Abstract
Background and Aims The typical rootless linear shoots of aquatic carnivorous plants exhibit clear, steep polarity associated with very rapid apical shoot growth. The aim of this study was to determine how auxin and cytokinin contents are related to polarity and shoot growth in such plants.
Methods The main auxin and cytokinin metabolites in separated shoot segments and turions of two carnivorous plants, Aldrovanda vesiculosa and Utricularia australis, were analysed using ultra-high-performance liquid chromatography coupled with triple quad mass spectrometry.
Key Results In both species, only isoprenoid cytokinins were identified. Zeatin cytokinins predominated in the apical parts, with their concentrations decreasing basipetally, and the trans isomer predominated in A. vesiculosa whereas the cis form was more abundant in U australis. Isopentenyladenine-type cytokinins, in contrast, increased basipetally. Conjugated cytokinin metabolites, the O-glucosides, were present at high concentrations in A. vesiculosa but only in minute amounts in U. australis. N9-glucoside forms were detected only in U. australis, with isopentenyladenine-9-glucoside (iP9G) being most abundant. In addition to free indole-3-acetic acid (IAA), indole-3-acetamide (IAM), IAA-aspartate (IAAsp), IAA-glutamate (IAGlu) and IAA-glycine (IAGly) conjugates were identified.
Conclusions Both species show common trends in auxin and cytokinin levels, the apical localization of the cytokinin biosynthesis and basipetal change in the ratio of active cytokinins to auxin, in favour of auxin. However, our detailed study of cytokinin metabolic profiles also revealed that both species developed different regulatory mechanisms of active cytokinin content; on the level of their degradation, in U. australis, or in the biosynthesis itself, in the case of A. vesiculosa. Results indicate that the rapid turnover of these signalling molecules along the shoots is essential for maintaining the dynamic balance between the rapid polar growth and development of the apical parts and senescence of the older, basal parts of the shoots.
Keywords: Auxin, Aldrovanda vesiculosa, cytokinin, growth polarity, phytohormones, rootless aquatic plants, Utricularia australis
INTRODUCTION
In plants, developmental and physiological processes are controlled by signalling molecules known as phytohormones. The plant hormones cytokinins and auxins play regulatory roles in many of these processes, and the nature and timing of a plant’s responses to environmental and endogenous factors are strongly dependent on the levels of these and/or other phytohormones and the relative amounts of the different hormones (Tarkowská et al., 2014).
Natural cytokinins are N6-substituted adenine derivatives with either isoprenoid or aromatic side-chains. In higher plants, the predominant cytokinins are the isoprenoid ones – the N6-isopentenyladenine (iP) type and cis/trans-zeatin (tZ, cZ) types with unsaturated aliphatic side chains, and the dihydrozeatin (DHZ) type with saturated aliphatic side chains. Variations in the structural substituents on the adenine moieties of cytokinins include nucleotide forms, ribosides and free bases, along with a wide range of other conjugates. It is widely believed that it is the cytokinin free bases that are the bioactive cytokinins (Sakakibara, 2005). However, in cytokinin receptor binding assays, either in vitro or in in vivo heterologous expression systems, some ribosides also display high affinity for cytokinin receptors (Spíchal, 2012). These phytohormones are involved in the regulation of various processes in plant growth and development, such as shoot differentiation, apical dominance, leaf senescence, nutrient distribution in tissues and plastid differentiation (Sakakibara, 2005; Spíchal, 2012). Indole-3-acetic acid (IAA), also referred to as auxin, is another important phytohormone, which plays crucial roles in virtually every aspect of the regulation of plant growth and development, including cell division and elongation (Perrot-Rechenmann, 2010), gravitropism and phototropism, and phyllotaxis (Kuhlemeier and Reinhardt, 2001; Vernoux et al., 2000), and also in the establishment of apical–basal polarity in individual cells, organs and the whole plant (Tanaka et al., 2006; Nakamura et al., 2012).
Aquatic carnivorous plants comprise the species Aldrovanda vesiculosa (Droseraceae), which has snapping traps, and about 50 species of the genus Utricularia (Lentibulariaceae) with suction traps (Juniper et al., 1989; Taylor, 1989). They usually grow in shallow, standing, nutrient-poor dystrophic waters (Adamec, 1997a; Guisande et al., 2007). Most species of aquatic carnivorous plants have linear, poorly branched shoots and are rootless. They usually exhibit very rapid apical shoot growth (1–4 leaf nodes d−1) and high relative growth rates, while their basal shoot segments decay at the same rate as new shoots are produced (Friday, 1989; Adamec, 2000, 2008a, 2009; Englund and Harms, 2003; Adamec and Kovářová, 2006). They show adaptations associated with very rapid growth, including mineral nutrient gain from carnivory, efficient N and P re-utilization (recycling) from senescent shoots, a very high net photosynthetic rate, a high affinity for mineral nutrient uptake from the ambient water, and, in Utricularia spp., possibly also the formation of commensal associations in the traps (Kamiński, 1987; Adamec, 1997b, 2000, 2008b, 2013, 2014; Richards, 2001; Englund and Harms, 2003; Sirová et al., 2009). Aldrovanda vesiculosa and Utricularia australis are perennial plants with linear shoots that form turions (winter buds), modified shoot apices that serve as vegetative dormant storage organs, enabling the plants to survive under ice over the winter (Adamec, 1999, 2010). Both species have often been used in ecophysiological studies describing the phenomenon of physiological polarity in their shoots, from perspectives including allocation of mineral nutrients and carbohydrate content, net photosynthetic rate, chlorophyll content, allocation of organic carbon derived from carnivory, and studies on trap function (Fabian-Galan and Salageanu, 1968; Kamiński, 1987; Kosiba, 1992a, b; Adamec, 1997a, b, 2000, 2008a, b, 2009, 2011a, 2013; Adamec and Kovářová, 2006). However, no direct phytohormonal screening has hitherto been carried out in aquatic carnivorous plants.
Hence, the aim of the work presented here was to determine levels of cytokinin and auxins and their localization in polar segments of different ages in linear shoots of two aquatic carnivorous plants, A. vesiculosa and U. australis, which exhibit steep gradients of physiological polarity, and to infer where, in these rootless plants, cytokinins and auxins may be synthesized. For comparison with growing shoots, phytohormone contents were also determined in dormant turions of these species. The levels and distribution of cytokinin and IAA derivatives are interpreted in terms of their possible roles in maintaining physiological and growth polarity.
MATERIALS AND METHODS
Plant material
Around 130 adult plants of Aldrovanda vesiculosa (originating from Poland) were collected from the dystrophic (humic) fen lake Karštejn in the Třeboň Basin Biosphere Reserve in South Bohemia, Czech Republic, on 26 July 2008 (Adamec and Kovářová, 2006). The plants were 7–15 cm long with 12–15 mature leaf nodes, and usually branched. They were immediately transferred, without water, to the laboratory for processing. The plants were thoroughly washed in tap water and all larger items of captured prey were taken out of their traps. All branches were removed. The main shoots were separated into four segments for phytohormone analyses: apices with immature traps; 1st–4th leaf nodes, representing the youngest functional traps; 7th–9th leaf nodes, representing medium-aged traps; and old segments with still-living leaf nodes, which were separated from the medium-aged segments by at least two leaf nodes. Sixty adult plants of Utricularia australis were collected from the dystrophic inlet of the Ruda fishpond in the same region as above on 28 July 2008 (Adamec, 2011b). The plants were 45–90 cm long, branched, but non-flowering. After rapid transfer to the laboratory, they were thoroughly washed and deprived of all attached macroorganisms, and their branches were removed. The fact that prey remnants were not removed from the traps should not influence the results. The main shoots were separated, in a similar manner to those of A. vesiculosa, into four segments for phytohormone analyses: apices with immature traps (here denoted ‘apices’); 1st–6th leaf nodes, representing the youngest functional traps (‘young’); 21st–26th leaf nodes, representing medium-aged traps (‘medium-aged’); and old segments with still-living leaf nodes but mostly lacking traps (‘old’). Thirty mature turions of A. vesiculosa, 6–7 mm long, and 12 mature turions of U. australis, 8–11 mm long, were collected from the sites on 15 October 2008. They were washed in tap water and all dead leaves were removed from their surfaces. All separated plant material was stored at −80 °C for some weeks prior to lyophilization. The freeze-dried biomass of each shoot segment or turion was 0·5–1·0 g. Lyophilized samples were homogenized under liquid nitrogen and split into duplicates for cytokinin analysis and auxin analysis, each replicate containing 100 mg of dry weight (d.w.) material.
Cytokinin analysis
The procedure for cytokinin purification was based on the method described by Novák et al. (2003), including modifications described by Novák et al. (2008). Isotope-labelled internal standards [13C5]tZ, [2H5]tZR, [2H5]tZ9G, [2H5]tZOG, [2H5]tZROG, [2H5]tZRMP, [13C5]cZ, [2H3]DHZ, [2H3]DHZR, [2H3]DHZ9G, [2H7]DHZOG, [2H3]DHZRMP, [2H6]iP, [2H6]iPR, [2H6]iP9G, [2H6]iPRMP, [2H7]BA, [2H7]BAR, [2H7]BA9G, [2H7]BARMP, [15N4]mT and [15N4]oT (Olchemim Ltd, Czech Republic) were added, each at 1 pmol per sample, to validate the accuracy of determination (Novák et al., 2008). The samples were purified using a combination of a cation (SCX cartridge) exchanger, an anion (DEAE-Sephadex-C18 cartridge) exchanger and immunoaffinity chromatography (IAC) based on monoclonal antibodies specific for a wide range of cytokinins (Novák et al., 2003). The eluates from the IAC columns were evaporated to dryness and dissolved in 20 µL of the mobile phase used for quantitative analysis. The samples were analysed by ultra-high-performance liquid chromatography (UHPLC; AcquityUPLC® System; Waters, Milford, MA, USA) coupled to a triple quadrupole mass spectrometer (MS/MS) equipped with an electrospray interface (Quattro Micro API™; Waters, Manchester, UK). The purified samples were injected onto a C18 reversed-phase column (BEH C18; 1·7 μm; 2·1× 50 mm; Waters). The column was eluted with a linear gradient (0 min, 10 % B; 0–8 min, 50 % B; flow rate 0·25 ml min−1; column temperature 40 °C) of 15 mm ammonium formate (pH 4.0, A) and methanol (B). Quantification was achieved by multiple reaction monitoring (MRM) of [M+H]+ and the appropriate product ion. For selective MRM experiments, the optimal conditions, dwell time, cone voltage and collision energy in the collision cell corresponding to the exact diagnostic transition were optimized for each cytokinin compound (Novák et al., 2008). Quantification was performed with MassLynx software using a standard isotope dilution method. The ratio of each endogenous cytokinin to an appropriate labelled standard was determined and used to quantify the level of endogenous compounds in the original extract, according to the known quantity of internal standard added (Novák et al., 2003).
Auxin analysis
Indole-3-acetic acid and its amide conjugates were analysed by a method described by Pěnčík et al. (2009). The compounds of interest were extracted with phosphate buffer, pre-purified by C8-based solid-phase extraction and further purified by an auxin-specific immunoaffinity extraction. Final analysis was done using UHPLC–MS/MS detection. The separation was performed on an Acquity UPLC™ System (Waters) equipped with a Symmetry C18 column (5 μm, 2·1 × 150 mm; Waters) at 30 °C by gradient elution with a flow rate of 0·25 ml min−1. The mobile phase consisted of 10 mm aqueous formic acid and methanol containing 10 mm formic acid. The content of methanol was held at 25 % during the first minute then increased linearly to 38 % (at 7 min), 40 % (12 min), 58 % (15 min) and 60 % (26 min). The effluent was introduced into the ion source of a Quattro Micro API triple quadrupole mass spectrometer (Waters). The ratio of endogenous IAA and IAA conjugates to appropriate labelled standards (10 pmol per sample of [2H5]IAA, [15N,2H5]IAM, [15N,2H5]IAAla, [15N,2H5]IAAsp, [15N,2H5]IAGlu, [15N,2H5]IAGly, [15N,2H5]IALeu, [15N,2H5]IAPhe, and [15N,2H5]IAVal) was determined and used to quantify the levels of endogenous compounds in the original extract (Pěnčík et al., 2009).
RESULTS
Cytokinins
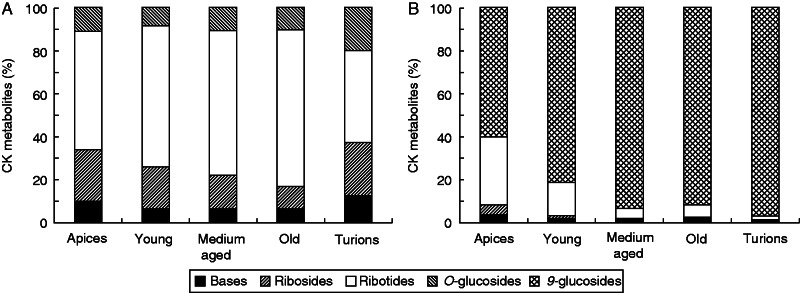
In both species, only isoprenoid cytokinins, the zeatin types (cZ, tZ, DHZ) and the iP type (Fig. 1A, B), were identified (Supplementary Data Tables S1 and S2). The zeatin-type cytokinins were dominant in the apical parts, with concentrations decreasing basipetally, whereas the iP-type cytokinins increased in the same direction (Fig. 1A, B). The cytokinin metabolites mainly responsible for these trends were cytokinin biosynthetic precursors, the ribotides, with the highest concentrations of both zeatin ribotides, cis-zeatin-9-riboside-5ʹ-monophosphate (cZR5ʹMP) and trans-zeatin-9-riboside-5ʹ-monophosphate (tZR5ʹMP), found in the apices, and the highest amount of N6-isopentenyladenosine-5ʹ-monophosphate (iPR5ʹMP) in the medium-aged and old segments (Fig. 2A, B). Interestingly, whereas in A. vesiculosa the dominant zeatin ribotide was the trans isomer (tZR5ʹMP; Fig. 2B), in U. australis it was the cis form (cZR5ʹMP; Fig. 2B). Conjugated cytokinin metabolites, the O-glucosides, were present at higher concentrations in A. vesiculosa, where they stayed at a fairly similar level throughout the whole plant. On the other hand, in U. australis the O-conjugated cytokinins were found in only minute concentrations. The N9-glucoside forms were detected only in U. australis, in which N6-isopentenyladenine-9-glucoside (iP9G) was found to be the most abundant metabolite (Fig. 3B) (Supplementary Data Table S2).
Fig. 1.
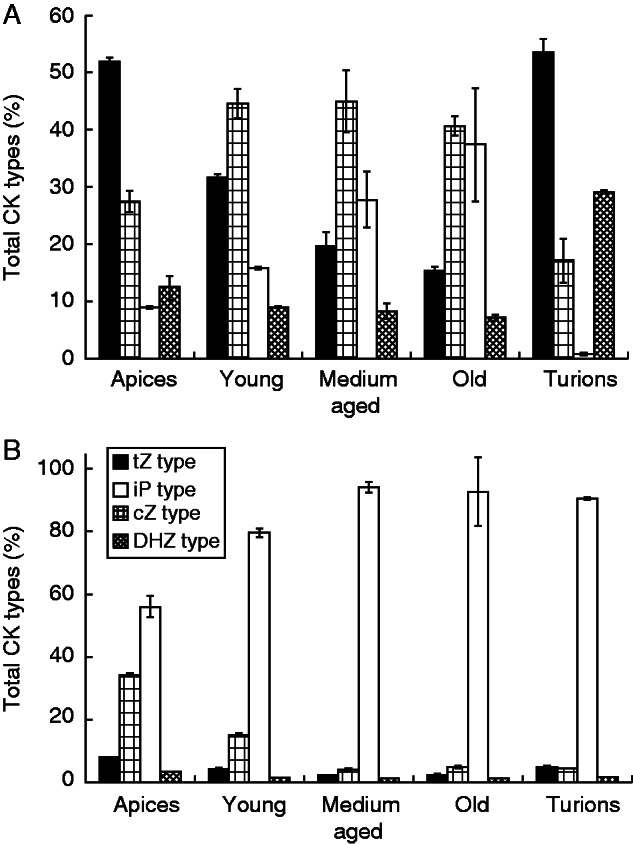
Percentages of different cytokinin (CK) types in Aldrovanda vesiculosa (A) and Utricularia australis (B) shoot segments and turions, grouped according to the structure of the N6-aliphatic side chain: trans-zeatin (tZ), cis-zeatin (cZ), dihydrozeatin (DHZ) and isopentenyladenine (iP). Error bars represent the standard deviation.
Fig. 2.
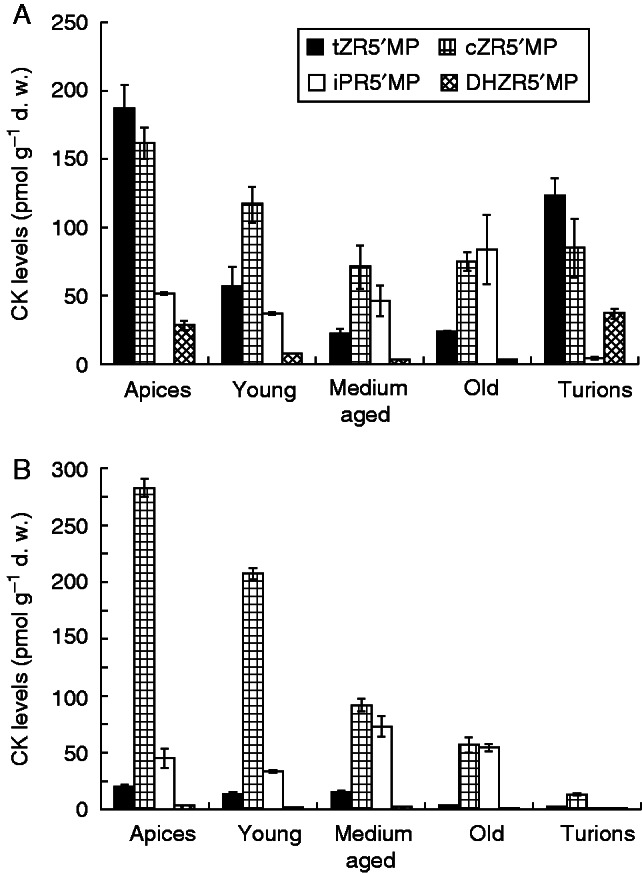
Content (pmol g−1 d.w.) of cytokinin (CK) biosynthetic precursors, the ribotides (R5ʹMP), in Aldrovanda vesiculosa (A) and Utricularia australis (B) shoot segments and turions presented according to the structure of the N6-aliphatic side chain: trans-zeatin (tZ), cis-zeatin (cZ), dihydrozeatin (DHZ), isopentenyladenine (iP). Error bars represent the standard deviation.
Fig. 3.
Composition of cytokinin (CK) metabolites (expressed as percentage) in Aldrovanda vesiculosa (A) and Utricularia australis (B) shoot segments and turions.
Turion samples from these species differed greatly in cytokinin content. In U. australis the N9-glucosides, specifically iP9G, were the predominant cytokinin metabolites (Fig. 3B, Supplementary Data Table S2). Other forms of cytokinin were present only in minute concentrations. In A. vesiculosa turions, the cytokinin spectrum was much broader, including O-glucosides and also ribotides. In comparison with U. australis turions, in A. vesiculosa higher amounts of free bases and ribosides, especially the tZ type, were found. Interestingly, no N9-glucosides were detected in A. vesiculosa turions (Fig. 3A), (Supplementary Data Table S1).
Auxins
In A. vesiculosa the most abundant metabolite from the auxin family was the amide conjugate with l-aspartic acid (IAAsp), which exceeded the concentration of free IAA, particularly in the apical part (Supplementary Data Table S3). IAA stayed at the same level of concentration throughout the whole plant (Fig. 4A). In A. vesiculosa the highest concentration of indole-3-acetamide (IAM; IAA precursor) was found in the medium-aged and old segments; however, its content showed no significant trend with respect to plant growth polarity. The amide conjugate with l-glycine (IAGly) was present only at minor levels. In U. australis the free IAA content increased basipetally, reaching a peak in the old segments (Fig. 4B). IAM and IAAsp predominated in the apex (Fig. 4B). The amide conjugate with l-glutamic acid (IAGlu) and IAGly were present only at low concentrations (Supplementary Data Table S4). No other amide conjugates with l-alanine, l-leucine, l-phenylalanine or l-valine (IAAla, IALeu, IAPhe and IAVal, respectively) were detected in A. vesiculosa or in U. australis.
Fig. 4.
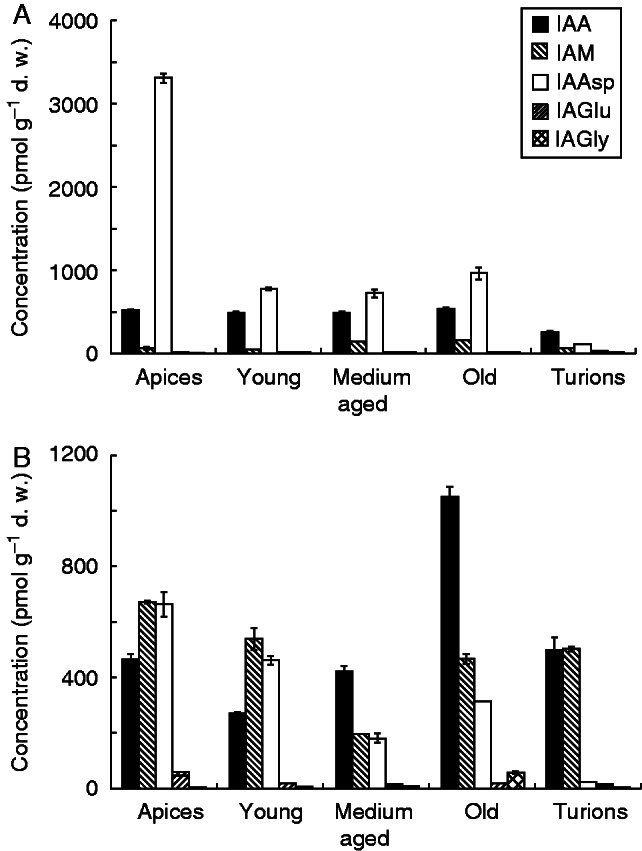
Content (pmol g–1 d.w.) of auxin metabolites in Aldrovanda vesiculosa (A) and Utricularia australis (B) shoot segments and turions. IAA, indole-3-acetic acid; IAM, indole-3-acetamide; IAAsp, IAA aspartate; IAGlu, IAA glutamate; IAGly, IAA-glycine.
Turions of both species showed high levels of free IAA, exceeding the levels of conjugated forms of IAA. In U. australis, IAM reached a level similar to that of free IAA, whereas in A. vesiculosa its content was lower compared with those of free IAA and IAAsp (Fig. 4A). The other two amide conjugates, IAGlu and IAGly, were present in both species only in minute concentrations or were not detected at all (Supplementary Data Tables S3 and S4).
DISCUSSION
In this study, we focused on the analysis of the levels and distribution of cytokinins and auxin in linear shoots of the rootless carnivorous plants A. vesiculosa and U. australis, which exhibit polar and very rapid linear growth. Plant growth and development are driven by both external environmental conditions and intrinsic growth regulators such as hormones. In this study we focused on two major phytohormone groups, the cytokinins and the auxins. Both groups can be biosynthesized in many, if not all, parts of the plant (Taiz and Zeiger, 2010). They can act as long-distance signalling substances as well as paracrine signals during plant development, and it is known that there is crosstalk between them. It has been reported that auxin regulates cytokinin levels and vice versa (Tanaka et al., 2006). For example, it was observed that cytokinin-overproducing tobacco had reduced levels of IAA, whereas overproduction of IAA in the same species led to a reduction in cytokinin content (Palni et al., 1988; Eklöf et al., 1997, 2000). On the other hand, another study showed that an elevation in cytokinin levels leads to a rapid increase in rates of auxin biosynthesis in young, developing Arabidopsis tissues, and a reduction in cytokinin levels leads to lower rates of endogenous auxin biosynthesis (Jones et al., 2010). Despite the difficulty of distinguishing between cause and effect in these experiments, it is obvious that plant developmental processes are driven by changes in hormone levels. Moreover, although an individual hormone may play a predominant role in a particular process, plant responses are likely to be influenced by multiple overlapping factors, including the presence of other hormones; tissue specific responses may depend not only on the absolute concentrations of these hormones but also on the ratios of each to the others (Nordström et al., 2004).
To our knowledge, no detailed study of the distribution of auxins and cytokinins in shoots of submerged aquatic plants has yet been published. Arthur et al. (2007) and Stirk and van Staden (2003) reported the contents of IAA and various types of cytokinins in whole shoots of the aquatic ferns Salvinia molesta and Azolla filiculoides, but the roles of these phytohormones in physiological and growth polarity have not been investigated. Winston and Gorham (1979) published results based on bioassays for gibberellins, cytokinins, auxins and abscisic acid-like activity during the formation and dormancy of turions in U. vulgaris. In this study we present cytokinin and auxin metabolite spectra throughout the rootless shoots of A. vesiculosa and U. australis.
Cytokinin biosynthesis starts with prenylation at the N6 position of an adenine nucleotide, catalysed by the enzyme isopentenyltransferase (IPT), to produce either iPR5ʹMP or tZR5ʹMP. The cZ cytokinin moiety is produced through enzymatic prenylation by tRNA-IPT of adenine residues adjacent to the anticodon in tRNA (Spíchal, 2012). The high levels of cZR5ʹMP and tZR5ʹMP in the apices in both species may indicate that these parts are the main sites of biosynthesis of the corresponding cytokinins. Nordström et al. (2004) observed that the greatest capacity for cytokinin biosynthesis in Arabidopsis is in young developing leaves with active cell division; our findings are consistent with this since in A. vesiculosa the apex is the only place where new leaves (whorls) are produced (Fig. 2A, B). Interestingly, the level of iPR5ʹMP showed a basipetal increase, reaching its peak in medium-aged segments in the case of U. australis and in old segments in A. vesiculosa. However, this increase was not as significant as in the case of zeatin ribotides. Moreover, in A. vesiculosa the higher content of iPR5ʹMP was not reflected in an increase in the active compounds, the bases (iP) and ribosides (iPR) (Supplementary Data Table S1). In U. australis we saw very large amounts of the conjugated form, iP9G, as well as an increase in active compounds (Supplementary Data Table S2). Cytokinin conjugation can be reversible in the case of the formation of O-glucosides, but it is irreversible in the case of N9-glucoside formation. Products of cytokinin conjugation serve purposes similar to those of auxins, playing roles as storage and transport forms because they are resistant to degradation by cytokinin oxidase/dehydrogenase, and because of their low, or lack of, biological activity. Thus, conjugation contributes to the regulation of cytokinin activity in plants (Bajguz and Piotrowska, 2009). In U. australis, conjugation resulted in a substantial increase in the total local cytokinin concentration in the older segments (Fig. 3B), and since very low concentrations of O-glucosides were found, the results suggest that N9-glucosylation plays an important role in the deactivation of cytokinin in U. australis. In A. vesiculosa the content of biologically active cytokinins is probably regulated differently, because almost no N9-glucosides were detected anywhere in the plant; only O-glucosides were present. Their levels showed no clear connection with possible sites of cytokinin biosynthesis, as they stayed at roughly the same level throughout the whole plant (Fig. 3A). Since the content of ribotides increased in segments further from the apex, we assume that cytokinin regulation may possibly lie at the level of biosynthesis itself. For example, in Arabidopsis thaliana the conversion of ribotides to biologically active ribosides and thence to active bases is carried out through enzymatic dephosphorylation via 5ʹ-ribonucleotide phosphohydrolase (EC 3.1.3.5) and adenosine nucleosidase (EC 3.2.2.7), respectively, or directly through cytokinin phosphoribohydrolase, known as ‘Lonely Guy’, from ribotides to the corresponding cytokinin free bases (Kurakawa et al., 2007). Negative regulation of these enzymes could lead to an increase in the contents of ribotides, as seen here (Fig. 3A). Moreover, active cytokinins in A. thaliana can also be degraded directly by cytokinin dehydrogenase (EC 1.5.99.12), as reviewed by Spíchal (2012); if such enzymatic activity also takes place in A. vesiculosa, it could explain the missing N9-glucosides.
The levels of those hormones having auxin activity are regulated by a combination of active transport, local biosynthesis, degradation and conjugation (Woodward and Bartel, 2005; Normanly, 2010). Ljung, 2013). IAA can be synthesized by the so-called l-tryptophan-dependent pathway, which is believed to be the main source of IAA in plants, and also through an l-tryptophan-independent pathway, by which, in A. thaliana, IAA is synthesized directly from indole-3-glycerol phosphate (IGP) (Ouyang et al., 2000). In our analysis we included one IAA precursor, IAM, which is an intermediate in the l-tryptophan-dependent pathway of IAA biosynthesis. Although the IAM content in the shoot of A. vesiculosa showed no pronounced trends, we can state that the presence of the compound indicates that the l-tryptophan-dependent pathway is active in this species (Fig. 4A, Supplementary Data Table S3). In U. australis, the highest IAM content was found in the apical part, although the highest IAA concentration was observed in the oldest segment. However, taking into account the high level of conjugates in the apex, we suggest that the apical part of this plant is the most active site of IAA biosynthesis (Fig. 4B, Supplementary Data Table S4). The two species exhibited different profiles of free IAA content. In A. vesiculosa, IAA was maintained at the same level throughout the whole shoot, while in U. australis the levels of free IAA differed greatly among segments, with the peak concentration being in the basal part (Fig. 4A, B); this variation in content in U. australis could be associated with the considerably more complex shoot structure and branching in this species compared with A. vesiculosa. IAA is conjugated mainly with sugars and amino acids. In general, dicotyledons mostly accumulate amide conjugates, but the formation and also the hydrolysis of conjugates are plant tissue-specific and, moreover, conjugate profiles differ significantly among plant species (Rampey et al., 2004). As with cytokinins, this conjugation can be either reversible or irreversible, and its products are involved in IAA storage and transport or form part of the auxin catabolism pathway. In general, conjugates serve as components in IAA homeostasis, detoxifying excess auxins (Bajguz and Piotrowska, 2009). Their other roles during plant development are still under investigation. In the present study, the most abundant of these compounds in both species was IAAsp. The other two conjugates that we identified, IAGlu and IAGly, were present in minute concentrations (Fig. 4A, B, Supplementary Data Tables S3 and S4).
Analysis of the apical parts of these plants has shown that the rapidly growing shoot apices, which have high cytokinin and auxin biosynthetic activity, act as a very strong physiological sink, attracting the allocation of both essential minerals and organic nutrients into the apex (Adamec 1997a, 2000), and thus promoting the growth and development of new tissues in this segment, while maintaining active meristems.
A basipetal decrease in the content of biologically active cytokinins led to a change in the cytokinin/auxin balance throughout the whole plant in favour of auxin in tissues further from the apex (Fig. 5A, B). Such a change in the cytokinin/auxin ratio may be reflected in a change in chlorophyll content, and thus in a progressive decrease in photosynthetic rate, in tissues (leaves and traps) located further from the apical part, as has been observed in A. vesiculosa (described in Adamec, 1997b), U. vulgaris, U. australis (Adamec, 2013) and U. macrorhiza (Knight, 1992).
Fig. 5.
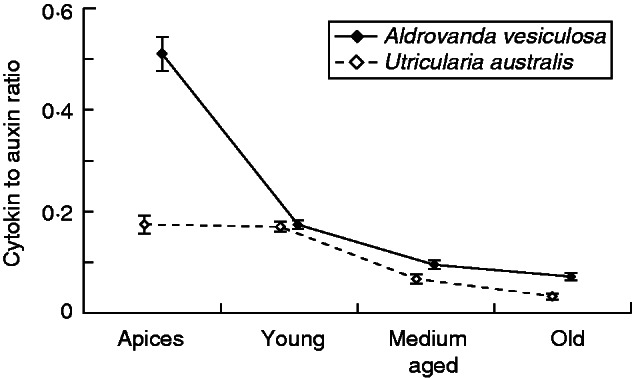
Ratio (CKs:IAA) of biologically active cytokinins (cytokinin bases and ribosides) to IAA from the apical to the basal part in Aldrovanda vesiculosa and Utricularia australis shoots.
A low content of active cytokinins is also associated with ageing processes in plants, such as maturation of leaves and traps, changes in leaf and trap function and senescence (Fabian-Galan and Salageanu, 1968; Friday, 1989; Knight, 1992; Adamec, 1997b, 2000, 2008a; Sirová et al., 2009). All of the above occur along the observed gradient of decrease in active cytokinin content, and contribute to the growth and physiological polarity of these plants (Fig. 6A, B). It should be stressed that both species in this study were collected under optimal growth conditions during a very warm, mid-summer period when the apical shoot growth rate was maximal for both species (Adamec, 2000, 2009). Therefore, the auxin and cytokinin spectra observed along the shoots of both species reflect these optimum growth conditions of maximal apical growth rate. Determination of the auxin and cytokinin spectra along the shoots of both species during the markedly slower apical growth under the sub-optimal conditions (i.e. low temperature, low light or CO2 or prey availability; Adamec, 2000, 2008b; Adamec and Kovářová, 2006) were not part of this study. However, Winston and Gorham (1979) observed that, 2 weeks before U. vulgaris turion formation and during the whole innate dormancy stage, the abscisic acid and bound gibberellin levels were high, while free gibberellin, cytokinin and auxin levels were low. It is most likely that cytokinin and auxin profiles will reflect changes in the environment and mediate the plant’s physiological responses, including turion formation.
Fig. 6.
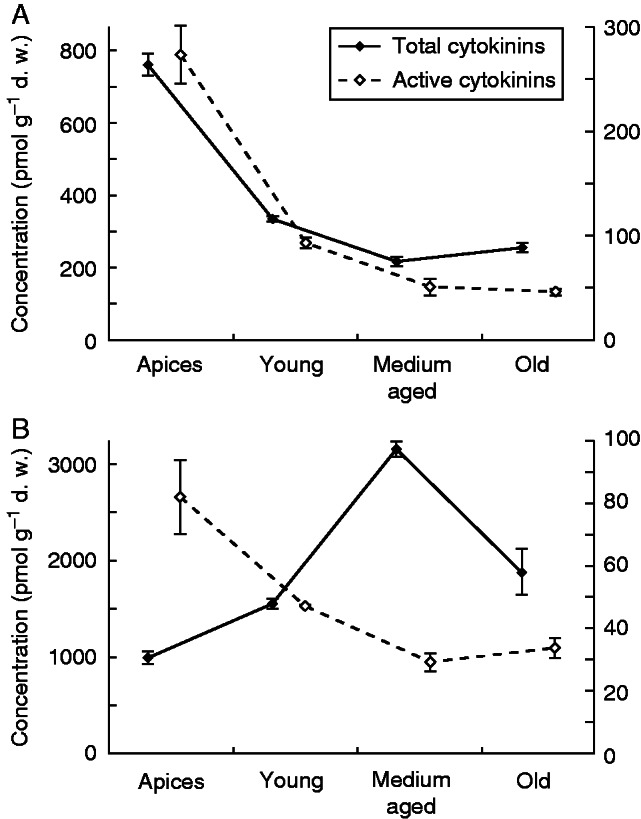
Total cytokinin content and active cytokinin content (pmol g–1 d.w.) from the apical to the basal part in Aldrovanda vesiculosa (A) and Utricularia australis (B) shoots.
Turions are vegetative dormant organs derived from the plant apex, with storage function. Formed in autumn, they accumulate starch, free sugars and also nitrogen in the form of amino acids, and survive winter on the bottom in warmer water, and at the end of winter they rise to the water surface, where they sprout (Adamec, 1999). One of the well-known effects of cytokinins is nutrient allocation, and it is interesting that mature turions from U. vulgaris had no detectable levels of cytokinins (Winston and Gorham, 1979), which corresponds also to our findings, where, in turions from U. australis >90 % of detected cytokinins were present in biologically inactive form, suggesting metabolomic dormancy of this organ. On the other hand, turions from A. vesiculosa in our study showed high contents of active cytokinins, with a similar cytokinin metabolic profile to growing apical parts collected in summer; this suggests still high metabolic activity and ongoing development, which could be explained by the different requirements of environmental conditions needed for mature A. vesiculosa turions to enter full dormancy (Adamec, 1999).
In conclusion, our analyses show that cytokinin and auxin activity, which were originally studied and described in model rooting terrestrial plant species (A. thaliana, Nicotiana tabacum), is strongly correlated with physiological aspects of the rapid and polar growth and the development of the rootless aquatic species A. vesiculosa and U. australis. Moreover, our results from the analysis of the active CKs and their metabolites combined with the results of auxin levels along the shoots point out the importance of the dynamic balance of these phytohormones, suggesting that physiological processes such as senescence are not controlled only by the presence of one particular hormone but rather through a combination of several compounds and their optimal ratio. The direct and detailed analysis of complex plant hormone metabolism is providing valuable information about the regulatory mechanisms and the roles played by phytohormone crosstalk in developmental processes such as rapid growth, flowering or formation of turions. In future studies, including more phytohormone classes in the analysis could also help us reveal interesting facts about the phylogenetic evolution of signalling pathways and/or species themselves.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: cytokinin content in extracted material of Aldrovanda vesiculosa. Table S2: cytokinin content in extracted material of Utricularia australis. Table S3: auxin content in extracted material of Aldrovanda vesiculosa. Table S4: auxin content in extracted material of Utricularia australis.
ACKNOWLEDGEMENTS
We thank Sees-editing Ltd. (UK) for the English editing of this manuscript. This study was partly supported by a Czech long-term research development project (project number RVO 67985939) (to L.A.); the Ministry of Education, Youth and Sports of the Czech Republic (National Program for Sustainability I: number LO1204, the ‘Návrat’ program LK21306) and the Internal Grant Agency of Palacký University (IGA_PrF_2016_011).
LITERATURE CITED
- Adamec L. 1997a. Mineral nutrition of carnivorous plants: a review. Botanical Review 63: 273–299. [Google Scholar]
- Adamec L. 1997b. Photosynthetic characteristics of the aquatic carnivorous plant Aldrovanda vesiculosa. Aquatic Botany 59: 297–306. [Google Scholar]
- Adamec L. 1999. Turion overwintering of aquatic carnivorous plants. Carnivorous Plant Newsletter 28: 19–24. [Google Scholar]
- Adamec L. 2000. Rootless aquatic plant Aldrovanda vesiculosa: physiological polarity, mineral nutrition, and importance of carnivory. Biologia Plantarum 43: 113–119. [Google Scholar]
- Adamec L. 2008a. Mineral nutrient relations in the aquatic carnivorous plant Utricularia australis and its investment in carnivory. Fundamental and Applied Limnology 171: 175–183. [Google Scholar]
- Adamec L. 2008b. The influence of prey capture on photosynthetic rate in two aquatic carnivorous plant species. Aquatic Botany 89: 66–70. [Google Scholar]
- Adamec L. 2009. Photosynthetic CO2 affinity of the aquatic carnivorous plant Utricularia australis (Lentibulariaceae) and its investment in carnivory. Ecological Research 24: 327–333. [Google Scholar]
- Adamec L. 2010. Tissue mineral nutrient content in turions of aquatic plants: does it represent a storage function? Fundamental and Applied Limnology 176: 145–151. [Google Scholar]
- Adamec L. 2011a. Functional characteristics of traps of aquatic carnivorous Utricularia species. Aquatic Botany 95: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamec L. 2011b. Shoot branching of the aquatic carnivorous plant Utricularia australis as the key process of plant growth. Phyton 51: 133–148. [Google Scholar]
- Adamec L. 2013. A comparison of photosynthetic and respiration rates in six aquatic carnivorous Utricularia species differing in morphology. Aquatic Botany 111: 89–94. [Google Scholar]
- Adamec L. 2014. Different reutilization of mineral nutrients in senescent leaves of aquatic and terrestrial carnivorous Utricularia species. Aquatic Botany 119: 1–6. [Google Scholar]
- Adamec L, Kovářová M. 2006. Field growth characteristics of two aquatic carnivorous plants, Aldrovanda vesiculosa and Utricularia australis. Folia Geobotanica 41: 395–406. [Google Scholar]
- Arthur GD, Stirk WA, Novak O, Hekera P, van Staden J. 2007. Occurrence of nutrients and plant hormones (cytokinins and IAA) in the water fern Salvinia molesta during growth and composting. Environmental and Experimental Botany 61: 137–144. [Google Scholar]
- Bajguz A, Piotrowska A. 2009. Conjugates of auxin and cytokinin. Phytochemistry 70: 957–969. [DOI] [PubMed] [Google Scholar]
- Eklöf S, Åstot C, Blackwell J, Moritz T, Olsson O, Sandberg G. 1997. Auxin-cytokinin interactions in wild-type and transgenic tobacco. Plant Cell Physiology 38: 225–235. [Google Scholar]
- Eklöf S, Åstot C, Sitbon F, Moritz T, Olsson O, Sandberg G. 2000. Transgenic tobacco plants co-expressing Agrobacterium iaa and ipt genes have wild-type hormone levels but display both auxin- and cytokinin-overproducing phenotypes. Plant Journal 23: 279–284. [DOI] [PubMed] [Google Scholar]
- Englund G, Harms S. 2003. Effects of light and microcrustacean prey on growth and investment in carnivory in Utricularia vulgaris. Freshwater Biology 48: 786–794. [Google Scholar]
- Fabian-Galan G, Salageanu N. 1968. Considerations on the nutrition of certain carnivorous plants (Drosera capensis and Aldrovanda vesiculosa). Revue Roumaine de Biologie Série Botanique 13: 275–280. [Google Scholar]
- Friday LE. 1989. Rapid turnover of traps in Utricularia vulgaris L. Oecologia 80: 272–277. [DOI] [PubMed] [Google Scholar]
- Guisande C, Granado-Lorencio C, Andrade-Sossa C, Duque SR. 2007. Bladderworts. Functional Plant Science and Biotechnology 1: 58–68. [Google Scholar]
- Jones B, Gunneras SA, Petersson SV, Tarkowski P. et al. 2010. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 22: 2956–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. 1989. The carnivorous plants. London, UK: Academic Press. [Google Scholar]
- Kamiński R. 1987. Studies on the ecology of Aldrovanda vesiculosa L. I. Ecological differentiation of A. vesiculosa population under the influence of chemical factors in the habitat. Ekologia Polska 35: 559–590. [Google Scholar]
- Kosiba P. 1992a. Studies on the ecology Utricularia vulgaris L. I. Ecological differentiation of Urticularia vulgaris L. population affected by chemical factors of the habitat. Ekologia Polska 40: 147–192. [Google Scholar]
- Kosiba P. 1992b. Studies on the ecology of Utricularia vulgaris L. II. Physical, chemical and biotic factors and the growth of Utricularia vulgaris L. in cultures in vitro. Ekologia Polska 40: 193–212. [Google Scholar]
- Knight SE. 1992. Costs of carnivory in the common bladderwort, Utricularia macrorhiza. Oecologia 89: 348–355. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C, Reinhardt D. 2001. Auxin and phyllotaxis. Trends in Plant Science 6: 187–189. [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, et al. 2007. Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445: 652–655. [DOI] [PubMed] [Google Scholar]
- Ljung K. 2013. Auxin metabolism and homeostasis during plant development. Development 140: 943–950. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Kiefer CS, Grebe M. 2012. Planar polarity, tissue polarity and planar morphogenesis in plants. Current Opinion in Plant Biology 15: 593–600. [DOI] [PubMed] [Google Scholar]
- Nordström A, Tarkowski P, Tarkowská D. et al. 2004. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proceedings of the National Academy of Sciences of the USA 101: 8039–8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normanly J. 2010. Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harbor Perspectives in Biology 2: a001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák O, Tarkowski P, Tarkowská D, Doležal K, Lenobel R, Strnad M. 2003. Quantitative analysis of cytokinins in plants by liquid chromatography–single-quadrupole mass spectrometry. Analytica Chimica Acta 480: 207–218. [Google Scholar]
- Novák O, Hauserová E, Amakorová P, Doležal K, Strnad M. 2008. Cytokinin profiling in plant tissues using ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Phytochemistry 69: 2214–2224. [DOI] [PubMed] [Google Scholar]
- Ouyang J, Shao X, Li J. 2000. Indole-3-glycerol phosphate, a branchpoint of indole-3-acetic acid biosynthesis from the tryptophan biosynthetic pathway in Arabidopsis thaliana. Plant Journal 24: 327–334. [DOI] [PubMed] [Google Scholar]
- Palni LMS, Burch L, Horgan R. 1988. The effect of auxin concentration on cytokinin stability and metabolism. Planta 174: 231–234. [DOI] [PubMed] [Google Scholar]
- Pěnčík A, Rolčík J, Novák O. et al. 2009. Isolation of novel indole-3-acetic acid conjugates by immunoaffinity extraction. Talanta 80: 651–655. [DOI] [PubMed] [Google Scholar]
- Perrot-Rechenmann C. 2010. Cellular responses to auxin: division versus expansion. Cold Spring Harbor Perspectives in Biology 2: a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampey RA, LeClere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B. 2004. A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiology 135: 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JH. 2001. Bladder function in Utricularia purpurea (Lentibulariaceae): is carnivory important? American Journal of Botany 88: 170–176. [PubMed] [Google Scholar]
- Sakakibara H. 2005. Cytokinin biosynthesis and regulation. Vitamins and Hormones 72: 271–287. [DOI] [PubMed] [Google Scholar]
- Sirová D, Borovec J, Černá B, Rejmánková E, Adamec L, Vrba J. 2009. Microbial community development in the traps of aquatic Utricularia species. Aquatic Botany 90: 129–136. [Google Scholar]
- Spíchal L. 2012. Cytokinins - recent news and views of evolutionally old molecules. Functional Plant Biology 39: 267–284. [DOI] [PubMed] [Google Scholar]
- Stirk WA, van Staden J. 2003. Occurrence of cytokinin-like compounds in two aquatic ferns and their exudates. Environmental and Experimental Botany 49: 77–85. [Google Scholar]
- Taiz L, Zeiger E. 2010. Plant physiology, 5th edn. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H. 2006. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant Journal 45: 1028–1036. [DOI] [PubMed] [Google Scholar]
- Tarkowská D, Novák O, Floková K. et al. 2014. Quo vadis plant hormone analysis? Planta 240: 55–76. [DOI] [PubMed] [Google Scholar]
- Taylor P. 1989. The genus Utricularia: a taxonomic monograph (Kew Bulletin Additional Series XIV). London, UK: HMSO. [Google Scholar]
- Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J. 2000. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127: 5157–5165. [DOI] [PubMed] [Google Scholar]
- Winston RD, Gorham PR. 1979. Roles of endogenous and exogenous growth regulators in dormancy of Utricularia vulgaris. Canadian Journal of Botany 57: 2750–2759. [Google Scholar]
- Woodward AW, Bartel B. 2005. Auxin: regulation, action, and interaction. Annals of Botany 95: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.