Abstract
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite generated by phosphorylation of sphingosine catalyzed by sphingosine kinase. S1P acts mainly through its high affinity G-protein-coupled receptors and participates in the regulation of multiple systems, including cardiovascular system. It has been shown that S1P signaling is involved in the regulation of cardiac chronotropy and inotropy and contributes to cardioprotection as well as cardiac remodeling; S1P signaling regulates vascular function, such as vascular tone and endothelial barrier, and possesses an anti-atherosclerotic effect; S1P signaling is also implicated in the regulation of blood pressure. Therefore, manipulation of S1P signaling may offer novel therapeutic approaches to cardiovascular diseases. As several S1P receptor modulators and sphingosine kinase inhibitors have been approved or under clinical trials for the treatment of other diseases, it may expedite the test and implementation of these S1P-based drugs in cardiovascular diseases.
Keywords: Review, Sphingosine Kinase, Blood Pressure, Cardiac Remodeling, Atherosclerosis, Sodium Excretion, Vascular Tone, Endothelial Permeability
2. INTRODUCTION
Sphingosin-1-phosphate (S1P) was originally considered merely an intermediate product during the degradation of sphingosine and now has been recognized as a very important signaling molecule since the discovery that S1P has bioactive activity (1-3). S1P signaling has been shown to be critically involved in diverse cellular behaviors such as cell adhesion, migration, proliferation and differentiation, participate in the development and homeostasis of many organs and tissues, regulate the functions of multiple systems such as immune system, central nervous system and cardiovascular system, and contribute to the pathogenesis of a broad range of diseases including atherosclerosis, respiratory distress, diabetes, cancer and inflammatory disorders (4). S1P signaling pathway is emerging as a novel drug target for the treatment of different diseases. A S1P agonist, fingolimod (FTY720), has been approved to treat multiple sclerosis as it produces potent anti-inflammatory effects. Interestingly, FTY720 shows cardiovascular side effects, further supporting the involvement of S1P signaling in the regulation of cardiovascular system. This review will summarize the role of S1P in the physiological and pathological processes in cardiovascular system, focusing on the most recent advances.
2.1. Biosynthesis of S1P
S1P is formed by phosphorylation of sphingosine catalyzed by sphingosine kinase (SPHK). Once formed, S1P can be either dephosphorylated back to sphingosine by two specific S1P phosphatases (SPP1 and SPP2) or irreversibly degraded to hexadecenal and phosphoethanolamine by S1P lyase (Figure 1) (5, 6). The immediate substrate for S1P generation, sphingosine, is formed from the hydrolysis of ceramide by ceramidase. Ceramide is the central building block of all sphingolipids and its de novo synthesis starts with the condensation of amino acid L-serine and palmitoyl-CoA in the endoplasmic reticulum (ER) through a series of reducing, acylating, and oxidizing reactions (7). Ceramide may also be converted by sphingomyelin synthase into sphingomyelin, which serves as an inert storage pool for ceramide. Although increased de novo synthesis may be a potential source for sphingolipid signaling, rapid generation of ceramide from sphingomyelin by sphingomyelinases has been proposed as the major mechanism for ceramide-mediated signaling (8).
Figure 1.
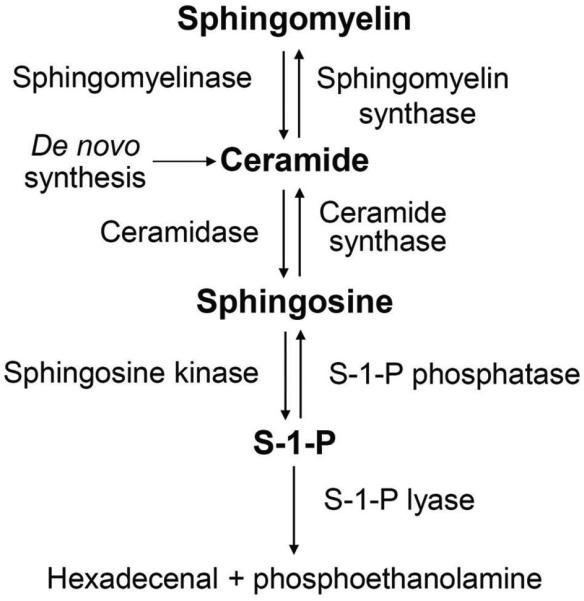
Pathway for the biosynthesis of S1P.
It should be noted that the levels of the various sphingolipids exhibit great differences. Concentrations of ceramide, sphingosine and S1P differ approximately by an order of magnitude, with ceramide presenting the highest and S1P the lowest level. A small change in ceramide can therefore drastically influences the levels of sphingosine and S1P (9). Therefore, the S1P generation is regulated not only by S1P biosynthetic and degradative enzymes but also by the availability of the substrate for SPHK (10).
2.2. S1P receptors
There are five high-affinity G-protein-coupled receptors for S1P, named S1P1–5 (11, 12). Although there are proposed intracellular roles for S1P, most of the effects of S1P are mediated by the activation of its receptors on cell membrane via an autocrine or paracrine manner (6, 11, 13, 14). Cellular and temporal expression of the S1P receptors (S1PRs) determines their specific roles in various organ systems. S1P1-3 are ubiquitously expressed, whereas the expression of S1P4 and S1P5 are highly restricted to distinct cell types (12). S1P4 has been reported to be primarily expressed in lymphoid tissues and that S1P5 shows restricted tissue distribution to brain and spleen. Binding of S1P to each of these receptors provokes distinctive signaling pathways and cellular responses that are, in some cases, antagonistic. Differential signaling of S1P mediated by different S1P receptors is due to distinct, though sometimes overlapping, coupling to diverse G proteins (Table 1). S1P1 couples exclusively to Gi/o; S1P2 can couple to Gi/o, Gs, Gq and G12/13, it couples most efficiently to G12/13; S1P3 can couple with Gq, Gi/o and G12/13, and most efficiently to the Gq protein; S1P4 and S1P5 both couple to Gs, Gq and G12/13. The expression patterns of S1P receptors along with their coupled G proteins dictate the activation of different intracellular signaling pathways upon the activation of these receptors, which result in remarkably diverse cellular responses (11, 12, 15).
Table 1.
S1P receptors and their coupled G proteins
| Receptor | Other name1 | Coupled G protein |
|---|---|---|
| S1P1 | EDG1 | Gi/o |
| S1P2 | EDG5 | G12/13, Gi/o, Gs, Gq |
| S1P3 | EDG3 | Gq, Gi/o, G12/13 |
| S1P4 | EDG6 | Gs, Gq, G12/13 |
| S1P5 | EDG8 | Gs, Gq, G12/13 |
: EDG, endothelial differentiation gene
2.3. S1P as an extracellular and intracellular signaling molecule
The effects of S1P are mostly mediated by its cell surface receptors. However, there is evidence, though limited, showing that S1P may also act as an intracellular messenger. Production of S1P is catalyzed by sphingosine kinase (SPHK). Two distinct SPHK isoforms, SPHK1 and SPHK2, have been identified (16, 17). It has been shown that agonist-induced activation of SPHK1 leads to S1P receptor-mediated signaling (18). Although a cytosolic protein, SPHK1 has been shown to undergo translocation to the plasma membrane following phosphorylation by ERK, which is thought to position SPHK1 into close proximity with its sphingosine substrate. When formed at the plasma membrane, S1P can then be easily exported from the cell and act in an autocrine or paracrine fashion (15). In this regard, several ATP-binding cassette transporters and Spinster 2 transporter have been implicated in S1P release (reviewed in REFERENCES (5, 19-21).
In contrast to SPHK1, SPHK2 is present in several intracellular compartments such as ER, nucleus and mitochondria. Although it remains unknown the direct mechanisms by which SPHK2 is regulated, S1P produced by SPHK2 acts on intracellular targets (18, 22). S1P produced by SPHK2 located in the ER is associated with inhibition of cell growth and pro-apoptosis, which is S1P receptor-independent (22). This is opposite to that of SPHK1, which promotes cell growth and inhibits apoptosis via S1P receptors. SPHK2 in the nucleus regulates gene transcription, at least in part by producing S1P, which acts as an endogenous inhibitor of histone deacetylases (18, 22).
3. S1P SIGNALING IN HEART
S1P1-3 receptors are all present in cardiomyocytes with S1P1 as the predominant subtype of S1P receptor and that the mRNA of S1P2 and S1P3 receptors present at much lower levels (16, 17). In addition, SPHK1 and SPHK2 are both expressed in heart (16, 17). S1P signaling has been shown to participate in the regulation of cardiac chronotropy and inotropy, and contribute to cardioprotection as well as cardiac remodeling.
3.1. S1P in cardiac physiology
For more than a decade, S1P has been shown to regulate cardiac electrophysiological and contractile activity (23, 24). In contrast to the observation in canine isolated heart preparation, in which S1P evokes a sinus tachycardia (25), in vivo administration of S1P and its agonists decreases heart rate and reduces ventricular contraction in rodent and human (23, 24, 26, 27). In isolated rabbit sinoatrial node cells S1P directly reduces the pacemaker activity (28). Similarly, in dissected rat atrioventricular nodes S1P agonist FTY720 is shown to inhibit atrioventricular node conduction (29). The chronotropic effect of S1P is mediated by S1P3 receptor and the inotropic effect by S1P1 receptor, as the bradycardia induced by S1P agonist is abolished in S1P3−/− mice and that selective activation of S1P3 reduces heart rate, whereas S1P1-specific agonist inhibits isoproterenol-induced positive inotropy (24, 26). The mechanism by which S1P3 mediates bradycardic effect is due to the activation of cardiac G protein-gated potassium channel IKACh (30, 31). The S1P1-mediated negative inotropic response is associated with inhibition of cAMP in cardiomyocytes (26), which reduces cAMP-mediated increases in L-type calcium channel current. In addition, Gi-mediated activation of IKACh may partially contribute to the negative inotropy following S1P activation of S1P1 (32), since the resulting outward K+ current increases the rate of repolarization and shortens the action potential duration, causing a concurrent decrease in Ca2+ current (33).
It should be noted that S1P receptor-mediated effects on heart rate exhibit species-specific differences. Although S1P3, not S1P1, mediates a bradycardic effect in rodent (24, 26, 34), selective activation of S1P1 can also induces a rapid and transient bradycardia in humans (35). S1P1 agonist-induced bradycardic response in human myocytes can be explained by the activation of G-protein-coupled inwardly rectifying potassium (GIRK) channels (35). Apparently, S1P signaling is involved in the regulation of cardiac physiology, which mainly involves S1P1 and S1P3 receptors. S1P2 KO mice do not show changes in cardiac function and blood pressure (36), indicating that S1P2 may play a minor role in cardiac function. These findings are helpful to understand the cardiac side effects of clinically approved S1P agonists. Details about the exact physiological role of S1P signaling pathway in normal cardiac function requires further investigation.
3.2. S1P in cardioprotection
It has been shown that S1P protects the cardiomycytes from hypoxic injury in cell culture. Pre-incubation of the cells with S1P prevents the hypoxia-induced cell death in neonatal rat cardiomyocytes (37). Moreover, inhibition of SPHK activity produces cell death and that exogenous S1P rescues the cell death induced by SPHK inhibition, suggesting a cardioprotective role of S1P (37, 38). S1P-ellicited cardioprotective action is later found mediated by S1P1 receptor (38-40). Recently, it has been found that High-Density Lipoprotein (HDL), a major carrier of S1P in the serum (41), protects mouse cardiomyocytes against injury induced by hypoxia-reoxygenation. This protective effect by HDL is dependent on both S1P1 and S1P3 subtype receptors (42). S1P agonist FTY720 also protects the cultured cardiomyocytes from hypoxic damage (43, 44). In addition to exogenous S1P or agonist, endogenous S1P also renders protective effect in cardiomyocytes. For example, production and accumulation of S1P is required in insulin-induced protection of rat cardiomyocytes against apoptosis induced by hypoxia-reoxygenaion (45).
In isolated and perfused heart, pretreatment with S1P significantly enhances the recovery of cardiac function after ischemia (46, 47). Similarly, S1P agonist FTY720 reduces the arrhythmic events associated with ischemia/reperfusion injury (48). Interestingly, in ex vivo rat heart antagonists of S1P1 and S1P3 receptors abolish the protective effect of ischemic preconditioning against ischemia/reperfusion injury, suggesting that S1P is an important endogenous cardioprotective factor released by ischemic preconditioning (49). This viewpoint is further supported by the observation that plasma levels of long-chain sphingoid base are dramatically increased following transient cardiac ischemia in humans(44, 50, 51) and that the protective effect of ischemic preconditioning and postconditoning against ischemia/reperfusion is abolished in SPHK1-null mice (52, 53). Further, inhibition of S1P lyase, an enzyme to degrade S1P, increases S1P levels and protects against ischemia/reperfusion injury in ex vivo heart (54), additionally supporting the cardiac protective role of S1P.
In addition to the findings that SHPK1 is cardioprotective, recent studies have revealed that SPHK2 also protects against ischemia/reperfusion injury in heart (55, 56). SPHK2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes the responsiveness to ischemic preconditioning (55). Unlike SPHK1-generated S1P, which acts in paracrine and autocrine manners via its receptors, SPHK2-produced S1P acts intracellularly as a second messenger. A deficiency in SPHK2 is shown to reduce mitochondrial S1P content, leading to a mismatch of the respiratory chain responsible for a defect in mitochondrial respiration as well as dysregulation of permeability transition pore (PTP). It is suggested that mitochondrial S1P produced by SPHK2 is the required downstream of SPHK1-activated signaling pathways for PTP-dependent preconditioning protection (56).
As pretreatment of the heart or cardiomyocytes with S1P has been shown to prevent cardiac injury, administration of the S1P or S1P agonists after ischemia during reperfusion can also effectively improves functional recovery in isolated and perfused rat heart, demonstrating the feasibility of pharmacologic S1P receptor modulation for treatment during reperfusion after myocardial ischemia (57, 58). While S1P has been shown to be cardioprotective, one study raises a concern that S1P and S1P1 agonist at a larger dose exacerbate reperfusion arrhythmias, although S1P improves recovery of left ventricular function and reduces infarct size in isolated and perfused rat heart (59). Given that selective agonists of S1P receptors are in clinical trial for various indications, the findings from this study suggest that extreme caution is warranted, especially in patients at risk for episodes of myocardial ischemia.
In vivo studies using S1P agonist FTY720 show different results in cardioprotection. In contrast to its cardioprotective effect in the in vitro and ex vivo experiments, an in vivo study using a myocardial ischemic rat model shows that FTY720 does not reduce infarct size when given before or after reperfusion, although it attenuates granulocyte infiltration and tumor necrosis factor (TNF)-α protein expression in reperfused myocardium. FTY720 even increases mortality due to induction of fatal ventricular tachyarrhythmias when administered once before reperfusion, but protects against reperfusion arrhythmias when given 24 h prior to ischemia without reducing infarct size (60). The discrepancy between the cardiac effects of FTY720 from the in vivo and ex vivo experiments is probably due to the systemic vs. local effect of the compound. One of the possible reasons may be the immuno-modulating property of FTY720 that reduces T-cell population in the blood (60). Induction of T-cell lymphopenia might be unfavourable, because there are particular subsets of T-cells that may favorably modulate the immune response in ischemia–reperfusion (60). It has been shown that depletion of regulatory T cells augments postischemic activation of resident and invading inflammatory cells, which results in production of deleterious cytokines and reduction of protective cytokines in ischemia-reperfusion injuries (60-62). Thus, interaction between systemic and local effects needs to be taken into account when assessing the effect of S1P agonist.
Failing to show cardioprotection by FTY720 in the above study does not exclude the protective role of S1P in myocardial ischemic injury in vivo. In a rat model of myocardial infarction the plasma levels of S1P are significantly decreased, but the plasma levels of ceramide significantly increased after myocardial infarction (63). Moreover, there is a dramatic reduction in S1P level and increase in the ceramide level in the uninfarcted area of the left ventricle after myocardial infarction (64). It is believed that the alteration of S1P/ceramide ratio may be responsible for apoptosis of the cardiomyocytes from the uninfarcted area of the myocardium (63, 64). Indeed, overexpression of SPHK1 to rescue the decreased production of S1P in the heart reduces the infarct size and preserves the cardiac function (65). Furthermore, inhibition of S1P lyase to increase the plasma level of S1P also reduces the infarct size and enhances the recovery of cardiac function in vivo (66), which is consistent with the results from the study using ex vivo heart (54). Inhibition of S1P lyase to increase the plasma level of S1P is accompanied by increased number of bone marrow-derived stem cells in the circulation, indicating that S1P-induced mobilization of bone marrow-derived stem cells may also contribute to the protective effect of S1P (66). Contrary to the previous study showing no cardioprotective effect of FTY720 in vivo, a more recent study, however, demonstrated that post-conditioning with FTY720 reduces infarct volume and improves short-term and long-term hemodynamic outcome (67). The possible explanations for these contradictory results compared with the previous study are differences in surgical procedures (i.e. open- vs. closed-chest model), species (rat vs. mouse) and dose of FTY720 (0.5. mg kg−1 vs. 1 mg kg−1) (67).
Using S1P2-null and/or S1P3-null mice (S1P1-null mice are embryonic lethal), it is shown that mice lacking either S1P2 or S1P3 receptor and subjected to 1-h coronary occlusion followed by 2 h of reperfusion develop infarcts equivalent to those of wild-type mice, whereas in S1P2 & 3 receptor double-knockout mice, infarct size following ischemia/reperfusion is increased by >50%, suggesting that S1P2 and S1P3 receptors plays a significant role in protecting cardiomyocytes from ischemia/reperfusion damage in vivo (68). In addition, in a mouse model of ischemia/reperfusion, post-conditioning with S1P significantly reduces the infarct size and improves cardiac function, which is shown to be S1P3 receptor-dependent, as the protective effect of S1P is absent in S1P3 knockout mice (69). Moreover, reconstituted (synthetic) HDL (rHDL) that is artificially added with S1P significantly potentiates the cardioprotective effect of rHDL and that the protective effect of rHDL appears to target directly the cardiomyocyte (70). S1P signaling is also shown to mediate the cardioprotective effect of adiponectin, as an antagonist of S1P1/3 abolishes the protective effect of adiponectin against the ischemia/reperfusion injury in mouse heart in vivo (71). Consistent with the reports that deletion of SPHK1 gene abolishes the cardioprotection of preconditioning, enhancing SPHK1 activity in SPHK1-transgenic mice protects the heart against ischemia/ reperfusion (72). Collectively, results from studies using cultured cells, isolated and perfused heart, and in vivo models have demonstrated that S1P is a cardioprotective mediator in ischemia/reperfusion injury.
3.3. S1P in cardiac remodeling
Cardiac remodeling is the change of heart structure to adapt to hemodynamic load (wall tension of myocardium) or in response to stress by various stimuli (73-75). The goal of cardiac remodeling is to reduce the increased wall tension and preserve or augment the heart pump function. Exercise, pregnancy, and postnatal growth are examples of physiologic remodeling, which causes alterations in the dimensions and function of the heart in response to physiologic stimuli. Cardiac remodeling may occur under pathologic conditions such as pressure overload, volume overload, following myocardial infarction, and inflammatory or idiopathic heart diseases. Pathological hypertrophic remodeling, which is initially to compensate and reduce ventricular wall stress and temporally preserve cardiac pump function, eventually progresses into maladaptive alterations and increases the risk of heart failure and malignant arrhythmia. The cardiac remodeling usually involves an increase in myocardial mass. As S1P stimulates cell proliferation and fibrosis (4), it is then not surprised that S1P has been implicated in cardiac remodeling.
Cardiac hypertrophy is a common type of cardiac remodeling that occurs when the heart has experienced persistently elevated workload or after myocardial infarction. In rat neonatal cardiomyocytes, incubation of S1P stimulates the cellular hypertrophy as shown by an increase in cell size, which is blocked by inhibiting S1P1 receptor (76). In vivo studies also show the involvement of S1P in cardiac hypertrophy. Patients with Fabry disease that exhibits left-ventricular hypertrophy have increased levels of plasma S1P and that there is a positive correlation between plasma S1P level and left-ventricular mass index (77). These observations in patients with Fabry disease lead a hypothesis that S1P plays a potential role in cardiac remodeling in these patients, and thereafter, an animal experiment that shows infusion of S1P induces cardiac hypertrophy without a hypertension in normal mice (77). However, in mice with established cardiac hypertrophy induced by aortic constriction, S1P agonist FTY720 reduces the ventricular mass, ameliorated fibrosis, and improves cardiac performance (78). It seems that S1P produces beneficial effects in disease models of cardiac hypertrophy, although induces cardiac hypertrophy in normal condition. S1P1 receptor has also been shown to mediate an improvement of cardiac function after myocardial infarction with increased end-diastolic diameter in rats (79). This study shows that left ventricle ejection fraction is dramatically decreased and end-diastolic diameter increased eight weeks after experimental myocardial infarction. However, at the eighth week after myocardial infarction, overexpression of S1P1 receptor in the heart by adenovirus infection significantly increases the wall thickness and mass of left ventricle, and decreases the left ventricular systolic and diastolic internal diameters, which is accompanied by increase of left ventricular systolic pressure and decrease of end- diastolic pressure compared with controls 12 weeks after the overexpression of S1P1 receptor by adenovirus infection. These results indicate that S1PR1 gene therapy induces a compensatory hypertrophic response able to counteract left ventricular dilation after myocardial infarction.
Cardiac remodeling is often accompanied by changes in the extracellular matrix (ECM), ECM remodeling and fibrosis (73-75, 80). Cardiac fibroblasts make up >50% of the total heart cells and provide a pivotal contribution to cardiac remodeling (80-82). It has been shown that cardiac fibroblasts express S1P1-3 receptors and that the levels of these receptors in cultured cardiac fibroblasts are significantly altered after stimulation by the factors associated with remodeling such as transforming growth factor (TGF)-β1, TNF-α, and platelet-derived growth factor (83). Furthermore, in cultured cardiac fibroblasts, TGF-β stimulates S1P production via SPHK1 and that S1P increases collagen expression via S1P2 receptor; TGF-β-stimulated collagen production is blocked by the inhibition of SPHK1 or S1P2, demonstrating that S1P is a critical mediator in TGF-β-induced fibrogenic effect in cardiac fibroblasts (84). The role of S1P in TGF-β-induced fibrogenic effect in cardiac fibroblasts is further supported by a study showing that apelin, an adipocyte-derived cardioprotective factor, prevents TGF-β-stimulated activation of cardiac fibroblasts and collagen accumulation through inhibiting SPHK1 (85). Another study demonstrates that relaxin, a peptide hormone that stimulates the turnover of ECM, increases S1P formation, whereas inhibition of SPHK prevents the effects of relaxin on ECM remodeling and cell differentiation in cultured mouse cardiac muscle cells and rat cardiomyoblasts, suggesting that S1P signaling may participate in the ECM remodeling (86). S1P is also shown to stimulate cardiac fibroblast proliferation and migration via S1P3 receptor in vitro (87). Consistently, in vivo activation of S1P signaling by overexpression of SPHK1 in SPHK1-transgenic mice produces myocardial degeneration and fibrosis, which is inhibited by deletion of S1P3 gene in SPHK1-transgenic mice, suggesting that S1P may cause cardiac fibrosis via S1P3 receptor (72). Overall, although S1P signaling is cardioprotective against ischemia/reperfusion injury, it may promote cardiac remodeling in the chronic course. Major cardiac effects of S1P are summarized in Table 2.
Table 2.
Summary of major cardiac effects of S1P
| Cardiac physiology | Cardioprotection | Cardiac remodeling |
|---|---|---|
| Negative inotropy (S1P1 mediated) |
S1P1, 2 & 3 are involved |
Hypertrophy (S1P1) |
| Negative chronotropy (S1P1 in human; S1P3 in rodent) |
Both SPHK1 and SPHK2 are required |
Fibrosis (S1P2, 3) |
4. S1P SIGNALING IN VASCULATURE
Regulation of vascular function by S1P signaling has been well recognized (88-91). S1P receptors are present in vasculature with different expression patterns in different cells. S1P1 and S1P3 are predominant S1P receptors expressed in endothelial cells, whereas S1P1, S1P2 and S1P3 receptors are present in vascular smooth muscle cells, and that S1P4 and S1P5 receptors are not detectable in the vascular system (89, 92). S1P induces different effects on vasculature, depending on the relative levels of dominant receptor subtypes in endothelial or smooth muscle cells and the accessibility of S1P to the receptors.
4.1. S1P in vascular tone
S1P1 or S1P3 receptor on the endothelium mediates a vasodilator effect through eNOS activation and NO production, whereas S1P2 or S1P3 receptor on the vascular smooth muscle cells mediates a vasoconstrictor effect (88-91). S1P-induced effects on vascular tone differ in different vascular beds. Using a wire myograph system, a preparation allows S1P to simultaneously access both endothelial cells and vascular smooth muscle cells, studies show that S1P constricts mesenteric, renal, cerebral, basilar and placental arteries. In large conduit arteries, such as aortas, S1P dilates the preconstricted vessels. Even within the same organ, S1P shows different effects in different segments of the vessels. In renal glomerular arterioles, S1P evokes concentration-dependent vasoconstriction in afferent arterioles, whereas it has no effect in efferent arterioles in an in vitro blood-perfused juxtamedullary nephron preparation (93). The different effects of S1P in different vascular beds are likely caused by the tissue-specific actions of different S1P receptors. For example, activation of S1P3 but not S1P2 produces constriction in cerebral arteries, whereas activation of S1P2 but not S1P3 induces vasoconstriction in the pulmonary vasculature. In addition to its effects in vessels, S1P signaling in penile cavernous tissue has recently been associated with erectile dysfunction in a rat model (94). Erectile dysfunction may be linked to a decrease of S1P1 and an increase of S1P2-3 in cavernous tissue that lead to a downregulation of NO pathway and upregulation of the RhoA/Rho kinase pathway.
S1P has also been shown to be a mediator of some vascular stimuli. For example, vascular endothelial growth factor, H2O2 and statins have all been shown to regulate vascular tone via activation of S1P/S1P1 signaling (90); anandamide induces vessel relaxation via a mechanism requiring SPHK1 and S1P/S1P3 (91); and hypoxic pulmonary vasoconstriction is dependent on SPHK1 and S1P/S1P2/4 signaling (95). Therefore, S1P is suggested as an important regulator of vascular tone (Figure 2).
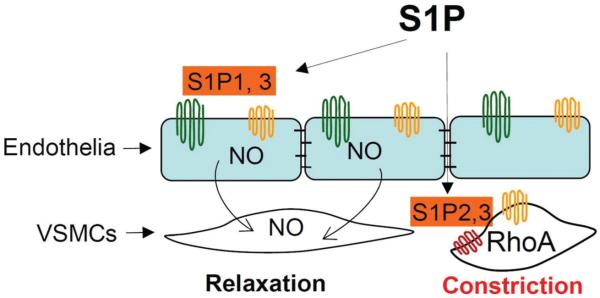
Figure 2.
Role of S1P signaling in vascular tone. S1P1 & 3 receptors on endothelial cells mediate a vasodilator effect, whereas S1P2 & 3 on vascular smooth muscle cells (VSMCs) mediate a vasoconstrictor effect.
4.2. S1P in endothelial permeability and vascular tone
S1P produces vasorelaxation via actions in endothelial cells and vasoconstriction in smooth muscle cells. What determines its overall effect on vascular tone? A novel mechanism for regulation of arterial vascular tone by control of endothelial permeability has been recently proposed (88). S1P signaling may regulate the endothelial permeability to control the access of circulating vasoconstrictors, including S1P, to vascular smooth muscle cells, which provides new insight into how S1P regulates vascular tone (88). Plasma contains a relatively high level of S1P compared with solid tissues and that there is a significant S1P concentration gradient between plasma and interstitial fluid (96). Therefore, control of the access of plasma S1P to vascular smooth muscle cells may play a more significant role than the local production of S1P in vascular smooth muscle cells in the regulation of vascular tone.
S1P is known to enhance endothelial barrier function at a low level via S1P1 and weaken endothelial barrier at a high level via S1P3 (Figure. 3). It has been suggested that S1P dynamically regulates barrier function as a means of controlling vascular tone at a dynamic range (88). Under physiological conditions, the levels of S1P in circulation maintain a relatively tight endothelial barrier, which prevents the access of circulating vasoconstrictors to the underlying vascular smooth muscle cells. Under pathophysiological conditions, such as inflammation, an increases of S1P level in plasma and/ or endothelia or the changes of S1P receptor expressions could lead to an enhanced vascular permeability through S1P3-mediated signaling that overrides S1P1-mediated signaling, allowing the access of circulating vasoconstrictors to the underlying vascular smooth muscle cells. Thus, the vasodilator and vasoconstrictor effects of S1P signaling may be switched by its actions on endothelial permeability under different conditions (88).
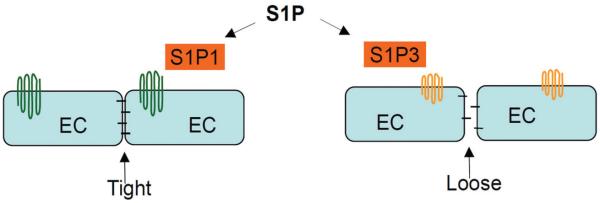
Figure 3.
Role of S1P signaling in endothelial barrier. A low level of S1P produces a tight endothelial barrier via S1P1 receptor on endothelial cells (EC), but a high level of S1P induces a loose barrier via S1P3 receptor on ECs.
4.3. S1P in atherosclerosis
It is well known that high-density lipoprotein (HDL) exerts anti-atherosclerotic effects. Recently, it has been recognized that the atheroprotective effect of HDL is mediated by S1P bound to HDL (97), as HDL that does not contain S1P has no protective effect and that deficiency of S1P receptors abolishes the beneficial effect of HDL. About 55% and 35% of S1P in plasma binds to HDL and albumin, respectively. HDL-bound S1P but not albumin-bond S1P shows protective effects, whereas albumin may serve as a reservoir and a molecular trap for S1P, preventing the over-stimulation of S1P receptors. S1P binding to and released from different proteins may be regulated differently. For example, HDL-associated S1P exhibits a half-life that is four-fold longer than albumin-bound S1P (97). Nevertheless, studies using HDL have revealed an important role of S1P in atherosclerosis.
The S1P levels in blood have been linked to coronary artery disease (CAD). Higher levels of S1P in serum have been reported in subjects with CAD and that S1P levels are closely correlated with severity of CAD. However, plasma levels of HDL-bound S1P are reversely correlated to CAD, whereas the levels of non-HDL-bound S1P increase proportionally with the severity of CAD. In general, the partitioning of S1P into HDL and non-HDL pool may be associated with CAD and that HDL-associated S1P has been shown to be beneficial. The atheroprotective role of S1P is also supported by studies that manipulate S1P levels by targeting S1P metabolic enzymes. SPHK1 inhibitor that significantly reduced plasma S1P levels increases atherosclerotic lesions in LDL receptor KO mice (98), whereas deficiency of S1P lyase, an enzyme that degrades S1P, decreases atherosclerotic lesion in LDL receptor KO Mice (99).
In addition to the above evidence that endogenous S1P may be involved in atherosclerosis, treatments using S1P agonists have also shown that S1P is protective against atherosclerosis. S1P agonists FTY720 and KRP-203 dramatically attenuate the development of atherosclerosis in ApoE KO mice and LDL receptor KO mice (97). FTY720 has also been shown to improve the coronary dysfunction and restore the reduced coronary flow reserve in diabetic rats (100).
Although S1P is generally atheroprotective, however, different S1P receptors induce different effects on atherosclerosis. It has been shown that S1P1 or S1P3 on endothelial cells mediates an anti-atherosclerotic effect via stimulation of endothelial nitric oxide synthase (eNOS) and inhibition of the expression of adhesion molecule and monocyte chemoattractant peptide-1, whereas S1P2 mediates a pro-atherosclerotic effect, as deletion of S1P2 in ApoE-KO mice results in less atherosclerotic lesion (101). The pro-atherogenic effect by S1P2 is attributed to the actions of S1P2 expressed on macrophages. S1P2 on smooth muscle cells, however, mediates contrary effects, which inhibit migration and suppress growth in arterial smooth muscle cells (97). Similarly, S1P3 on vessel shows anti-atherogenic effect, whereas S1P3 on macrophages shows pro-atherogenic effects (97). Both S1P2 and S1P3 may play opposing roles in the atherosclerosis depending on the site of expression. S1P1 KO is embryonic lethal, which limits the use of S1P1 KO model for studying its role in atherogenesis. However, studies using selective S1P1 agonist suggest that S1P1 signaling mediates the atheroprotective effects of S1P in vivo (97). Figure 4 briefly illustrates the role of S1P signaling in atherosclerosis.
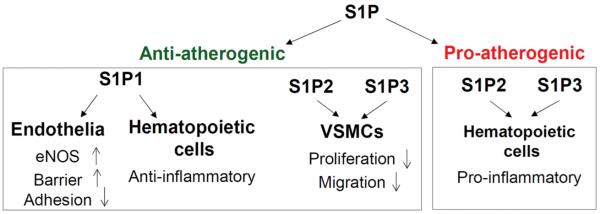
Figure 4.
Role of S1P signaling in atherosclerosis. S1P mainly induces an atheroprotective effect via S1P1 receptor on endothelial and hematopoietic cells and S1P2 & 3 on VSMCs, although S1P2&3 receptors on macrophages have been shown to mediate a pro-atherosclerotic effect.
5. S1P SIGNALING IN BLOOD PRESSURE
5.1. S1P regulation of vascular tone and blood pressure
The significant role of S1P signaling pathway in vascular tone may contribute to the regulation of blood pressure. Indeed, analysis of gene interaction network in human population suggests that sphingolipid metabolic network are highly involved in the regulation of blood pressure (102) and that genetics of ceramide/ S1P rheostat may be involved in hypertension (103), supporting the potential role of S1P signaling in the regulation of blood pressure. It has been shown that administration of S1P and its agonist produces a transit reduction of blood pressure initially and a mild increase in blood pressure after a long-term use in both animal and human studies (104). The initial decrease in blood pressure is likely due to S1P1/S1P3-dependent activation of eNOS, whereas later increase in blood pressure is probably related to down-regulation of S1P1 receptors on endothelial cells, which reduces S1P1-dependent eNOS activation and shifts the balance of S1P effects to favor S1P2- and/or S1P3-mediated signaling in vascular smooth muscle cells, leading to vasoconstriction (104). A transient decrease in heart rate may also contribute to the transient reduction of blood pressure (104). Although the effects of exogenous S1P or its agonist on blood pressure are mild and clinically insignificant, studies have revealed a significant role of endogenous S1P pathway in blood pressure regulation.
A recent study has demonstrated that S1P-S1P1-eNOS signaling plays a critical role in the regulation of blood pressure and in the development of hypertension, probably via actions in vasculature (105). Nogo-B is a membrane protein of the endoplasmic reticulum that negatively controls endothelial S1P levels via inhibiting serine palmitoyltransferase, a rate-limiting enzyme of the de novo sphingolipid biosynthetic pathway. Mice lacking Nogo-B either systemically or specifically in endothelial cells are hypotensive and resistant to angiotensin II-induced hypertension, which is accompanied by upregulated eNOS activation and NO production. Antagonist of S1P1, but not S1P2 or S1P3 reverses the enhanced vasodilation in Nogo-B-null mice. Moreover, the selective S1P1 agonist normalizes blood pressure in angiotensin II-induced hypertension. These results suggest that S1P-S1P1-mediated signaling in endothelia plays a critical role in the regulation of blood pressure in both physiological and hypertensive conditions and that it may participate in the pathogenesis of hypertension.
The above study demonstrates a protective role of S1P signaling against angiotensin II-induced hypertension. However, other study has shown a controversial result, in which genetic deletion of SPHK1 attenuates the transmembrane Ca2+ influx in vascular smooth muscle cells and significantly inhibits both the acute hypertensive response to angiotensin II in anaesthetized mice and the sustained hypertensive response to continuous infusion of angiotensin II in conscious mice, indicating that S1P produced by SPHK1 may have a pro-hypertensive role (106). More puzzling, another study also using SPHK1 knock mice and angiotensin II-induced hypertension does not observe any difference in blood pressure between wild type and SPHK1 knock mice (107). These discrepancies are probably due to the different experiment conditions or the distinct roles of different enzymes manipulating S1P production, adding more complexity to the role of S1P signaling in the control of blood pressure. As S1P signaling induces both vasodilation and vasoconstriction, it is possible that S1P produces opposite effects under certain circumstances. Although there is no doubt that S1P signaling may play a critical role in the regulation of blood pressure, reports on this topic are currently very limited, comparing to the numerous studies in vasculature. Apparently, more investigations are required to clarify the role of S1P signaling in the control of blood pressure and in the pathogenesis of hypertension.
5.2. S1P regulation of renal sodium excretion and blood pressure
S1P1, S1P2 and S1P3 receptors are present in the kidneys (108). SPHKs are also detected in the kidneys (13, 109, 110). Acute infusion of S1P intravenously or into renal artery transiently reduces renal blood flow without altering blood pressure and endogenous creatinine clearance (111-113). Despite a reduction in renal blood flow S1P causes dramatic increase in urine flow and sodium excretion, indicating a tubular effect of S1P on sodium excretion (112, 113). Our lab has recently determined the effect of S1P on urinary Na+ excretion in anesthetized rats (114). Our results show that S1P1-3 receptors are most abundantly expressed in the renal medulla and predominantly located in collecting ducts. Infusion of S1P agonist FTY720 into the renal medulla produces a substantial increase in urinary Na+ excretion and a small increase in medullary blood flow, also suggesting that S1P-induced natriuretic effect is mainly through its tubular action. Although S1P has been shown to stimulate the production of nitric oxide, our results indicate that S1P-induced natriuresis is NO-independent. Our results also show that the tubular effect of S1P agonist may be through inhibition of epithelial sodium channel (ENaC) activity, as S1P agonist in combination with different Na+ transport inhibitors produces additive natriuretic effect except amiloride, an inhibitor of ENaC. It is suggested that S1P targets ENaC to inhibit Na+ reabsorption.
Our recent results have further demonstrated that the natriuretic effect of S1P is through the activation of S1P1, as the antagonist of S1P1 abolishes the effect of S1P agonist, while the antagonist of S1P2 or S1P3 has no effect. Additionally, S1P1 antagonist alone significantly reduces the urinary Na+ excretion, suggesting that endogenous S1P plays an important role in the regulation of Na+ excretion, whereas S1P2 or S1P3 antagonist alone has no effect on urinary Na+ excretion. Our findings that S1P produces natriuretic effect by inhibiting ENaC activity via S1P1 receptor are consistent with the facts that natriuretic effect of S1P is blocked by pertussis toxin, a Gi protein inhibitor, and that activation of Gi signaling reduces cAMP levels (115, 116). It is well known that cAMP stimulates ENaC activity (117). S1P1-mediated activation of Gi protein (11, 12) would inhibit ENaC activity via reducing cAMP levels. Given the essential role of the kidneys in the control of blood pressure via regulating sodium balance, it is very possible that renal S1P pathway importantly participates in the regulation of blood pressure, especially the salt-sensitivity of blood pressure, and may be potentially involved in the mechanism of hypertension. This notion is supported by the finding that S1P1 is a candidate gene for salt sensitive hypertension in the spontaneous hypertensive stroke prone rats (SHRSPs), as renal S1P1 is significantly reduced from salt-loaded SHRSPs (118). Detailed role of S1P1-mediated natriuretic effect in the regulation of blood pressure requires further investigations.
Taken together, studies have shown strong effects of S1P on vascular functions and renal sodium excretion, and changes of blood pressure in mice with deletion of genes that are related to S1P signaling, though controversial. These reports suggest a significant role of S1P signaling in the control of blood pressure (Figure 5), and maybe, in the mechanisms of hypertension. Currently, our knowledge about the role of S1P signaling in the control of blood pressure is still very limited. More investigations on this important topic are required in the future.
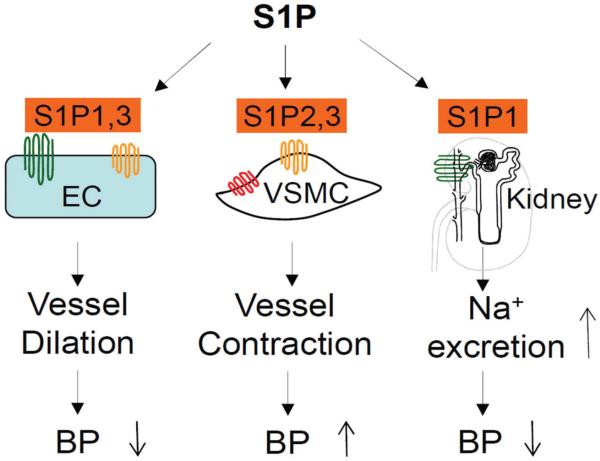
Figure 5.
Role of S1P signaling in blood pressure. S1P1&3-mediated vasodilation reduces blood pressure (BP), whereas S1P2&3-mediated vasoconstriction increases BP. Strong natriuretic effect of S1P via S1P1 on renal tubules may contribute to the long-term control of blood pressure.
6. CONCLUSION
Evidently, S1P signaling is critically involved in cardiovascular function and pathogenesis of various cardiovascular diseases. Manipulation of S1P signaling may offer novel therapeutic approaches to cardiovascular diseases. This review focuses on the direct effects of S1P on cardiovascular system. The actions of S1P signaling appear complicated and target a large variety of organ systems. Therefore, interactions among the effects of S1P in different systems may exist. For example, S1P signaling has been shown to play an important role in immune system. The regulatory role of S1P in immune and inflammation may also affect cardiovascular functions. As evidence has indicated that there are differences between the local and systemic effects of S1P on cardiovascular system, the overall effects of pharmacological interferences with S1P signaling on specific cardiovascular diseases remain to be clarified. Nevertheless, applications of S1P-based drugs for cardiovascular diseases should be attractive, especially as FDA has approved FTY720 for the treatment of relapsing multiple sclerosis and that several other S1P receptor modulators and SPHK inhibitors are under clinical trials for the treatment of different diseases. The approved clinical use of these S1P-based drugs may expedite the test and implementation of these drugs in cardiovascular diseases. On the other hand, due to the significant role of S1P signaling in cardiovascular system, the potential cardiovascular problems of S1P-based therapy need to be considered for the use of such drugs to treat diseases of other systems.
7. ACKNOWLEDGEMENT
Source of Funding: National Institutes of Health Grant HL-89563, HL106042, and HL115068.
Footnotes
Authors declare no conflict of Interest.
8. REFERENCES
- 1.Ghosh TK, Bian J, Gill DL. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990;248(4963):1653–6. doi: 10.1126/science.2163543. DOI: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114(1):155–67. doi: 10.1083/jcb.114.1.155. DOI: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadahira Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci U S A. 1992;89(20):9686–90. doi: 10.1073/pnas.89.20.9686. DOI: 10.1073/pnas.89.20.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12(9):688–702. doi: 10.1038/nrd4099. DOI: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Zhang QH, Yi GH. Regulation of metabolism and transport of sphingosine-1-phosphate in mammalian cells. Mol Cell Biochem. 2012;363(1-2):21–33. doi: 10.1007/s11010-011-1154-1. DOI: 10.1007/s11010-011-1154-1. [DOI] [PubMed] [Google Scholar]
- 6.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. 2008;60(2):181–95. doi: 10.1124/pr.107.07113. DOI: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannun YA, Luberto C, Argraves KM. Enzymes of Sphingolipid Metabolism: From Modular to Integrative Signaling. Biochemistry. 2001;40(16):4893–4903. doi: 10.1021/bi002836k. DOI: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 8.Claus RA, Dorer MJ, Bunck AC, Deigner HP. Inhibition of sphingomyelin hydrolysis: targeting the lipid mediator ceramide as a key regulator of cellular fate. Curr Med Chem. 2009;16(16):1978–2000. doi: 10.2174/092986709788682182. DOI: 10.2174/092986709788682182. [DOI] [PubMed] [Google Scholar]
- 9.Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. J Lipid Res. 2009;50(Suppl):S91–6. doi: 10.1194/jlr.R800080-JLR200. DOI: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jolly PS, Bektas M, Watterson KR, Sankala H, Payne SG, Milstien S, Spiegel S. Expression of SphK1 impairs degranulation and motility of RBL-2H3 mast cells by desensitizing S1P receptors. Blood. 2005;105(12):4736–4742. doi: 10.1182/blood-2004-12-4686. DOI: 10.1182/blood-2004-12-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55(8):1596–1608. doi: 10.1194/jlr.R046300. DOI: 10.1194/jlr.R046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2010;62(4):579–87. doi: 10.1124/pr.110.003111. DOI: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwalm S, Pfeilschifter J, Huwiler A. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway to treat chronic inflammatory kidney diseases. Basic Clin Pharmacol Toxicol. 2014;114(1):44–9. doi: 10.1111/bcpt.12103. DOI: 10.1111/bcpt.12103. [DOI] [PubMed] [Google Scholar]
- 14.Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology. 2011;76(8 Suppl 3):S3–8. doi: 10.1212/WNL.0b013e31820d5ec1. DOI: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- 15.Orr Gandy KA, Obeid LM. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: review of sphingosine kinase inhibitors. Biochim Biophys Acta. 2013;1831(1):157–66. doi: 10.1016/j.bbalip.2012.07.002. DOI: 10.1016/j.bbalip.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohama T, Olivera A, Edsall L, Nagiec MM, Dickson R, Spiegel S. Molecular cloning and functional characterization of murine sphingosine kinase. J Biol Chem. 1998;273(37):23722–8. doi: 10.1074/jbc.273.37.23722. DOI: 10.1074/jbc.273.37.23722. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Sugiura M, Nava VE, Edsall LC, Kono K, Poulton S, Milstien S, Kohama T, Spiegel S. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J Biol Chem. 2000;275(26):19513–20. doi: 10.1074/jbc.M002759200. DOI: 10.1074/jbc.M002759200. [DOI] [PubMed] [Google Scholar]
- 18.Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012;22(1):50–60. doi: 10.1016/j.tcb.2011.09.003. DOI: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagahashi M, Takabe K, Terracina KP, Soma D, Hirose Y, Kobayashi T, Matsuda Y, Wakai T. Sphingosine-1-phosphate transporters as targets for cancer therapy. Biomed Res Int. 2014;2014:651727. doi: 10.1155/2014/651727. DOI: 10.1155/2014/651727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takabe K, Spiegel S. Export of sphingosine-1-phosphate and cancer progression. J Lipid Res. 2014;55(9):1839–46. doi: 10.1194/jlr.R046656. DOI: 10.1194/jlr.R046656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishi T, Kobayashi N, Hisano Y, Kawahara A, Yamaguchi A. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim Biophys Acta. 2014;1841(5):759–65. doi: 10.1016/j.bbalip.2013.07.012. DOI: 10.1016/j.bbalip.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, Collier C, Zhang M, Satin LS, Merrill AH, Jr., Milstien S, Spiegel S. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280(44):37118–29. doi: 10.1074/jbc.M502207200. DOI: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama A, Aye NN, Yatomi Y, Ozaki Y, Hashimoto K. Effects of sphingosine 1-phosphate, a naturally occurring biologically active lysophospholipid, on the rat cardiovascular system. Jpn J Pharmacol. 2000;82(4):338–42. doi: 10.1254/jjp.82.338. DOI: 10.1254/jjp.82.338. [DOI] [PubMed] [Google Scholar]
- 24.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279(14):13839–48. doi: 10.1074/jbc.M311743200. DOI: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 25.Sugiyama A, Yatomi Y, Ozaki Y, Hashimoto K. Sphingosine 1-phosphate induces sinus tachycardia and coronary vasoconstriction in the canine heart. Cardiovasc Res. 2000;46(1):119–25. doi: 10.1016/s0008-6363(00)00013-4. DOI: 10.1016/S0008-6363(00)00013-4. [DOI] [PubMed] [Google Scholar]
- 26.Means CK, Miyamoto S, Chun J, Brown JH. S1P1 receptor localization confers selectivity for Gi-mediated cAMP and contractile responses. J Biol Chem. 2008;283(18):11954–63. doi: 10.1074/jbc.M707422200. DOI: 10.1074/jbc.M707422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budde K, Schmouder RL, Brunkhorst R, Nashan B, Lucker PW, Mayer T, Choudhury S, Skerjanec A, Kraus G, Neumayer HH. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J Am Soc Nephrol. 2002;13(4):1073–83. doi: 10.1681/ASN.V1341073. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, MacDonell KL, Giles WR. Effects of sphingosine 1-phosphate on pacemaker activity in rabbit sino-atrial node cells. Pflugers Arch. 1999;438(5):642–8. doi: 10.1007/s004249900067. DOI: 10.1007/s004249900067. [DOI] [PubMed] [Google Scholar]
- 29.Egom EE, Kruzliak P, Rotrekl V, Lei M. The effect of the sphingosine-1-phosphate analogue FTY720 on atrioventricular nodal tissue. J Cell Mol Med. 2015;19(7):1729–34. doi: 10.1111/jcmm.12549. DOI: 10.1111/jcmm.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koyrakh L, Roman MI, Brinkmann V, Wickman K. The heart rate decrease caused by acute FTY720 administration is mediated by the G protein-gated potassium channel I. Am J Transplant. 2005;5(3):529–36. doi: 10.1111/j.1600-6143.2005.00754.x. DOI: 10.1111/j.1600-6143.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 31.Bunemann M, Brandts B, zu Heringdorf DM, van Koppen CJ, Jakobs KH, Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J Physiol. 1995;489:701–7. doi: 10.1113/jphysiol.1995.sp021084. Pt 3. DOI: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landeen LK, Dederko DA, Kondo CS, Hu BS, Aroonsakool N, Haga JH, Giles WR. Mechanisms of the negative inotropic effects of sphingosine-1-phosphate on adult mouse ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294(2):H736–49. doi: 10.1152/ajpheart.00316.2007. DOI: 10.1152/ajpheart.00316.2007. [DOI] [PubMed] [Google Scholar]
- 33.Yamada M. The role of muscarinic K(+) channels in the negative chronotropic effect of a muscarinic agonist. J Pharmacol Exp Ther. 2002;300(2):681–7. doi: 10.1124/jpet.300.2.681. DOI: 10.1124/jpet.300.2.681. [DOI] [PubMed] [Google Scholar]
- 34.Demont EH, Andrews BI, Bit RA, Campbell CA, Cooke JW, Deeks N, Desai S, Dowell SJ, Gaskin P, Gray JR, Haynes A, Holmes DS, Kumar U, Morse MA, Osborne GJ, Panchal T, Patel B, Perboni A, Taylor S, Watson R, Witherington J, Willis R. Discovery of a Selective S1P1 Receptor Agonist Efficacious at Low Oral Dose and Devoid of Effects on Heart Rate. ACS Med Chem Lett. 2011;2(6):444–9. doi: 10.1021/ml2000214. DOI: 10.1021/ml2000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, Pan S, Gray NS, Hinterding K, Cooke NG, Groenewegen A, Vitaliti A, Sing T, Luttringer O, Yang J, Gardin A, Wang N, Crumb WJ, Jr., Saltzman M, Rosenberg M, Wallstrom E. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharmacol. 2012;167(5):1035–47. doi: 10.1111/j.1476-5381.2012.02061.x. DOI: 10.1111/j.1476-5381.2012.02061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ. Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R440–6. doi: 10.1152/ajpregu.00085.2006. DOI: 10.1152/ajpregu.00085.2006. [DOI] [PubMed] [Google Scholar]
- 37.Karliner JS, Honbo N, Summers K, Gray MO, Goetzl EJ. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33(9):1713–7. doi: 10.1006/jmcc.2001.1429. DOI: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- 38.Tao R, Zhang J, Vessey DA, Honbo N, Karliner JS. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74(1):56–63. doi: 10.1016/j.cardiores.2007.01.015. DOI: 10.1016/j.cardiores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Tao R, Hoover HE, Zhang J, Honbo N, Alano CC, Karliner JS. Cardiomyocyte S1P1 receptor-mediated extracellular signal-related kinase signaling and desensitization. J Cardiovasc Pharmacol. 2009;53(6):486–94. doi: 10.1097/FJC.0b013e3181a7b58a. DOI: 10.1097/FJC.0b013e3181a7b58a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293(5):H3150–8. doi: 10.1152/ajpheart.00587.2006. DOI: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 41.Argraves KM, Argraves WS. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J Lipid Res. 2007;48(11):2325–33. doi: 10.1194/jlr.R700011-JLR200. DOI: 10.1194/jlr.R700011-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Tao R, Hoover HE, Honbo N, Kalinowski M, Alano CC, Karliner JS, Raffai R. High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia-reoxygenation through lipoprotein-associated sphingosine 1-phosphate. Am J Physiol Heart Circ Physiol. 2010;298(3):H1022–8. doi: 10.1152/ajpheart.00902.2009. DOI: 10.1152/ajpheart.00902.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang M, Lu L, Liu Y, Gu G, Tao R. FTY720 attenuates hypoxia-reoxygenation-induced apoptosis in cardiomyocytes. Exp Mol Pathol. 2014;97(2):218–24. doi: 10.1016/j.yexmp.2014.07.008. DOI: 10.1016/j.yexmp.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Egom EE, Mohamed TM, Mamas MA, Shi Y, Liu W, Chirico D, Stringer SE, Ke Y, Shaheen M, Wang T, Chacko S, Wang X, Solaro RJ, Fath-Ordoubadi F, Cartwright EJ, Lei M. Activation of Pak1/Akt/eNOS signaling following sphingosine-1-phosphate release as part of a mechanism protecting cardiomyocytes against ischemic cell injury. Am J Physiol Heart Circ Physiol. 2011;301(4):H1487–95. doi: 10.1152/ajpheart.01003.2010. DOI: 10.1152/ajpheart.01003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H, Che X, Xu X, Zheng M, Zhao Y, He W, Yu J, Xiong J, Li W. Insulin Protects Apoptotic Cardiomyocytes from Hypoxia/ Reoxygenation Injury through the Sphingosine Kinase/Sphingosine 1-Phosphate Axis. PLoS ONE. 2013;8(12):e80644. doi: 10.1371/journal.pone.0080644. DOI: 10.1371/journal.pone.0080644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin ZQ, Zhou HZ, Zhu P, Honbo N, Mochly-Rosen D, Messing RO, Goetzl EJ, Karliner JS, Gray MO. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282(6):H1970–7. doi: 10.1152/ajpheart.01029.2001. DOI: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 47.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34(5):509–18. doi: 10.1006/jmcc.2002.1533. DOI: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 48.Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, Solaro RJ, Lei M. FTY720 prevents ischemia/reperfusion injury-associated arrhythmias in an ex vivo rat heart model via activation of Pak1/Akt signaling. J Mol Cell Cardiol. 2010;48(2):406–14. doi: 10.1016/j.yjmcc.2009.10.009. DOI: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297(4):H1429–35. doi: 10.1152/ajpheart.00358.2009. DOI: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egom EE, Mamas MA, Chacko S, Stringer SE, Charlton-Menys V, El-Omar M, Chirico D, Clarke B, Neyses L, Cruickshank JK, Lei M, Fath-Ordoubadi F. Serum sphingolipids level as a novel potential marker for early detection of human myocardial ischaemic injury. Front Physiol. 2013;4:130. doi: 10.3389/fphys.2013.00130. DOI: 10.3389/fphys.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deutschman DH, Carstens JS, Klepper RL, Smith WS, Page MT, Young TR, Gleason LA, Nakajima N, Sabbadini RA. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146(1):62–8. doi: 10.1016/S0002-8703(03)00118-2. DOI: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 52.Jin ZQ, Karliner JS, Vessey DA. Ischaemic postconditioning protects isolated mouse hearts against ischaemia/reperfusion injury via sphingosine kinase isoform-1 activation. Cardiovasc Res. 2008;79(1):134–40. doi: 10.1093/cvr/cvn065. DOI: 10.1093/cvr/cvn065. [DOI] [PubMed] [Google Scholar]
- 53.Jin ZQ, Zhang J, Huang Y, Hoover HE, Vessey DA, Karliner JS. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76(1):41–50. doi: 10.1016/j.cardiores.2007.05.029. DOI: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 54.Bandhuvula P, Honbo N, Wang GY, Jin ZQ, Fyrst H, Zhang M, Borowsky AD, Dillard L, Karliner JS, Saba JD. S1P lyase: a novel therapeutic target for ischemia-reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011;300(5):H1753–61. doi: 10.1152/ajpheart.00946.2010. DOI: 10.1016/j.cardiores.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vessey DA, Li L, Jin ZQ, Kelley M, Honbo N, Zhang J, Karliner JS. A sphingosine kinase form 2 knockout sensitizes mouse myocardium to ischemia/reoxygenation injury and diminishes responsiveness to ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:961059. doi: 10.1155/2011/961059. DOI: 10.1155/2011/961059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez L, Paillard M, Price M, Chen Q, Teixeira G, Spiegel S, Lesnefsky EJ. A novel role for mitochondrial sphingosine-1-phosphate produced by sphingosine kinase-2 in PTP-mediated cell survival during cardioprotection. Basic Res Cardiol. 2011;106(6):1341–53. doi: 10.1007/s00395-011-0223-7. DOI: 10.1007/s00395-011-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vessey DA, Li L, Imhof I, Honbo N, Karliner JS. FTY720 postconditions isolated perfused heart by a mechanism independent of sphingosine kinase 2 and different from S1P or ischemic postconditioning. Med Sci Monit Basic Res. 2013;19:126–32. doi: 10.12659/MSMBR.883877. DOI: 10.12659/MSMBR.883877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hofmann U, Burkard N, Vogt C, Thoma A, Frantz S, Ertl G, Ritter O, Bonz A. Protective effects of sphingosine-1-phosphate receptor agonist treatment after myocardial ischaemia-reperfusion. Cardiovasc Res. 2009;83(2):285–93. doi: 10.1093/cvr/cvp137. DOI: 10.1093/cvr/cvp137. [DOI] [PubMed] [Google Scholar]
- 59.Tsukada YT, Sanna MG, Rosen H, Gottlieb RA. S1P1-selective agonist SEW2871 exacerbates reperfusion arrhythmias. J Cardiovasc Pharmacol. 2007;50(6):660–9. doi: 10.1097/FJC.0b013e318157a5fe. DOI: 10.1097/FJC.0b013e318157a5fe. [DOI] [PubMed] [Google Scholar]
- 60.Hofmann U, Hu K, Walter F, Burkard N, Ertl G, Bauersachs J, Ritter O, Frantz S, Bonz A. Pharmacological pre- and post-conditioning with the sphingosine-1-phosphate receptor modulator FTY720 after myocardial ischaemia-reperfusion. Br J Pharmacol. 2010;160(5):1243–51. doi: 10.1111/j.1476-5381.2010.00767.x. DOI: 10.1111/j.1476-5381.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2):192–9. doi: 10.1038/nm.1927. DOI: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 62.Monteiro RM, Camara NO, Rodrigues MM, Tzelepis F, Damiao MJ, Cenedeze MA, Teixeira Vde P, dos Reis MA, Pacheco-Silva A. A role for regulatory T cells in renal acute kidney injury. Transpl Immunol. 2009;21(1):50–5. doi: 10.1016/j.trim.2009.02.003. DOI: 10.1016/j.trim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Knapp M, Zendzian-Piotrowska M, Blachnio-Zabielska A, Zabielski P, Kurek K, Gorski J. Myocardial infarction differentially alters sphingolipid levels in plasma, erythrocytes and platelets of the rat. Basic Res Cardiol. 2012;107(6):294. doi: 10.1007/s00395-012-0294-0. DOI: 10.1007/s00395-012-0294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knapp M, Żendzian-Piotrowska M, Kurek K, Błachnio-Zabielska A. Myocardial Infarction Changes Sphingolipid Metabolism in the Uninfarcted Ventricular Wall of the Rat. Lipids. 2012;47(9):847–853. doi: 10.1007/s11745-012-3694-x. DOI: 10.1007/s11745-012-3694-x. [DOI] [PubMed] [Google Scholar]
- 65.Duan HF, Wang H, Yi J, Liu HJ, Zhang QW, Li LB, Zhang T, Lu Y, Wu CT, Wang LS. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/ reperfusion-induced injury and attenuates its postischemic failure. Hum Gene Ther. 2007;18(11):1119–28. doi: 10.1089/hum.2007.036. DOI: 10.1089/hum.2007.036. [DOI] [PubMed] [Google Scholar]
- 66.Klyachkin YM, Nagareddy PR, Ye S, Wysoczynski M, Asfour A, Gao E, Sunkara M, Brandon JA, Annabathula R, Ponnapureddy R, Solanki M, Pervaiz ZH, Smyth SS, Ratajczak MZ, Morris AJ, Abdel-Latif A. Pharmacological Elevation of Circulating Bioactive Phosphosphingolipids Enhances Myocardial Recovery After Acute Infarction. Stem Cells Transl Med. 2015 doi: 10.5966/sctm.2014-0273. DOI: 10.5966/sctm.2014-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goltz D, Huss S, Ramadori E, Buttner R, Diehl L, Meyer R. Immunomodulation by splenectomy or by FTY720 protects the heart against ischemia reperfusion injury. Clin Exp Pharmacol Physiol. 2015;42(11):1168–77. doi: 10.1111/1440-1681.12465. DOI: 10.1111/1440-1681.12465. [DOI] [PubMed] [Google Scholar]
- 68.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, Ishii I, Chun J, Brown JH. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292(6):H2944–51. doi: 10.1152/ajpheart.01331.2006. DOI: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 69.Theilmeier G, Schmidt C, Herrmann J, Keul P, Schäfers M, Herrgott I, Mersmann J, Larmann J, Hermann S, Stypmann J, Schober O, Hildebrand R, Schulz R, Heusch G, Haude M, Lipinski KvW, Herzog C, Schmitz M, Erbel R, Chun J, Levkau B. High-Density Lipoproteins and Their Constituent, Sphingosine-1-Phosphate, Directly Protect the Heart Against Ischemia/Reperfusion Injury In vivo via the S1P3 Lysophospholipid Receptor. Circulation. 2006;114(13):1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. DOI: 10.1161/CIRCULATIONAHA.105. 607135. [DOI] [PubMed] [Google Scholar]
- 70.Brulhart-Meynet M-C, Braunersreuther V, Brinck J, Montecucco F, Prost J-C, Thomas A, Galan K, Pelli G, Pedretti S, Vuilleumier N, Mach F, Lecour S, James RW, Frias MA. Improving Reconstituted HDL Composition for Efficient Post-Ischemic Reduction of Ischemia Reperfusion Injury. PLoS ONE. 2015;10(3):e0119664. doi: 10.1371/journal.pone.0119664. DOI: 10.1371/journal.pone.0119664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan W, Zhang F, Zhang R, Zhang X, Wang Y, Zhou F, Xia Y, Liu P, Gao C, Wang H, Zhang L, Zhou J, Gao F, Gao E, Koch WJ, Wang H, Cheng H, Qu Y, Tao L. Adiponectin regulates SR Ca(2+) cycling following ischemia/ reperfusion via sphingosine 1-phosphate-CaMKII signaling in mice. J Mol Cell Cardiol. 2014;74:183–92. doi: 10.1016/j.yjmcc.2014.05.010. DOI: 10.1016/j.yjmcc.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Takuwa N, Ohkura S, Takashima S, Ohtani K, Okamoto Y, Tanaka T, Hirano K, Usui S, Wang F, Du W, Yoshioka K, Banno Y, Sasaki M, Ichi I, Okamura M, Sugimoto N, Mizugishi K, Nakanuma Y, Ishii I, Takamura M, Kaneko S, Kojo S, Satouchi K, Mitumori K, Chun J, Takuwa Y. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc Res. 2010;85(3):484–93. doi: 10.1093/cvr/cvp312. DOI: 10.1093/cvr/cvp312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13):1370–80. doi: 10.1056/NEJMra072139. DOI: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 74.Spaich S, Katus HA, Backs J. Ongoing controversies surrounding cardiac remodeling: is it black and white-or rather fifty shades of gray? Front Physiol. 2015;6:202. doi: 10.3389/fphys.2015.00202. DOI: 10.3389/fphys.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–82. doi: 10.1016/s0735-1097(99)00630-0. DOI: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 76.Robert P, Tsui P, Laville MP, Livi GP, Sarau HM, Bril A, Berrebi-Bertrand I. EDG1 receptor stimulation leads to cardiac hypertrophy in rat neonatal myocytes. J Mol Cell Cardiol. 2001;33(9):1589–606. doi: 10.1006/jmcc.2001.1433. DOI: 10.1006/jmcc.2001.1433. [DOI] [PubMed] [Google Scholar]
- 77.Brakch N, Dormond O, Bekri S, Golshayan D, Correvon M, Mazzolai L, Steinmann B, Barbey F. Evidence for a role of sphingosine-1 phosphate in cardiovascular remodelling in Fabry disease. Eur Heart J. 2010;31(1):67–76. doi: 10.1093/eurheartj/ehp387. DOI: 10.1093/eurheartj/ehp387. [DOI] [PubMed] [Google Scholar]
- 78.Liu W, Zi M, Tsui H, Chowdhury SK, Zeef L, Meng QJ, Travis M, Prehar S, Berry A, Hanley NA, Neyses L, Xiao RP, Oceandy D, Ke Y, Solaro RJ, Cartwright EJ, Lei M, Wang X. A novel immunomodulator, FTY-720 reverses existing cardiac hypertrophy and fibrosis from pressure overload by targeting NFAT (nuclear factor of activated T-cells) signaling and periostin. Circ Heart Fail. 2013;6(4):833–44. doi: 10.1161/CIRCHEARTFAILURE.112.000123. DOI: 10.1161/CIRCHEARTFAILURE.112.000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cannavo A, Rengo G, Liccardo D, Pagano G, Zincarelli C, De Angelis MC, Puglia R, Di Pietro E, Rabinowitz JE, Barone MV, Cirillo P, Trimarco B, Palmer TM, Ferrara N, Koch WJ, Leosco D, Rapacciuolo A. β1-Adrenergic Receptor and Sphingosine-1-Phosphate Receptor 1 (S1PR1) Reciprocal Downregulation Influences Cardiac Hypertrophic Response and Progression to Heart Failure: Protective Role of S1PR1 Cardiac Gene Therapy. Circulation. 2013;128(15):1612–1622. doi: 10.1161/CIRCULATIONAHA.113.002659. DOI: 10.1161/CIRCULATIONAHA.113.002659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis & Tissue Repair. 2012;5(1):15. doi: 10.1186/1755-1536-5-15. DOI: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kehat I, Molkentin JD. Molecular Pathways Underlying Cardiac Remodeling During Pathophysiological Stimulation. Circulation. 2010;122(25):2727–2735. doi: 10.1161/CIRCULATIONAHA.110.942268. DOI: 10.1161/CIRCULATIONAHA.110. 942268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujiu K, Nagai R. Fibroblast-mediated pathways in cardiac hypertrophy. J Mol Cell Cardiol. 2014;70:64–73. doi: 10.1016/j.yjmcc.2014.01.013. DOI: 10.1016/j.yjmcc.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 83.Landeen LK, Aroonsakool N, Haga JH, Hu BS, Giles WR. Sphingosine-1-phosphate receptor expression in cardiac fibroblasts is modulated by in vitro culture conditions. Am J Physiol Heart Circ Physiol. 2007;292(6):H2698–711. doi: 10.1152/ajpheart.01065.2006. DOI: 10.1152/ajpheart.01065.2006. [DOI] [PubMed] [Google Scholar]
- 84.Gellings Lowe N, Swaney JS, Moreno KM, Sabbadini RA. Sphingosine-1-phosphate and sphingosine kinase are critical for transforming growth factor-beta-stimulated collagen production by cardiac fibroblasts. Cardiovasc Res. 2009;82(2):303–12. doi: 10.1093/cvr/cvp056. DOI: 10.1093/cvr/cvp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pchejetski D, Foussal C, Alfarano C, Lairez O, Calise D, Guilbeau-Frugier C, Schaak S, Seguelas MH, Wanecq E, Valet P, Parini A, Kunduzova O. Apelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1. Eur Heart J. 2012;33(18):2360–9. doi: 10.1093/eurheartj/ehr389. DOI: 10.1093/eurheartj/ehr389. [DOI] [PubMed] [Google Scholar]
- 86.Frati A, Ricci B, Pierucci F, Nistri S, Bani D, Meacci E. Role of sphingosine kinase/ S1P axis in ECM remodeling of cardiac cells elicited by relaxin. Mol Endocrinol. 2015;29(1):53–67. doi: 10.1210/me.2014-1201. DOI: 10.1210/me.2014-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benamer N, Fares N, Bois P, Faivre JF. Electrophysiological and functional effects of sphingosine-1-phosphate in mouse ventricular fibroblasts. Biochem Biophys Res Commun. 2011;408(1):6–11. doi: 10.1016/j.bbrc.2011.03.072. DOI: 10.1016/j.bbrc.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 88.Kerage D, Brindley DN, Hemmings DG. Review: novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta. 2014;35(Suppl):S86–92. doi: 10.1016/j.placenta.2013.12.006. DOI: 10.1016/j.placenta.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Takuwa Y, Okamoto Y, Yoshioka K, Takuwa N. Sphingosine-1-phosphate signaling and biological activities in the cardiovascular system. Biochim Biophys Acta. 2008;1781(9):483–8. doi: 10.1016/j.bbalip.2008.04.003. DOI: 10.1016/j.bbalip.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 90.Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res. 2009;82(2):212–20. doi: 10.1093/cvr/cvp064. DOI: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schuchardt M, Tolle M, Prufer J, van der Giet M. Pharmacological relevance and potential of sphingosine 1-phosphate in the vascular system. Br J Pharmacol. 2011;163(6):1140–62. doi: 10.1111/j.1476-5381.2011.01260.x. DOI: 10.1111/j.1476-5381.2011.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skoura A, Hla T. Lysophospholipid receptors in vertebrate development, physiology, and pathology. J Lipid Res. 2009;50(Suppl):S293–8. doi: 10.1194/jlr.R800047-JLR200. DOI: 10.1194/jlr.R800047-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guan Z, Singletary ST, Cook AK, Hobbs JL, Pollock JS, Inscho EW. Sphingosine-1-phosphate evokes unique segment-specific vasoconstriction of the renal microvasculature. J Am Soc Nephrol. 2014;25(8):1774–85. doi: 10.1681/ASN.2013060656. DOI: 10.1681/ASN.2013060656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang B, Jiang J, Fan Z, Jiang R, Wang R, Lin H. Expression of Sphingosine 1-Phosphate 1-3 on Penile Cavernous Tissue in Hypertensive and Normotensive Rats. Urology. 2014;84(2):490.e7–490.e13. doi: 10.1016/j.urology.2014.04.039. DOI: 10.1016/j.urology.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 95.Tabeling C, Yu H, Wang L, Ranke H, Goldenberg NM, Zabini D, Noe E, Krauszman A, Gutbier B, Yin J, Schaefer M, Arenz C, Hocke AC, Suttorp N, Proia RL, Witzenrath M, Kuebler WM. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci U S A. 2015;112(13):E1614–23. doi: 10.1073/pnas.1421190112. DOI: 10.1073/pnas.1421190112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ksiazek M, Chacinska M, Chabowski A, Baranowski M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J Lipid Res. 2015;56(7):1271–81. doi: 10.1194/jlr.R059543. DOI: 10.1194/jlr.R059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poti F, Simoni M, Nofer JR. Atheroprotective role of high-density lipoprotein (HDL)-associated sphingosine-1-phosphate (S1P) Cardiovasc Res. 2014;103(3):395–404. doi: 10.1093/cvr/cvu136. DOI: 10.1093/cvr/cvu136. [DOI] [PubMed] [Google Scholar]
- 98.Poti F, Ceglarek U, Burkhardt R, Simoni M, Nofer JR. SKI-II--a sphingosine kinase 1 inhibitor--exacerbates atherosclerosis in low-density lipoprotein receptor-deficient (LDL-R−/−) mice on high cholesterol diet. Atherosclerosis. 2015;240(1):212–5. doi: 10.1016/j.atherosclerosis.2015.03.020. DOI: 10.1016/j.atherosclerosis.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 99.Bot M, Van Veldhoven PP, de Jager SC, Johnson J, Nijstad N, Van Santbrink PJ, Westra MM, Van Der Hoeven G, Gijbels MJ, Muller-Tidow C, Varga G, Tietge UJ, Kuiper J, Van Berkel TJ, Nofer JR, Bot I, Biessen EA. Hematopoietic sphingosine 1-phosphate lyase deficiency decreases atherosclerotic lesion development in LDL-receptor deficient mice. PLoS One. 2013;8(5):e63360. doi: 10.1371/journal.pone.0063360. DOI: 10.1371/journal.pone.0063360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu H, Jin Y, Ni H, Hu S, Zhang Q. Sphingosine-1-phosphate receptor agonist, FTY720, restores coronary flow reserve in diabetic rats. Circ J. 2014;78(12):2979–86. doi: 10.1253/circj.cj-14-0521. DOI: 10.1253/circj.CJ-14-0521. [DOI] [PubMed] [Google Scholar]
- 101.Jiang XC, Liu J. Sphingolipid metabolism and atherosclerosis. Handb Exp Pharmacol. 2013;(216):133–46. doi: 10.1007/978-3-7091-1511-4_7. [DOI] [PubMed] [Google Scholar]
- 102.Fenger M, Linneberg A, Jeppesen J. Network-based analysis of the sphingolipid metabolism in hypertension. Front Genet. 2015;6:84. doi: 10.3389/fgene.2015.00084. DOI: 10.3389/fgene.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fenger M, Linneberg A, Jorgensen T, Madsbad S, Sobye K, Eugen-Olsen J, Jeppesen J. Genetics of the ceramide/ sphingosine-1-phosphate rheostat in blood pressure regulation and hypertension. BMC Genet. 2011;12:44. doi: 10.1186/1471-2156-12-44. DOI: 10.1186/1471-2156-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Camm J, Hla T, Bakshi R, Brinkmann V. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am Heart J. 2014;168(5):632–44. doi: 10.1016/j.ahj.2014.06.028. DOI: 10.1016/j.ahj.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 105.Cantalupo A, Zhang Y, Kothiya M, Galvani S, Obinata H, Bucci M, Giordano FJ, Jiang X-C, Hla T, Di Lorenzo A. Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat Med. 2015;21(9):1028–1037. doi: 10.1038/nm.3934. DOI: 10.1038/nm.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilson PC, Fitzgibbon WR, Garrett SM, Jaffa AA, Luttrell LM, Brands MW, El-Shewy HM. Inhibition of Sphingosine Kinase 1 Ameliorates Angiotensin II-Induced Hypertension and Inhibits Transmembrane Calcium Entry via Store-Operated Calcium Channel. Mol Endocrinol. 2015;29(6):896–908. doi: 10.1210/me.2014-1388. DOI: 10.1210/me.2014-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Furuya H, Wada M, Shimizu Y, Yamada PM, Hannun YA, Obeid LM, Kawamori T. Effect of sphingosine kinase 1 inhibition on blood pressure. Faseb j. 2013;27(2):656–64. doi: 10.1096/fj.12-219014. DOI: 10.1096/fj.12-219014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kluk MJ, Hla T. Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim Biophys Acta. 2002;1582(1-3):72–80. doi: 10.1016/s1388-1981(02)00139-7. DOI: 10.1016/S1388-1981(02)00139-7. [DOI] [PubMed] [Google Scholar]
- 109.Facchinetti MM, Leocata Nieto F, Marquez MG, Sterin-Speziale N. Stratification of sphingosine kinase-1 expression and activity in rat kidney. Cells Tissues Organs. 2008;188(4):384–92. doi: 10.1159/000139770. DOI: 10.1159/000139770. [DOI] [PubMed] [Google Scholar]
- 110.Alemany R, van Koppen CJ, Danneberg K, Ter Braak M, Meyer Zu Heringdorf D. Regulation and functional roles of sphingosine kinases. Naunyn Schmiedebergs Arch Pharmacol. 2007;374(5-6):413–28. doi: 10.1007/s00210-007-0132-3. DOI: 10.1007/s00210-007-0132-3. [DOI] [PubMed] [Google Scholar]
- 111.Bischoff A, Czyborra P, Meyer Zu Heringdorf D, Jakobs KH, Michel MC. Sphingosine-1-phosphate reduces rat renal and mesenteric blood flow in vivo in a pertussis toxin-sensitive manner. Br J Pharmacol. 2000;130(8):1878–83. doi: 10.1038/sj.bjp.0703516. DOI: 10.1038/sj.bjp.0703516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bischoff A, Meyer Zu Heringdorf D, Jakobs KH, Michel MC. Lysosphingolipid receptor-mediated diuresis and natriuresis in anaesthetized rats. Br J Pharmacol. 2001;132(8):1925–33. doi: 10.1038/sj.bjp.0703969. DOI: 10.1038/sj.bjp.0703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Czyborra C, Bischoff A, Michel MC. Indomethacin differentiates the renal effects of sphingosine-1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2006;373(1):37–44. doi: 10.1007/s00210-006-0037-6. DOI: 10.1007/s00210-006-0037-6. [DOI] [PubMed] [Google Scholar]
- 114.Zhu Q, Xia M, Wang Z, Li PL, Li N. A novel lipid natriuretic factor in the renal medulla: sphingosine-1-phosphate. Am J Physiol Renal Physiol. 2011;301(1):F35–41. doi: 10.1152/ajprenal.00014.2011. DOI: 10.1152/ajprenal.00014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuentes E, Palomo I. Regulatory mechanisms of cAMP levels as a multiple target for antiplatelet activity and less bleeding risk. Thromb Res. 2014;134(2):221–6. doi: 10.1016/j.thromres.2014.04.027. DOI: 10.1016/j.thromres.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 116.Stojilkovic SS, Murano T, Gonzalez-Iglesias AE, Andric SA, Popovic MA, Van Goor F, Tomic M. Multiple roles of Gi/o protein-coupled receptors in control of action potential secretion coupling in pituitary lactotrophs. Ann N Y Acad Sci. 2009;1152:174–86. doi: 10.1111/j.1749-6632.2008.03994.x. DOI: 10.1111/j.1749-6632.2008.03994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benos DJ, Awayda MS, Berdiev BK, Bradford AL, Fuller CM, Senyk O, Ismailov Diversity and regulation of amiloride-sensitive Na+ channels. Kidney Int. 1996;49(6):1632–7. doi: 10.1038/ki.1996.237. DOI: 10.1038/ki.1996.237. [DOI] [PubMed] [Google Scholar]
- 118.Graham D, McBride MW, Gaasenbeek M, Gilday K, Beattie E, Miller WH, McClure JD, Polke JM, Montezano A, Touyz RM, Dominiczak AF. Candidate Genes That Determine Response to Salt in the Stroke-Prone Spontaneously Hypertensive Rat: Congenic Analysis. Hypertension. 2007;50(6):1134–1141. doi: 10.1161/HYPERTENSIONAHA.107.095349. DOI: 10.1161/HYPERTENSIONAHA.107.095349. [DOI] [PubMed] [Google Scholar]