Abstract
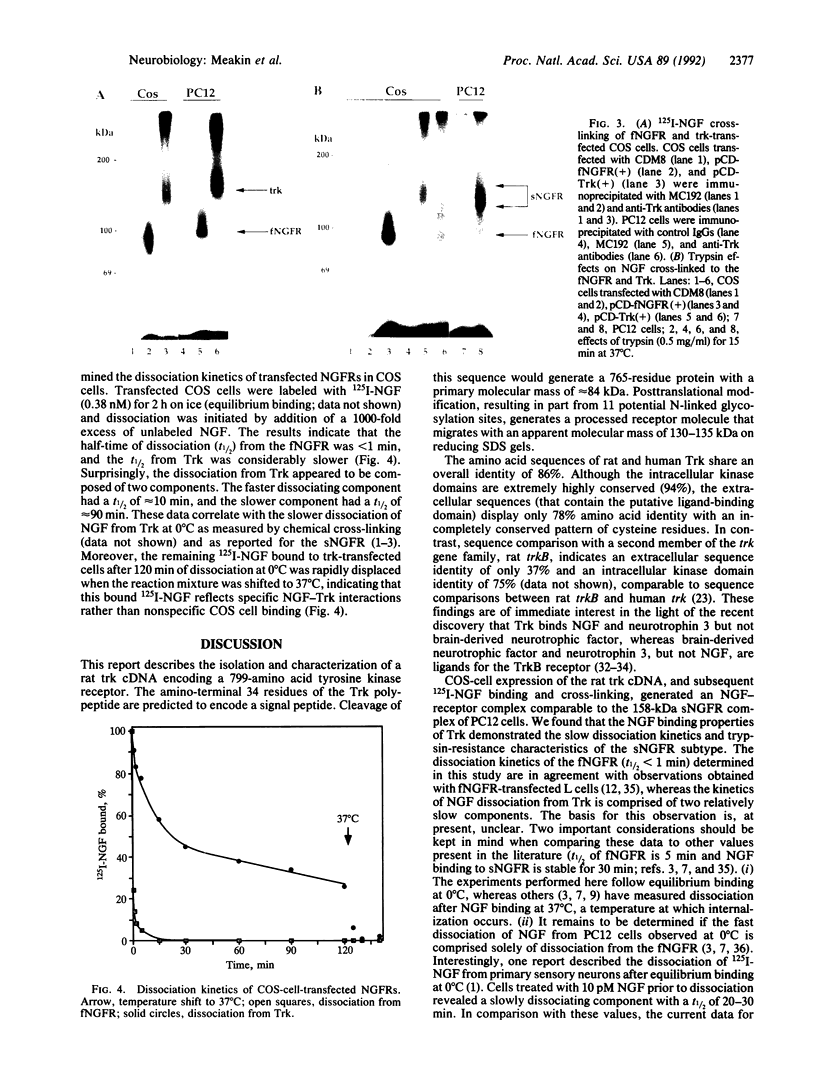
Two distinct nerve growth factor receptor (NGFR) complexes are present on NGF-responsive cell types; these correspond to 100 kDa and 158 kDa for the fast (fNGFR) and the slow (sNGFR) NGFRs, respectively. Previous studies indicate that each complex is derived from a separate gene product and that the sNGFR contains tyrosine kinase activity. The cDNA encoding the fNGFR has previously been cloned. In this report, a rat trk protooncogene cDNA has been isolated from PC12 cells and Trk has been shown to bind NGF, generating a complex of 158 kDa. Characterization of NGF-Trk interactions indicates that Trk and NGF dissociate more slowly than do NGF and the fNGFR. Moreover, NGF-bound Trk is not destroyed by trypsin digestion whereas the NGF-fNGFR complex is sensitive to trypsin digestion. These observations suggest that the trk protooncogene product, expressed in the absence of the fNGFR, binds NGF with properties characteristic of the sNGFR, which was identified as the high-affinity NGFR on primary neurons and PC12 cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg M. M., Sternberg D. W., Hempstead B. L., Chao M. V. The low-affinity p75 nerve growth factor (NGF) receptor mediates NGF-induced tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7106–7110. doi: 10.1073/pnas.88.16.7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernd P., Greene L. A. Association of 125I-nerve growth factor with PC12 pheochromocytoma cells. Evidence for internalization via high-affinity receptors only and for long-term regulation by nerve growth factor of both high- and low-affinity receptors. J Biol Chem. 1984 Dec 25;259(24):15509–15516. [PubMed] [Google Scholar]
- Bernd P., Martinez H. J., Dreyfus C. F., Black I. B. Localization of high-affinity and low-affinity nerve growth factor receptors in cultured rat basal forebrain. Neuroscience. 1988 Jul;26(1):121–129. doi: 10.1016/0306-4522(88)90131-5. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Keeping track of neurotrophin receptors. Cell. 1991 Jun 14;65(6):915–918. doi: 10.1016/0092-8674(91)90540-f. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Tapley P., Jing S. Q., Nanduri V., O'Rourke E., Lamballe F., Kovary K., Klein R., Jones K. R., Reichardt L. F. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991 Jul 12;66(1):173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F. H., Fisher L. J. Intracerebral grafting: a tool for the neurobiologist. Neuron. 1991 Jan;6(1):1–12. doi: 10.1016/0896-6273(91)90116-h. [DOI] [PubMed] [Google Scholar]
- Glass D. J., Nye S. H., Hantzopoulos P., Macchi M. J., Squinto S. P., Goldfarb M., Yancopoulos G. D. TrkB mediates BDNF/NT-3-dependent survival and proliferation in fibroblasts lacking the low affinity NGF receptor. Cell. 1991 Jul 26;66(2):405–413. doi: 10.1016/0092-8674(91)90629-d. [DOI] [PubMed] [Google Scholar]
- Godfrey E. W., Shooter E. M. Nerve growth factor receptors on chick embryo sympathetic ganglion cells: binding characteristics and development. J Neurosci. 1986 Sep;6(9):2543–2550. doi: 10.1523/JNEUROSCI.06-09-02543.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. A. A quantitative bioassay for nerve growth factor (NGF) activity employing a clonal pheochromocytoma cell line. Brain Res. 1977 Sep 16;133(2):350–353. doi: 10.1016/0006-8993(77)90770-3. [DOI] [PubMed] [Google Scholar]
- Green S. H., Greene L. A. A single Mr approximately 103,000 125I-beta-nerve growth factor-affinity-labeled species represents both the low and high affinity forms of the nerve growth factor receptor. J Biol Chem. 1986 Nov 15;261(32):15316–15326. [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Schleifer L. S., Chao M. V. Expression of functional nerve growth factor receptors after gene transfer. Science. 1989 Jan 20;243(4889):373–375. doi: 10.1126/science.2536190. [DOI] [PubMed] [Google Scholar]
- Hosang M., Shooter E. M. Molecular characteristics of nerve growth factor receptors on PC12 cells. J Biol Chem. 1985 Jan 10;260(1):655–662. [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Martin-Zanca D., Parada L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Klein R., Nanduri V., Jing S. A., Lamballe F., Tapley P., Bryant S., Cordon-Cardo C., Jones K. R., Reichardt L. F., Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991 Jul 26;66(2):395–403. doi: 10.1016/0092-8674(91)90628-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma S. C., Redmond S. M., Fu X. C., Saurer S. M., Groner B., Hynes N. E. Activation of the receptor kinase domain of the trk oncogene by recombination with two different cellular sequences. EMBO J. 1988 Jan;7(1):147–154. doi: 10.1002/j.1460-2075.1988.tb02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamballe F., Klein R., Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991 Sep 6;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- Landreth G. E., Shooter E. M. Nerve growth factor receptors on PC12 cells: ligand-induced conversion from low- to high-affinity states. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4751–4755. doi: 10.1073/pnas.77.8.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large T. H., Weskamp G., Helder J. C., Radeke M. J., Misko T. P., Shooter E. M., Reichardt L. F. Structure and developmental expression of the nerve growth factor receptor in the chicken central nervous system. Neuron. 1989 Feb;2(2):1123–1134. doi: 10.1016/0896-6273(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin S. O., Shooter E. M. Molecular investigations on the high-affinity nerve growth factor receptor. Neuron. 1991 Jan;6(1):153–163. doi: 10.1016/0896-6273(91)90130-r. [DOI] [PubMed] [Google Scholar]
- Meakin S. O., Shooter E. M. Tyrosine kinase activity coupled to the high-affinity nerve growth factor-receptor complex. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5862–5866. doi: 10.1073/pnas.88.13.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemas D. S., Lindberg R. A., Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991 Jan;11(1):143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. D., Mathew T. C., Toma J. G. Regulation of nerve growth factor receptor gene expression by nerve growth factor in the developing peripheral nervous system. J Cell Biol. 1991 Jan;112(2):303–312. doi: 10.1083/jcb.112.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda A. R., Martin-Zanca D., Kaplan D. R., Parada L. F., Santos E. Induction by NGF of meiotic maturation of Xenopus oocytes expressing the trk proto-oncogene product. Science. 1991 Apr 26;252(5005):558–561. doi: 10.1126/science.1850550. [DOI] [PubMed] [Google Scholar]
- Radeke M. J., Feinstein S. C. Analytical purification of the slow, high affinity NGF receptor: identification of a novel 135 kd polypeptide. Neuron. 1991 Jul;7(1):141–150. doi: 10.1016/0896-6273(91)90082-b. [DOI] [PubMed] [Google Scholar]
- Radeke M. J., Misko T. P., Hsu C., Herzenberg L. A., Shooter E. M. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987 Feb 12;325(6105):593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tébar A., Dechant G., Barde Y. A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990 Apr;4(4):487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Schechter A. L., Bothwell M. A. Nerve growth factor receptors on PC12 cells: evidence for two receptor classes with differing cytoskeletal association. Cell. 1981 Jun;24(3):867–874. doi: 10.1016/0092-8674(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Soppet D., Escandon E., Maragos J., Middlemas D. S., Reid S. W., Blair J., Burton L. E., Stanton B. R., Kaplan D. R., Hunter T. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991 May 31;65(5):895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Squinto S. P., Stitt T. N., Aldrich T. H., Davis S., Bianco S. M., Radziejewski C., Glass D. J., Masiakowski P., Furth M. E., Valenzuela D. M. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991 May 31;65(5):885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Sutter A., Riopelle R. J., Harris-Warrick R. M., Shooter E. M. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979 Jul 10;254(13):5972–5982. [PubMed] [Google Scholar]
- Vale R. D., Ignatius M. J., Shooter E. M. Association of nerve growth factor receptors with the triton X-100 cytoskeleton of PC12 cells. J Neurosci. 1985 Oct;5(10):2762–2770. doi: 10.1523/JNEUROSCI.05-10-02762.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Shooter E. M. Assaying binding of nerve growth factor to cell surface receptors. Methods Enzymol. 1985;109:21–39. doi: 10.1016/0076-6879(85)09073-5. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Müller R. G., Fu X. C., Hynes N. E., Kozma S. Oncogenic activation of the human trk proto-oncogene by recombination with the ribosomal large subunit protein L7a. EMBO J. 1990 Jan;9(1):191–196. doi: 10.1002/j.1460-2075.1990.tb08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]