Abstract
Introduction
Consumption of cotoneaster may reduce neonatal jaundice.
Aim
Hence this study was undertaken to determine the effect of mothers’ cotoneaster consumption on treatment of their neonates’ jaundice.
Materials and Methods
In this randomized clinical trial study, 120 neonates with jaundice referred to a hospital in southwest Iran were enrolled by nonprobability sampling and divided randomly into four groups. In the first group both mothers and neonates received cotoneaster; in the second group only mothers; in the third group only neonates; and in the fourth group the neonates received distilled water as placebo. Phototherapy was done under the same condition for all neonates.
Results
The reduction of bilirubin was significantly higher in treatment groups compared to control group (p<0.05). Bilirubin in the group of neonates whose mothers consumed cotoneaster was less compared to control group at 24 and 36 hours (p<0.05) and the highest reduction in bilirubin was observed in the first group. The mean duration of hospitalization was longer for the control group (p<0.05).
Conclusion
Consumption of cotoneaster by both mothers and neonates caused a decrease in neonatal jaundice more rapidly compared to other groups and decreased the duration of hospitalization. Cotoneaster consumption by mothers, neonates, or both may be useful in treatment of neonatal jaundice.
Keywords: Bilirubin, Medicinal herbs, Phototherapy
Introduction
Jaundice is the most prevalent clinical difficulty of neonates which may even lead to brain damage to normal term neonates in severe cases [1,2]. The increased production of bilirubin impairment of conjugation, decrease in hepatic uptake, and increase in bilirubin enterohepatic cycle are some factors contributing to causing unconjugated hyperbilirubinaemia [3].
The preferred treatment for this condition in modern medicine is phototherapy with blue light. Transfusion exchange may be considered as the last way to reduce the bilirubin level in cases where jaundice has not shown acceptable response to other therapies [4]. Transient erythaematous rash, hyperthermia, loose stool, and blood transfusion complications including blood transmitted infections, coagulation disorders, thrombocytopenia, necrotizing enterocolitis, graft-versus-host disease, electrolyte abnormalities, portal vein thrombosis, cardiac arrhythmias, and death are some of the side effects of phototherapy [5].
Methods of traditional medicine especially consumption of medicinal herbs have been considered by some researchers [6,7]. Since ancient times, cotoneaster has been used for the treatment of neonatal jaundice in Iran. Cotoneaster is a white rather yellow and sweet substance, known as “Purgative manna”, from Rosaceae family. This plant has been mentioned in famous traditional medicine books such as “Canon of Medicine” by Avicenna, “Alhavi” by Rhazes and “Makhzan-ol-Advieh” by Aghili Khorasani [8–10]. Although this plant has been traditionally used in treating neonatal jaundice, there is a need for more scientific study on this issue. In addition, the difficulty in administration can be a concern.
Aim
This study aims to investigate the effect of cotoneaster intake by neonates and mothers on the improvement of neonatal jaundice in neonates under phototherapy.
Materials and Methods
This randomized clinical trial study was conducted on 120 exclusively breastfeeding neonates with jaundice who were hospitalized in Neonatal Unit of Hajar Hospital, Shahrekord, Iran in 2010 after obtaining the approval of Ethics Committee of Shahrekord University of Medical Sciences (87-8-3) and registering in Iran Registry of Clinical Trials (IRCT). Sampling was done by nonprobability method. Sample size was determined to compare mean bilirubin of each treatment group with control group. The sample size formula was used to compare the mean levels between two independent samples. By this formula, the sample size is determined as 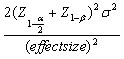 , so to detect any difference more than 0.75 standard deviation in bilirubin between each treatment group and control group, 30 patients were required in each group with 95% confidence and 80% power. Then the total sample size included 120 patients.
, so to detect any difference more than 0.75 standard deviation in bilirubin between each treatment group and control group, 30 patients were required in each group with 95% confidence and 80% power. Then the total sample size included 120 patients.
Neonates were assigned into four 30-member groups. In the first group both mothers and neonates received cotoneaster; in the second group only mothers received cotoneaster; in the third group only neonates received cotoneaster; and in the fourth group neonates received distilled water as placebo. Phototherapy was done under the same condition for all neonates under study. Phototherapy cuts had 6 lamps with the wavelength of 420-460 nm and an average age of less than 2000 hours, and the neonates’ distance from the light source was 40 cm. The duration of phototherapy was at least four days and the phototherapy was performed on the basis of indirect bilirubin levels of higher than 14 mg/dl [5].
Inclusion criteria
Infants aged 37-42 weeks including pregnancy period with the birth weight of 2.5-4 kg and the total bilirubin level of 14-20 mg/dl who were breastfed and their jaundice began after the second day of life were included. The infants had no previous jaundice in life history.
Exclusion criteria
Patients with conjugated hyperbilirubinaemia, history of jaundice, phototherapy or blood exchange for the previous child, maternal-infant blood group incompatibility, history of traditional medical treatment, any apparent anomaly in the physical examination, haematocrit levels of more than 65% or the haemoglobin levels of more than 20, and abnormal blood tests (reticulocyte count above 5%, disorder in glucose-6 phosphate dehydrogenase {G6PD}, positive Coombs test, positive blood smear with respect to spherocytosis, ovalocytosis, and elliptocytosis) that indicated pathologic jaundice or an underlying disease were excluded.
Treatment and measurements
Infants were given 3 drops/kg of Bilineaster, a cotoneaster drop (Barij Essence Pharmaceutical Co., Tehran, Iran) every eight hours and their mothers received the drops three times more than their neonates.
Total bilirubin, direct bilirubin, blood levels of G6PD, complete blood count, blood group, and Rh factor were checked at the time of admission, and direct and total bilirubin were measured at the beginning of study and 12, 24, 36, 48, and 72 hours after admission.
Statistical Analysis
The mean ± standard deviation was used for descriptive statistics. Chi-square test was used for comparing the categorical variables. The normal distribution was evaluated using Kolmogorov test. The ANOVA test was used in case of normally distributed variables; otherwise, Kruskal-Wallis test was used. The bilirubin levels at the beginning of study and 12, 24, and 36 hours after admission were normally distributed in each group so the parametric test ANOVA was used to compare to compare it between groups, and because bilirubin levels at 48 and 72 hour were non-normally distributed, the nonparametric test Kruskal-Wallis was used. The parametric repeated measures ANOVA was applied to determine whether any change exists in bilirubin during the study. Data analysis was done by SPSS and p<0.05 was considered as the level of significance.
Results
Overall 120 neonates (2 to 23-day-old) with the mean age of 3.4±6.9 days were enrolled. Forty nine neonates (40.8%) were female and the rest were male. For birth order, the infants were 1st-6th (median: 1) child of family. There was no significant difference in gender, history of hospitalization, birth weight, and weight at the time of admission among the neonates in different groups (p>0.05). Based on ANOVA, there was no statistically significant difference in total bilirubin and direct bilirubin at the time of admission among the groups (p>0.05) [Table/Fig-1].
[Table/Fig-1]:
The mean of direct and total bilirubin (mg/dl) in neonates at admission.
| Group | Direct Bilirubin | Total Bilirubin |
|---|---|---|
| Cotoneaster taken by mother and infant | 0.53 ± 0.08 | 16.64 ± 2.81 |
| Cotoneaster taken by mother | 0.54 ± 0.16 | 16.49 ± 2.44 |
| Cotoneaster taken by infant | 0.58 ± 0.21 | 16.21 ± 2.56 |
| Control group | 0.51 ± 0.17 | 15.97 ± 2.91 |
| Overall | 0.54 ± 0.16 | 16.33 ± 2.67 |
Repeated measures ANOVA showed a significant decrease in bilirubin level (p<0.05) during the study. In addition, the trend of reduction in treatment groups was different based on this test (p<0.05). ANOVA showed a significant difference in the bilirubin level among the groups at 12, 24, 36, 48 and 72 hours (p<0.05). Dunnett’s post-hoc test showed that only the bilirubin level of the first group was different from that of the control group at the 12 hours. At 24 and the 36 hours, the bilirubin level of all treatment groups was significantly lower than that of the control group and the first group had the lowest bilirubin level in this study. Kruskal-Wallis test did not show any difference among the groups at 48 and 72 hours [Table/Fig-2]. The mean duration of hospitalization was 70±21.2 hours for the control group and 35.3±15 hours for treatment groups with a significant difference (p<0.001).
[Table/Fig-2]:
Comparison of the mean neonatal bilirubin (mg/dl) in the different groups.
| Group | Interval (hour) | ||||
|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | 72 | |
| Cotoneaster taken by mother and infant | 13.95 ± 2.31 | 12.32 ± 2.09 | 11.50 ± 1.73 | 11.55 ± 0.79 | 11.20 ± 0.98 |
| Cotoneaster taken by mother | 14.7 ± 2.5 | 12.23 ± 2.07 | 11.32 ± 1.57 | 12 ± 1.23 | 11.47 ± 0.49 |
| Cotoneaster taken by infant | 13.17 ± 2.31 | 11.01 ± 1.74 | 11.14 ± 1.47 | 11.20 ± 1.12 | 10.75 ± 0.77 |
| Control group | 14.88 ± 2.65 | 13.82 ± 2.03 | 12.99 ± 1.96 | 12.06 ± 1.71 | 11.39 ± 1.55 |
| p-value | 0.03 | < 0.001 | < 0.001 | 0.59 | 0.77 |
Discussion
Jaundice is the most prevalent clinical difficulty of neonates which may even lead to brain damage to normal term neonates in severe cases. Selective treatment of jaundice is phototherapy with blue light that was used for treatment for all groups of neonates in the present study. At the beginning of this study the total bilirubin level of newborn infants was 16.33±2.66 mg/dl that showed the necessity of immediate treatment. The difference in the mean bilirubin level among the studied groups was not significant at the time of admission. However, the total bilirubin at 12, 24, and 36 hours after the treatment with cotoneaster and phototherapy in the studied groups decreased significantly compared to the control group that received placebo and phototherapy.
In addition, the range of hospitalization duration for the studied groups was between 12 and 72 (mean: 35.3±15) hours and for the control group from 12 to 96 (mean: 70±21.2) hours. The duration of hospitalization in the treatment groups was significantly shorter than that in the control group. Generally, a significant decrease was observed in bilirubin levels during the study in all groups that showed the general effect of phototherapy. The results of this study confirm the effect of cotoneaster on further reduction of bilirubin along with phototherapy. The rate of bilirubin was lower in the group that both mothers and neonates received cotoneaster. In the present study, the trend of bilirubin reduction was faster in the first two days of study compared to the next two days because higher levels of bilirubin are more affected by phototherapy and cotoneaster. The present study showed that this plant was very effective on reducing the neonatal jaundice, which is consistent with the studies of Etebari et al., and Azadbakht et al., but is inconsistent with Ahmad Shah et al., study [11–13].
To the best of our knowledge few studies have so far examined the effect of cotoneaster intake by neonates and/or mothers on the improvement of neonatal jaundice in neonates under phototherapy. Moreover, mothers also underwent the administration of cotoneaster in the present study, which led to a significant decrease in bilirubin after 24 and 36 hours. After 12 hours of receiving cotoneaster by mothers the rate of bilirubin did not decrease significantly. However, the effect on reducing bilirubin was significant after 24 and 48 hours. This issue strongly suggests the probability of excretion of cotoneaster in the breast milk; in addition, lack of appropriate blood level of cotoneaster because of administering inappropriate doses of medicine may be a reason for no effect of cotoneaster used by mothers on reducing jaundice in the first 12 hours of treatment compared to the control group, which needs more investigation. The effect mechanism of cotoneaster is not clear. The major components of cotoneaster are manitol, glucose, and sucrose [14]. The laxative effect of cotoneaster may increase the frequency of stool and hence the excretion of billirubin. But in Ahmad Shah et al., study the frequency of defecation and weight of neonates were not different between the two groups. In our study we did not determine the billirubin in stool of neonates but cotoneaster might increase the excretion of billirubin in stool without change in its frequency [13].
There are various herbal medications worldwide for treatment of neonatal jaundice. In East Asia, mixture of four plants (Artemisia capillaris, Rheum officinale, Scutellaria baicalensis root and Gardenia) is used for the treatment of neonatal jaundice. The mechanism of its effect is through the activation of hepatic receptors that cause the clearance of bilirubin [15]. In order to accurately determine the mechanism through which cotoneaster plays its role in reduction of the bilirubin, further research is needed. However, because of its consumption by mothers and its effect on neonatal jaundice, the latter is more probable.
No special side effect was observed after consumption of cotoneaster in the present study. Besides, the potential effect of cotoneaster on the neonatal jaundice showed that the use of cotoneaster by mother could reduce the bilirubin in neonates. Since mothers and physicians are worried about administering any type of medicine during the neonatal period and the rate of bilirubin in the present study reduced after consumption of cotoneaster by mothers as with its consumption by neonates, this medicine could be recommended to be taken by mothers in case of neonatal jaundice.
Limitations
Short hospital stay of the studied infants and failure to include preterm newborns, and use of phototherapy in addition to cotoneaster in case group due to ethical considerations are some of the limitations of the present work.
Conclusion
Based on the results of this study, consumption of cotoneaster leads to faster reduction of neonatal jaundice and reduces infants hospitalization period. In addition, its consumption by mothers can be useful in reduction of neonatal jaundice in neonates who are breastfed. However, more research is needed on this issue.
Acknowledgments
Authors gratefully thank the staff of Neonatal Unit of Hajar Hospital who collaborated in conducting this study, Research and Technology Department of Shahrekord University of Medical Sciences for funding (grant no. 617) this study, and all people for helping us.
Financial or Other Competing Interests
As declared above.
References
- [1].Ho NK. Understanding traditional Chinese medicine–a doctor’s viewpoint. Singapore Med J. 2001;42(10):487–92. [PubMed] [Google Scholar]
- [2].Maisels MJ, Newman TB. Kernicterus in otherwise healthy, breast-fed term newborns. Pediatrics. 1995;96(4):730–33. [PubMed] [Google Scholar]
- [3].Stevenson DK, Wong RJ, Hintz SR, Vreman HJ. The jaundiced newborn. understanding and managing transitional hyperbilirubinaemia. Minerva Pediatr. 2002;54(5):373–82. [PubMed] [Google Scholar]
- [4].Murki S, Kumar P. Blood exchange transfusion for infants with severe neonatal hyperbilirubinaemia. Semin Perinatol. 2011;35(3):175–84. doi: 10.1053/j.semperi.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [5].Kliegman RM, Behrman RE, Jenson HB, et al. Phototherapy: Nelson Textbook of Pediatrics. ed. 18. Philadelphia: Saunders; 2007. pp. 262–63. [Google Scholar]
- [6].Kappas A, Drummond GS, Henschke C, Valaes T. Direct comparison of Sn-mesoporphyrin, an inhibitor of bilirubin production and phototherapy in controlling hyperbilirubinaemia in term and near-term newborns. Pediatrics. 1995;95(4):468–74. [PubMed] [Google Scholar]
- [7].Lazar MA. East meets West: an herbal tea finds a receptor. J Clin Invest. 2004;113(1):23–25. doi: 10.1172/JCI200420661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Avicenna. Canon of Medicine: Re-printed by The Institute of Medical History, Islamic and Complementary Medicine Publication, Volume 2; 2004:314.
- [9].Rhazes MZ. Alhavi (The first Encyclopedia of Traditional Medicine): Ministry of Health and Medical Education. 1979:297–98. [Google Scholar]
- [10].Aghili Khorasani MH. Shams Ardakani MR Rahimi R, Farjadmand F (Ed.) Tehran: Tehran University of Medical Sciences; 2009. Makhzan ol Advieh (Drugs Reservoir) p. 560. [Google Scholar]
- [11].Etebari M, Ghannadi A, Jafarian-Dehkordi A, Ahmadi F. Genotoxicity evaluation of aqueous extracts of Cotoneaster discolor and Alhagi pseudalhagi by comet assay. J Res Med Sci. 2012;17:S238–S42. [Google Scholar]
- [12].Azdbakht M, Pishiva N, Mohammadi Samani S, Alinejad F. Effect of manna from cotoneaster discolour on infant jaundice (Effect on blood bilirubin level) J Med Plants. 2005 Spring;4(14):36–44. [Google Scholar]
- [13].Ahmad Shah F, Mohammadzadeh A, Amiri M, Ramazani M. Effect of cotoneaster tricolor pojark Mana on serum billirubin levels in neonates. Int J Pharmacol. 2006;2(4):455–58. [Google Scholar]
- [14].Amin G. Popular Medicinal Plants of Iran. Tehran University of Medical Sciences Press. 2005:192–93. [Google Scholar]
- [15].Huang W, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest. 2004;113(1):137–43. doi: 10.1172/JCI200418385. [DOI] [PMC free article] [PubMed] [Google Scholar]


