to the protection conferred by SIRT3 activation (58, 67). However, at the level of total protein acetylation, we did not find a consistent correlation between NAD+ levels and changes in protein acetylation (data not shown), which could indicate that only a small number of targets are changing acetylation status. In agreement with our results, a recent study has found that redistributing cellular NAD+ from the cytosol to the mitochondria decreases oxidative phosphorylation without significantly affecting global protein acetylation patterns (68). Alternatively, the observed decrease in mitochondrial ROS production could also be explained by a direct effect of shifting the NAD+/NADH ratio. Superoxide production by complex I is favored when the concentration of NADH is significantly higher than the levels of NAD+, whereas superoxide production decreases as the relative NAD+ concentration increases (69, 70). In astrocytes, treatment with NAD+ precursors leads to a much larger increase in mitochondrial NAD+ levels than NADH and to a decrease in mitochondrial oxygen consumption, suggesting that this mechanism could be responsible for the decrease in ROS production.
In our co-culture model, only 40% of the motor neurons seem to be susceptible to the underlying toxic mechanism (39, 40), and decreasing SIRT1 or SIRT3 expression in hSOD1G93A does not cause further reduction in motor neuron survival. However, decreasing SIRT1 or SIRT3 expression in non-transgenic astrocytes decreases motor neuron survival to the levels observed in hSOD1G93A co-cultures. This prevented us from directly testing whether a sirtuin-dependent mechanism is responsible for the observed protection conferred by an increase in NAD+ availability. SIRT1 activation has been shown to be protective in models of ALS (59, 60). However, overexpression of SIRT1 does not revert the toxicity of hSOD1G93A astrocytes and could suggest that SIRT1 protection is a cell-autonomous mechanism mediated by SIRT1 activation directly in the neurons. On the contrary, SIRT3 overexpression is able to revert the neurotoxic phenotype of hSOD1G93A. However, the protection conferred by overexpression of SIRT3 does not directly address whether a sirtuin-dependent mechanism is responsible for the protection conferred by an increase in NAD+ availability. Moreover, the increase in NAD+ availability caused by the different treatments could lead to the activation of other members of the sirtuin family with potential beneficial effects. Nevertheless, the decrease in IDH2 acetylation following NMN treatment, the reversion of the toxic phenotype by only increasing mitochondrial NAD+ salvage (mNAMPT overexpression), and the protective effect of the mitochondrial SIRT3 overexpression point out to a critical role of the mitochondrial NAD+ pool in the protection observed. Regardless, our findings demonstrate that it is possible to modify astrocyte-motor neuron interaction by enhancing NAD+ salvage pathway in the astrocytes. Interestingly, aminopropyl carbazole derivatives previously shown to preserve motor function in hSOD1G93A mice have been recently identified as NAMPT activators (71, 72). In summary, because we have shown that therapeutic targets identified in this co-culture system may have a beneficial effect when translated into animal models of ALS (45), our data suggest that further investigation regarding the therapeutic potential of increasing NAD+ availability to prevent astrocyte-mediated motor neuron death in ALS is deserved.
Author Contributions
B. A. H., M. P, D. R. S., G. B., and M. R. V. performed experiments. B. A. H., M. P, G. B., C. C. B., and M. R. V. analyzed data. B. A. H., M. P., and M. R. V. wrote the paper. All authors reviewed the content of the manuscript.
Acknowledgments
We thank Dr. Mathias Ziegler for the mitoPARP and mitoEGFP plasmids. We also thank the depositing laboratories of the SIRT1 and SIRT3 plasmids. This work utilized a facility constructed with support from National Institutes of Health Grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources to the Medical University of South Carolina.
This study was supported by National Institutes of Health Grants NS089640 and GM103542. The authors declare that they have no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- PARP
- poly(ADP-ribose) polymerase
- ALS
- amyotrophic lateral sclerosis
- NAM
- nicotinamide
- NAMPT
- nicotinamide phosphoribosyltransferase
- NMN
- nicotinamide mononucleotide
- NMNAT
- nicotinamide mononucleotide adenylyltransferase
- NR
- nicotinamide riboside
- SOD1
- superoxide dismutase 1
- SIRT
- sirtuin
- hSOD1
- human superoxide dismutase 1
- SALS
- sporadic ALS
- FALS
- familial ALS
- EGFP
- enhanced GFP
- PAR
- poly(ADP-ribose)
- mNAMPT
- mitochondrially targeted NAMPT
- ROS
- reactive oxygen species
- IDH2
- isocitrate dehydrogenase 2
- mitoEGFP
- mitochondrially targeted EGFP
- mitoPARP
- mitochondrially targeted PARP1 catalytic domain.
References
- 1. Berger F., Ramírez-Hernández M. H., and Ziegler M. (2004) The new life of a centenarian: signalling functions of NAD(P). Trends Biochem. Sci. 29, 111–118 [DOI] [PubMed] [Google Scholar]
- 2. Cantó C., Menzies K. J., and Auwerx J. (2015) NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 22, 31–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bai P. (2015) Biology of poly(ADP-ribose) polymerases: the factotums of cell maintenance. Mol. Cell 58, 947–958 [DOI] [PubMed] [Google Scholar]
- 4. Imai S., and Guarente L. (2014) NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24, 464–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aksoy P., White T. A., Thompson M., and Chini E. N. (2006) Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 345, 1386–1392 [DOI] [PubMed] [Google Scholar]
- 6. Bai P., and Cantó C. (2012) The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 16, 290–295 [DOI] [PubMed] [Google Scholar]
- 7. Cantó C., Sauve A. A., and Bai P. (2013) Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol. Aspects Med. 34, 1168–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malavasi F., Deaglio S., Funaro A., Ferrero E., Horenstein A. L., Ortolan E., Vaisitti T., and Aydin S. (2008) Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 88, 841–886 [DOI] [PubMed] [Google Scholar]
- 9. Mayo L., Jacob-Hirsch J., Amariglio N., Rechavi G., Moutin M. J., Lund F. E., and Stein R. (2008) Dual role of CD38 in microglial activation and activation-induced cell death. J. Immunol. 181, 92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Partidá-Sánchez S., Rivero-Nava L., Shi G., and Lund F. E. (2007) CD38: an ecto-enzyme at the crossroads of innate and adaptive immune responses. Adv. Exp. Med. Biol. 590, 171–183 [DOI] [PubMed] [Google Scholar]
- 11. Feldman J. L., Dittenhafer-Reed K. E., and Denu J. M. (2012) Sirtuin catalysis and regulation. J. Biol. Chem. 287, 42419–42427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomes A. P., Price N. L., Ling A. J., Moslehi J. J., Montgomery M. K., Rajman L., White J. P., Teodoro J. S., Wrann C. D., Hubbard B. P., Mercken E. M., Palmeira C. M., de Cabo R., Rolo A. P., Turner N., Bell E. L., and Sinclair D. A. (2013) Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 155, 1624–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dittenhafer-Reed K. E., Richards A. L., Fan J., Smallegan M. J., Fotuhi Siahpirani A., Kemmerer Z. A., Prolla T. A., Roy S., Coon J. J., and Denu J. M. (2015) SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 21, 637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Price N. L., Gomes A. P., Ling A. J., Duarte F. V., Martin-Montalvo A., North B. J., Agarwal B., Ye L., Ramadori G., Teodoro J. S., Hubbard B. P., Varela A. T., Davis J. G., Varamini B., Hafner A., Moaddel R., Rolo A. P., Coppari R., Palmeira C. M., de Cabo R., Baur J. A., and Sinclair D. A. (2012) SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 15, 675–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bause A. S., and Haigis M. C. (2013) SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 48, 634–639 [DOI] [PubMed] [Google Scholar]
- 16. Houtkooper R. H., Cantó C., Wanders R. J., and Auwerx J. (2010) The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31, 194–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Belenky P., Bogan K. L., and Brenner C. (2007) NAD+ metabolism in health and disease. Trends Biochem. Sci. 32, 12–19 [DOI] [PubMed] [Google Scholar]
- 18. Bieganowski P., and Brenner C. (2004) Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell 117, 495–502 [DOI] [PubMed] [Google Scholar]
- 19. Ruddick J. P., Evans A. K., Nutt D. J., Lightman S. L., Rook G. A., and Lowry C. A. (2006) Tryptophan metabolism in the central nervous system: medical implications. Expert Rev. Mol. Med. 8, 1–27 [DOI] [PubMed] [Google Scholar]
- 20. Garten A., Petzold S., Körner A., Imai S., and Kiess W. (2009) Nampt: linking NAD biology, metabolism and cancer. Trends Endocrinol. Metab. 20, 130–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Stefano M., and Conforti L. (2013) Diversification of NAD biological role: the importance of location. FEBS J. 280, 4711–4728 [DOI] [PubMed] [Google Scholar]
- 22. Revollo J. R., Grimm A. A., and Imai S. (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 [DOI] [PubMed] [Google Scholar]
- 23. Yang H., Yang T., Baur J. A., Perez E., Matsui T., Carmona J. J., Lamming D. W., Souza-Pinto N. C., Bohr V. A., Rosenzweig A., de Cabo R., Sauve A. A., and Sinclair D. A. (2007) Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell 130, 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nikiforov A., Dölle C., Niere M., and Ziegler M. (2011) Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J. Biol. Chem. 286, 21767–21778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakagawa T., Lomb D. J., Haigis M. C., and Guarente L. (2009) SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell 137, 560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pittelli M., Formentini L., Faraco G., Lapucci A., Rapizzi E., Cialdai F., Romano G., Moneti G., Moroni F., and Chiarugi A. (2010) Inhibition of nicotinamide phosphoribosyltransferase: cellular bioenergetics reveals a mitochondrial insensitive NAD pool. J. Biol. Chem. 285, 34106–34114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cantó C., Houtkooper R. H., Pirinen E., Youn D. Y., Oosterveer M. H., Cen Y., Fernandez-Marcos P. J., Yamamoto H., Andreux P. A., Cettour-Rose P., Gademann K., Rinsch C., Schoonjans K., Sauve A. A., and Auwerx J. (2012) The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stein L. R., and Imai S. (2012) The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol. Metab. 23, 420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barile M., Passarella S., Danese G., and Quagliariello E. (1996) Rat liver mitochondria can synthesize nicotinamide adenine dinucleotide from nicotinamide mononucleotide and ATP via a putative matrix nicotinamide mononucleotide adenylyltransferase. Biochem. Mol. Biol. Int. 38, 297–306 [PubMed] [Google Scholar]
- 30. Felici R., Lapucci A., Ramazzotti M., and Chiarugi A. (2013) Insight into molecular and functional properties of NMNAT3 reveals new hints of NAD homeostasis within human mitochondria. PLoS One 8, e76938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Renton A. E., Chiò A., and Traynor B. J. (2014) State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 17, 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X., Rahmani Z., Krizus A., McKenna-Yasek D., Cayabyab A., Gaston S. M., et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 [DOI] [PubMed] [Google Scholar]
- 33. Al-Chalabi A., Jones A., Troakes C., King A., Al-Sarraj S., and van den Berg L. H. (2012) The genetics and neuropathology of amyotrophic lateral sclerosis. Acta Neuropathol. 124, 339–352 [DOI] [PubMed] [Google Scholar]
- 34. Ravits J., Appel S., Baloh R. H., Barohn R., Brooks B. R., Elman L., Floeter M. K., Henderson C., Lomen-Hoerth C., Macklis J. D., McCluskey L., Mitsumoto H., Przedborski S., Rothstein J., Trojanowski J. Q., van den Berg L. H., and Ringel S. (2013) Deciphering amyotrophic lateral sclerosis: what phenotype, neuropathology and genetics are telling us about pathogenesis. Amyotroph. Lateral Scler. Frontotemporal Degener. 14, Suppl. 1, 5–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gurney M. E., Pu H., Chiu A. Y., Dal Canto M. C., Polchow C. Y., Alexander D. D., Caliendo J., Hentati A., Kwon Y. W., Deng H. X., Deng H. X., Chen W., Zhai P., Sufit R. L., and Siddique T. (1994) Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 [DOI] [PubMed] [Google Scholar]
- 36. Boillée S., Yamanaka K., Lobsiger C. S., Copeland N. G., Jenkins N. A., Kassiotis G., Kollias G., and Cleveland D. W. (2006) Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312, 1389–1392 [DOI] [PubMed] [Google Scholar]
- 37. Clement A. M., Nguyen M. D., Roberts E. A., Garcia M. L., Boillée S., Rule M., McMahon A. P., Doucette W., Siwek D., Ferrante R. J., Brown R. H. Jr., Julien J. P., Goldstein L. S., and Cleveland D. W. (2003) Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302, 113–117 [DOI] [PubMed] [Google Scholar]
- 38. Yamanaka K., Chun S. J., Boillee S., Fujimori-Tonou N., Yamashita H., Gutmann D. H., Takahashi R., Misawa H., and Cleveland D. W. (2008) Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 11, 251–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vargas M. R., Pehar M., Cassina P., Beckman J. S., and Barbeito L. (2006) Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J. Neurochem. 97, 687–696 [DOI] [PubMed] [Google Scholar]
- 40. Nagai M., Re D. B., Nagata T., Chalazonitis A., Jessell T. M., Wichterle H., and Przedborski S. (2007) Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 10, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Giorgio F. P., Carrasco M. A., Siao M. C., Maniatis T., and Eggan K. (2007) Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat. Neurosci. 10, 608–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haidet-Phillips A. M., Hester M. E., Miranda C. J., Meyer K., Braun L., Frakes A., Song S., Likhite S., Murtha M. J., Foust K. D., Rao M., Eagle A., Kammesheidt A., Christensen A., Mendell J. R., Burghes A. H., and Kaspar B. K. (2011) Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat. Biotechnol. 29, 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meyer K., Ferraiuolo L., Miranda C. J., Likhite S., McElroy S., Renusch S., Ditsworth D., Lagier-Tourenne C., Smith R. A., Ravits J., Burghes A. H., Shaw P. J., Cleveland D. W., Kolb S. J., and Kaspar B. K. (2014) Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc. Natl. Acad. Sci. U.S.A. 111, 829–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang J., Xu G., Gonzales V., Coonfield M., Fromholt D., Copeland N. G., Jenkins N. A., and Borchelt D. R. (2002) Fibrillar inclusions and motor neuron degeneration in transgenic mice expressing superoxide dismutase 1 with a disrupted copper-binding site. Neurobiol. Dis. 10, 128–138 [DOI] [PubMed] [Google Scholar]
- 45. Vargas M. R., Johnson D. A., Sirkis D. W., Messing A., and Johnson J. A. (2008) Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J. Neurosci. 28, 13574–13581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lowry O. H., Passonneau J. V., and Rock M. K. (1961) The stability of pyridine nucleotides. J. Biol. Chem. 236, 2756–2759 [PubMed] [Google Scholar]
- 47. Li W., and Sauve A. A. (2015) NAD+ content and its role in mitochondria. Methods Mol. Biol. 1241, 39–48 [DOI] [PubMed] [Google Scholar]
- 48. Rizzuto R., Simpson A. W., Brini M., and Pozzan T. (1992) Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358, 325–327 [DOI] [PubMed] [Google Scholar]
- 49. Niere M., Kernstock S., Koch-Nolte F., and Ziegler M. (2008) Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix. Mol. Cell. Biol. 28, 814–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dölle C., Niere M., Lohndal E., and Ziegler M. (2010) Visualization of subcellular NAD pools and intra-organellar protein localization by poly-ADP-ribose formation. Cell. Mol. Life Sci. 67, 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. North B. J., Marshall B. L., Borra M. T., Denu J. M., and Verdin E. (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol. Cell 11, 437–444 [DOI] [PubMed] [Google Scholar]
- 52. Bao J., Lu Z., Joseph J. J., Carabenciov D., Dimond C. C., Pang L., Samsel L., McCoy J. P. Jr., Leclerc J., Nguyen P., Gius D., and Sack M. N. (2010) Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. J. Cell. Biochem. 110, 238–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vargas M. R., Pehar M., Cassina P., Martínez-Palma L., Thompson J. A., Beckman J. S., and Barbeito L. (2005) Fibroblast growth factor-1 induces heme oxygenase-1 via nuclear factor erythroid 2-related factor 2 (Nrf2) in spinal cord astrocytes: consequences for motor neuron survival. J. Biol. Chem. 280, 25571–25579 [DOI] [PubMed] [Google Scholar]
- 54. Pehar M., Ball L. E., Sharma D. R., Harlan B. A., Comte-Walters S., Neely B. A., and Vargas M. R. (2016) Changes in protein expression and lysine acetylation induced by decreased glutathione levels in astrocytes. Mol. Cell. Proteomics 15, 493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pehar M., Beeson G., Beeson C. C., Johnson J. A., and Vargas M. R. (2014) Mitochondria-targeted catalase reverts the neurotoxicity of hSOD1G93A astrocytes without extending the survival of ALS-linked mutant hSOD1 mice. PLoS One 9, e103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Medeiros D. M. (2008) Assessing mitochondria biogenesis. Methods 46, 288–294 [DOI] [PubMed] [Google Scholar]
- 57. Schlicker C., Gertz M., Papatheodorou P., Kachholz B., Becker C. F., and Steegborn C. (2008) Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J. Mol. Biol. 382, 790–801 [DOI] [PubMed] [Google Scholar]
- 58. Yu W., Dittenhafer-Reed K. E., and Denu J. M. (2012) SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J. Biol. Chem. 287, 14078–14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kim D., Nguyen M. D., Dobbin M. M., Fischer A., Sananbenesi F., Rodgers J. T., Delalle I., Baur J. A., Sui G., Armour S. M., Puigserver P., Sinclair D. A., and Tsai L. H. (2007) SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 26, 3169–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Watanabe S., Ageta-Ishihara N., Nagatsu S., Takao K., Komine O., Endo F., Miyakawa T., Misawa H., Takahashi R., Kinoshita M., and Yamanaka K. (2014) SIRT1 overexpression ameliorates a mouse model of SOD1-linked amyotrophic lateral sclerosis via HSF1/HSP70i chaperone system. Mol. Brain 7, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Song W., Song Y., Kincaid B., Bossy B., and Bossy-Wetzel E. (2013) Mutant SOD1G93A triggers mitochondrial fragmentation in spinal cord motor neurons: neuroprotection by SIRT3 and PGC-1alpha. Neurobiol. Dis. 51, 72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wilhelm F., and Hirrlinger J. (2011) The NAD+/NADH redox state in astrocytes: independent control of the NAD+ and NADH content. J. Neurosci. Res. 89, 1956–1964 [DOI] [PubMed] [Google Scholar]
- 63. Ying W., Sevigny M. B., Chen Y., and Swanson R. A. (2001) Poly(ADP-ribose) glycohydrolase mediates oxidative and excitotoxic neuronal death. Proc. Natl. Acad. Sci. U.S.A. 98, 12227–12232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tang K. S., Suh S. W., Alano C. C., Shao Z., Hunt W. T., Swanson R. A., and Anderson C. M. (2010) Astrocytic poly(ADP-ribose) polymerase-1 activation leads to bioenergetic depletion and inhibition of glutamate uptake capacity. Glia 58, 446–457 [DOI] [PubMed] [Google Scholar]
- 65. Bai P., Cantó C., Oudart H., Brunyánszki A., Cen Y., Thomas C., Yamamoto H., Huber A., Kiss B., Houtkooper R. H., Schoonjans K., Schreiber V., Sauve A. A., Menissier-de Murcia J., and Auwerx J. (2011) PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qiu X., Brown K., Hirschey M. D., Verdin E., and Chen D. (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12, 662–667 [DOI] [PubMed] [Google Scholar]
- 67. Someya S., Yu W., Hallows W. C., Xu J., Vann J. M., Leeuwenburgh C., Tanokura M., Denu J. M., and Prolla T. A. (2010) Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. VanLinden M. R., Dölle C., Pettersen I. K., Kulikova V. A., Niere M., Agrimi G., Dyrstad S. E., Palmieri F., Nikiforov A. A., Tronstad K. J., and Ziegler M. (2015) Subcellular distribution of NAD+ between cytosol and mitochondria determines the metabolic profile of human cells. J. Biol. Chem. 290, 27644–27659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kussmaul L., and Hirst J. (2006) The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. U.S.A. 103, 7607–7612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pryde K. R., and Hirst J. (2011) Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J. Biol. Chem. 286, 18056–18065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang G., Han T., Nijhawan D., Theodoropoulos P., Naidoo J., Yadavalli S., Mirzaei H., Pieper A. A., Ready J. M., and McKnight S. L. (2014) P7C3 neuroprotective chemicals function by activating the rate-limiting enzyme in NAD salvage. Cell 158, 1324–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tesla R., Wolf H. P., Xu P., Drawbridge J., Estill S. J., Huntington P., McDaniel L., Knobbe W., Burket A., Tran S., Starwalt R., Morlock L., Naidoo J., Williams N. S., Ready J. M., McKnight S. L., and Pieper A. A. (2012) Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U.S.A. 109, 17016–17021 [DOI] [PMC free article] [PubMed] [Google Scholar]