Abstract
Background and Aims Seedling herbivory is an important factor underlying plant community diversity and structure. While considerable research has characterized seedling defence in terms of resistance, very little is known about seedling tolerance of herbivory. Moreover, few studies have attempted to identify mechanisms of tolerance across a range of plant species.
Methods Seedling tolerance of simulated herbivory was tested in a diverse pool of ten Hawaiian plant species, including several lobeliad species (family Campanulaceae), a grass, a herb and common woody trees and shrubs. Tolerance was measured as the relative survival and growth of damaged plants receiving 50 % defoliation with simultaneous jasmonic acid application compared with undamaged control plants, assessed 1·5 and 5 weeks after damage. Putative mechanisms of tolerance were measured, including photosynthetic parameters, light use efficiency, and biomass allocation reflecting growth priorities, and analysed using species-level regression analyses on tolerance indices.
Key Results No species fully tolerated 50 % defoliation at either harvest date, and simulated herbivory significantly reduced shoot as well as root biomass. Lobeliad species had particularly low tolerance. Species varied considerably in size, biomass allocation parameters and their constitutive (pre-damage) and induced (post-damage) photosynthetic parameters. However, only constitutive levels of non-photochemical quenching were significantly related to tolerance, indicating that species with more efficient light use (and less heat dissipation) are better at tolerating damage than species with high levels of heat dissipation.
Conclusions Native Hawaiian plants expressed low tolerance to a conservative level of simulated herbivory. Root growth decreased in response to damage, but this was not associated with greater tolerance, suggesting this response may be due to allocation constraints following defoliation and not due to adaptive plasticity. Conservation of native island plants threatened by invasive herbivores should prioritize protection for seedlings for improved regeneration and the persistence of native plants in disturbed habitats.
Keywords: Plant defence, island ecology, pulse-amplitude fluorometry, root/shoot ratio, plant allocation, compensation, phenotypic plasticity, non-photochemical quenching
INTRODUCTION
Seedling herbivory is common and widespread, contributing to recruitment-driven patterns in plant diversity and driving the evolution of defence traits that enhance seedling performance (Barton and Hanley, 2013). Despite considerable evidence that seedling defence differs from that of older ontogenetic stages (Boege and Marquis, 2005; Barton and Koricheva, 2010), there remain some important gaps in our knowledge about the mechanisms underlying seedling defence. In particular, very little is known about seedling tolerance, with examples of species demonstrating both weak (Weltzin et al., 1998; Lucas et al., 2013; Hoque and Avila-Sakar, 2015) and strong (Thomson et al., 2003; Salgado-Luarte and Gianoli, 2012; Barton, 2013) recovery following seedling herbivory. Because allocating resources to growth has a high priority and because escape is common for seedlings due to their small size and the unpredictability of herbivores finding them, it is likely that many plants forego resistance during seedling establishment, and indeed the production of secondary compounds is generally lower in seedlings compared with juvenile and mature stages (Barton and Koricheva, 2010; Hanley et al., 2013). Tolerance may thus be particularly important for seedlings, providing cost savings and maximizing fitness for herbivory they cannot avoid.
Tolerance is broadly defined as the maintenance of fitness in damaged versus undamaged plants and can be measured by comparing some aspect of performance, such as survival, growth, and reproduction, of damaged and undamaged plants (Strauss and Agrawal, 1999; Simms, 2000). When damaged plants have levels of fitness similar to those of undamaged plants, full tolerance has been expressed. Often, damaged plants have higher mortality, are smaller, or produce fewer seeds, reflecting low tolerance. Because tolerance is a relative measure, assessed from damaged versus undamaged plant comparisons, and because it depends on the type and amount of damage, it can be difficult to generalize tolerance among species and across studies (Haukioja and Koricheva, 2000; Nykänen and Koricheva, 2004; Massad, 2013). Moreover, for seedling tolerance, particularly of woody plants, it is not feasible to measure lifetime fitness. Nonetheless, survival and growth following damage are important aspects of performance during seedling establishment when size is a key determinant of competitive outcomes (Connolly and Wayne, 1996). Seedlings that die in response to herbivory or fail to grow as big as or bigger than competitors will fail to establish, and their fitness will be zero (Hanley et al., 1995; Hanley and Sykes, 2009). Survival and growth are thus meaningful fitness metrics for seedlings and can be used to quantify tolerance to damage (Hanley and May, 2006; Hanley and Fegan, 2007; Hanley, 2012).
Like induced resistance, tolerance is expressed following herbivory. Although there is no clear consensus on the most important mechanisms driving herbivory tolerance (Tiffin, 2000; Fornoni, 2011), changes in photosynthesis (Thomson et al., 2003; Fang et al., 2006; Barton, 2013), mobilization of stored reserves (Armstrong and Westoby, 1993; Bossdorf et al., 2004; Gruntman and Novoplansky, 2011; Latzel et al., 2011) and phenological shifts to earlier flowering (Freeman et al., 2003; Barton, 2013) are the traits most commonly associated with tolerance. Only a subset of these mechanisms pertain to seedlings, given their small storage tissues, minimal bud bank and lack of flowering potential.
Recent evidence reveals that seedling source–sink dynamics are particularly important for seedling tolerance, and that herbivore-induced shifts in biomass allocation are distinct from those of older plants, shedding light on why seedling tolerance differs from that of older ontogenetic stages (Gruntman and Novoplansky, 2011; Barton, 2013). Seedlings lack the substantial storage tissues that are common in mature perennial plants with well developed root systems and stem storage. What little storage seedlings do have is found in cotyledons or roots, and there is clear evidence that these tissues can enhance tolerance to seedling herbivory (Hanley and Fegan, 2007; Myers and Kitajima, 2007; Barton, 2013; Hoque and Avila-Sakar, 2015). Nonetheless, these stored reserves are small, and quickly depleted when source tissues are reduced by leaf herbivory (Myers and Kitajima, 2007; Willaume and Pages, 2011). Moreover, the translocation of newly fixed carbohydrates is often reduced to seedling roots following herbivory, reflecting a growth priority of new leaves to replace those lost to herbivores (Schmidt et al., 2015). As a consequence of this shift in source–sink dynamics to favour shoots, seedling root growth rates decrease following herbivory, leading to smaller root biomass in damaged versus undamaged control plants (Barton, 2008; Yoshizuka and Roach, 2011; Lucas et al., 2013; Schmidt et al., 2015). This response is very different from what has been observed in older juvenile and mature plants, which often increase root biomass following herbivory due to the sequestration of resources belowground as a strategy to escape further losses to herbivores (Schwachtje et al., 2006; Orians et al., 2011; Rivera-Solis et al., 2012).
While evidence has accumulated for herbivore-induced decreases in root growth of seedlings, it remains unclear whether this response is a mechanism of tolerance that enhances seedling performance or whether it merely reflects a source–sink-driven constraint due to the minimal storage in these small plants. To test whether decreases in root growth are mechanisms of tolerance, genotypic regressions are the most useful approach (Fornoni, 2011). Generally, genotypic regressions are conducted on genotype-level means to test whether the trait of interest explains variation among genotypes in their level of tolerance (Muola et al., 2010; Barton, 2013). While intraspecific genotypic regressions are useful for explaining tolerance at the species level, it is also desirable to assess broader patterns across species. In this case, a similar approach of regression analyses on species-level means of the potential mechanistic trait and tolerance can be used (Bloor and Grubb, 2003; Myers and Kitajima, 2007).
I examined seedling tolerance to simulated herbivory in a range of native Hawaiian plant species, including a grass, a herb and several shrubs and small trees (Table 1). Although many herbivore guilds are absent from the native Hawaiian fauna (such as mammals, reptiles and slugs), other guilds were historically very abundant, including insects and large flightless ducks (Carlquist, 1970). Invasive herbivores are currently very abundant and pose serious threats to native plants, largely due to their selective damage of native seedlings (Joe and Daehler, 2008; Drake and Hunt, 2009; Nogueira-Filho et al., 2009). Thus, native Hawaiian plants have likely experienced strong selection pressure for seedling defences, although the agents of selection have clearly shifted from native to non-native herbivores, a potentially important shift if there is specificity in herbivory tolerance, as has been found in other plant species (Carrillo et al., 2014; Moreira et al., 2015). To avoid this complication, herbivory was simulated with mechanical defoliation to mimic herbivory. Because mechanical defoliation alone does not induce responses (including both resistance and tolerance traits) of the same magnitude as real herbivory (Korpita et al., 2013; Barton, 2016), plants receiving simulated herbivory were also sprayed with jasmonic acid. Mechanical damage plus jasmonic acid treatment more closely mimics herbivory, inducing full responses in plants (van Kleunen et al., 2004; Barton, 2013; Hoan et al., 2014). Tolerance was quantified in terms of seedling survival and growth, important early components of fitness, and was calculated as the relative difference in survival and growth of undamaged control and damaged plants. Although low seedling survival and growth may be more detrimental to the population dynamics of herbs than long-lived woody plants (Silvertown et al., 1993), comparing a diverse species pool with varying life-history strategies allowed me to test whether there are universal traits underlying seedling tolerance to damage. Several potential mechanisms of seedling tolerance in native Hawaiian plants were examined, including photosynthetic parameters relating to light absorption and efficiency, and biomass allocation to roots; both constitutive (before damage) and induced (after damage) levels of traits were tested (Hochwender et al., 2012; Barton, 2013).
Table 1.
Hawaiian species tested for tolerance to simulated herbivory imposed as 50 % defoliation with simultaneous jasmonic acid application. Lobeliad species (family Campanulaceae) are listed first. Species are described with respect to phylogeny, growth form, and habitat type
| Species | Family | Growth form | Habitat type |
|---|---|---|---|
| Clermontia hawaiiensis | Campanulaceae | Shrub, small tree | Mesic and wet forest (mid to high elevation) |
| Clermontia kakeana | Campanulaceae | Shrub | Mesic forest (mid to high elevation) |
| Clermontia oblongifolia | Campanulaceae | Shrub, small tree | Wet forest (high elevation) |
| Cyanea angustifolia | Campanulaceae | Shrub | Mesic and wet forest (mid to high elevation) |
| Lobelia hypoleuca | Campanulaceae | Shrub | Mesic and wet forest (high elevation) |
| Eragrostis grandis | Poaceae | Grass, perennial | Moist and wet slopes of mesic and wet forest |
| Kadua affinis | Rubiaceae | Shrub, small tree | Mesic and wet forest (mid to high elevation) |
| Metrosideros polymorpha | Myrtaceae | Tree | Dry, mesic and wet forests (mid and high elevation) |
| Peperomia blanda | Piperaceae | Herb, perennial | Rocks and cliffs in dry to mesic forest |
| Pipturus albidus | Urticaceae | Shrub, small tree | mesic and wet forest (mid and high elevation) |
MATERIALS AND METHODS
Study system
Seeds for all species were provided by the Lyon Arboretum Seed Conservation Laboratory from collections made in Manoa Valley or private gardens on Oahu. Seeds were in storage from 1 to 15 years prior to the study. Although frequencies and intensities of seedling herbivory are not documented for these ten species, they are distributed in wet and mesic forests where native insects were historically abundant and where non-native molluscs are common today (Carlquist, 1970; Joe and Daehler, 2008). In controlled choice tests, non-native snails and slugs have shown variable preferences for two of these species (Metrosideros polymorpha and Pipturus albidus), as well as four other native and non-native Hawaiian species, defoliating juvenile plants between 5 and 100 %. As a starting point, an intermediate level of defoliation (50 %) was selected for this experiment to provide a consistent and reasonable level of damage across the focal species, irrespective of their potential differences in resistance (and thus palatability to herbivores). Because herbivores may often damage seedlings at much higher intensities (Hanley et al., 1995, 1996b; Weltzin et al., 1998; Joe and Daehler, 2008), this study provides a conservative test for seedling tolerance to damage. Future research on field populations could provide more specific information about contemporary levels of herbivory for these plant species, but unfortunately it is impossible to determine historical herbivore intensities due to the extinction of most native herbivores in Hawaii.
Experimental design
In 2013, seeds were removed from storage at the Seed Laboratory and sown in germination trays filled with equal-parts Promix BX (65–75 % Canadian sphagnum peat moss, perlite, dolomitic and calcitic limestone, macro- and micronutrients, Glomus intraradices mycorrhizae inoculum) and black cinder with a top layer of sphagnum moss to prevent very small seeds from falling below the soil surface. Because of their small seed size, the lobeliad species were also germinated on agar in sterile Petri dishes in the laboratory. Germination rates were very high both in Petri dishes and on soil, and seedlings were transplanted from both sources within a week of germination into 540-mL pots filled with equal-parts Promix BX and black cinder with a single application of slow-release fertilizer (Osmocote®). Seeds of all species were sown in May 2013, but due to interspecific variation in germination timing, species were transplanted at different times, undergoing experimental treatments at various times over the next 5 months.
At the time of transplant, seedlings were randomly assigned to three treatment groups: (1) ‘pre-harvest’ plants to be harvested at the time of the herbivory treatment for measuring pre-damage traits that enhance tolerance (constitutive mechanisms of tolerance); (2) ‘damage’ plants that received simulated herbivory with 50 % defoliation by removing half of all leaves with scissors and were sprayed with 0·5 mM jasmonic acid solution prepared with distilled water (Rasmann et al., 2009); and (3) ‘control’ plants that were sprayed with distilled water at the time when simulated herbivory treatments were made. Damage treatments were applied at the two to four true leaf ontogenetic stage. Ten days after damage, half of all damage and control plants were harvested; these plants are referred to as ‘early harvests’. Five weeks after damage, the remaining plants in both groups were harvested (‘late harvests’). Two harvest times allow temporary shifts in growth and biomass allocation to be detected and provide the means for testing how initial changes in growth influence longer-term tolerance. At all three harvest times (pre-, early and late), shoot and roots were extracted from pots and cleaned of potting media, then oven-dried at 60 °C to constant weight. Dry biomass was measured to the nearest 0·01 mg with an analytical balance. Sample sizes ranged from 5 to 15 plants per treatment × harvest group, varying among species due to differences in germination rates. The total sample size was n=405 seedlings.
To test whether damaged plants induced changes in their photosynthesis as a mechanism of tolerance, pulse-amplitude modulated chlorophyll fluorometry (Junior PAM; Walz) was used on damaged and control plants 2 d following simulated herbivory treatments. Photosynthesis was measured on the youngest fully expanded leaf during peak photosynthetic times, i.e. 0900–1300 Hawaii-Aleutian Standard Time (Geiger and Servaites, 1994). PAM fluorometry measures the fluorescence generated by plants absorbing light for photosynthesis to produce light saturation curves, as well as the heat dissipation when excess light has been absorbed. PAM light saturation curves produce several photosynthetic variables, including: photosynthetic light efficiency (also called potential quantum yield; Y0), which represents the proportion of light that a plant absorbs of the total light available; α,which is the slope of the light-limited part of the curve; the maximum photosynthetic electron transport rate under saturating light (ETRmax), which correlates linearly with net photosynthesis under most environmental conditions (Genty et al., 1989; Beer and Axelsson, 2004; Pasquini and Santiago, 2012); the light saturation parameter (Ek); and the amount of excess light that is dissipated as heat (non-photochemical quenching, NPQ), a measure of photoprotection in leaves. PAM fluorometry has the advantage of producing photosynthetic data much faster than gas exchange methods (1–2 versus 12–15 min per sample) and has been broadly used for studies of algae and terrestrial plants (Genty et al., 1989; Baker, 2008), including previous studies on herbivore induction and tolerance (Thomson et al., 2003; Barton, 2013). In addition to photosynthesis, chlorophyll content was measured using a Minolta SPAD-502 chlorophyll meter. Due to their small size, photosynthetic data are missing for some of the smallest seedlings.
Statistical analyses
Data were analysed using SAS for Windows version 9·2 (SAS Institute, Cary, NC). Residuals were examined, and shoot and root biomass were log-transformed to meet assumptions of normality and homoscedasticity. Species were analysed together in order to test for species differences in tolerance and induced responses in photosynthesis and growth. Shoot and root biomass were each analysed with linear mixed models in PROC MIXED, with the main fixed effects of damage and harvest time, and species nested within plant family and interactions with species as random factors. Significance of factors was assessed in a step-down approach in which likelihood ratio tests were conducted on maximum likelihood values for fixed effects and restricted maximum likelihood values for random effects using a χ2 distribution with 1 degree of freedom (West et al., 2014). The covariance structure used was variance components, and degrees of freedom were calculated using the Kenward–Roger method (Kenward and Roger, 1997). Significant effects of damage in which control plants are significantly larger than damaged plants will reveal low tolerance of seedlings. A null effect of damage would indicate similar size of damaged and control plants, reflecting full tolerance. A significant interaction between damage and time would reveal that the degree of tolerance changes over time; I predict an increase in tolerance for plants harvested late compared with those harvested early as damaged plants catch up in size with control plants (Barton, 2008, 2013). A significant interaction between species and damage would indicate significant variation among species in their damage tolerance. If damage (and species × damage) significantly influences root size, this reflects an indirect effect of damage whereby simulated herbivory on leaves alters root growth.
Photosynthetic variables were analysed with linear mixed models similar to those described for shoot and root biomass. Significant effects of damage reveal induced changes in photosynthesis, and a significant interaction between damage and species reflects variation among species in their induction of photosynthesis.
To test whether specific plant traits act as mechanisms of tolerance, regression analyses were conducted with species as the unit of replication using PROC REG. Species-level means were calculated for a tolerance index as the ratio of the mean total biomass damaged/control plants (Strauss and Agrawal, 1999) from the late harvest, and for all putative mechanisms of tolerance, including ‘constitutive mechanisms’ quantified using plants harvested at the time damage was inflicted on plants (total biomass, root/shoot ratio) as well as ‘induced mechanisms’ calculated as the ratio between damaged/control plants for root biomass of plants harvested early. Species-level means were also calculated for photosynthetic variables such that means of control plants were treated as constitutive traits and the ratio of damaged/control plant means were treated as induced traits. Regression analyses were conducted with n=7–10; there were missing data for species with low sample sizes (which did not include a pre-harvest control for constitutive biomass data) or very small leaves (which could not be measured for photosynthesis). Significant positive relationships would indicate mechanisms of tolerance whereby traits enhance tolerance.
RESULTS
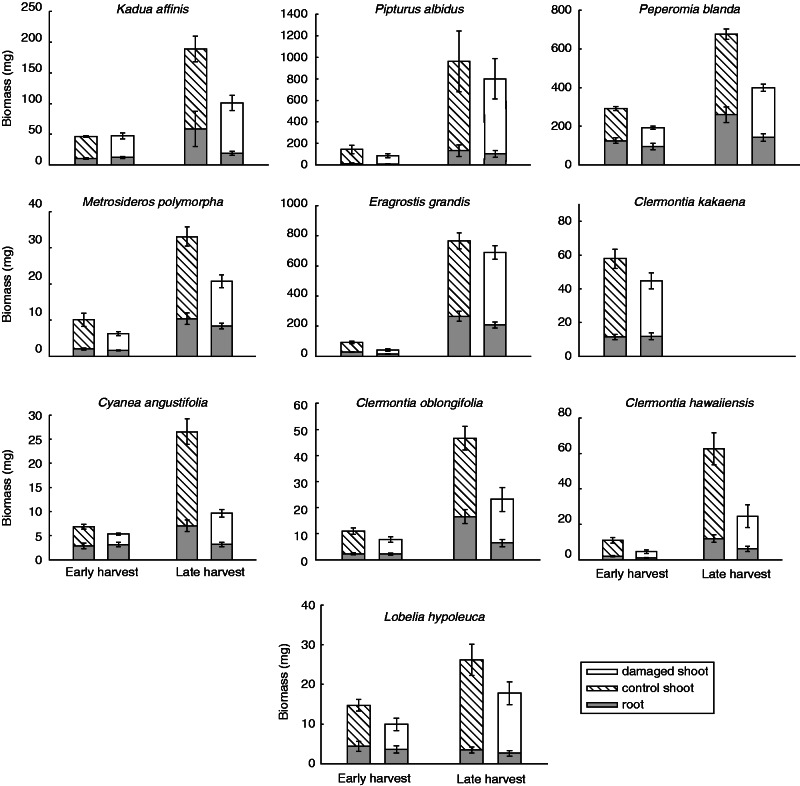
Seedling survival was 100 % for most species, with all instances of mortality occurring before damage treatments were even applied. In terms of growth, seedlings did not fully tolerate simulated herbivory, with significant reductions in both shoot and root biomass in damaged plants compared with control plants (Table 2, Fig. 1). This pattern persisted through both harvest times, leading to a non-significant damage × time interaction (Table 2), although clearly plants grew considerably during the intervening 3 weeks (significant harvest time effect; Table 2). Species varied by over an order of magnitude in size, an effect that was amplified over time (highly significant species and species × time effects; Table 2, Fig. 1). However, damage effects were consistent across species, revealing that all species expressed low tolerance after 50 % defoliation and jasmonic acid application (non-significant species × damage effect; Table 2). Interestingly, effects of damage, harvest time, species and species × time were significant for both shoot and root biomass, revealing that removing above-ground biomass has strong cascading effects on belowground biomass. For all species, damage treatments reduced root biomass, an effect that persisted through the late harvest 5 weeks after damage (Fig. 2).
Table 2.
Summary of mixed model ANOVA results for dried shoot and root biomass in response to damage to simulate herbivory (50 % defoliation with simultaneous jasmonic acid application), harvest time (early versus late), species nested within family, and all interactions retained in the model using a step-down model-building approach. Factors were assessed using likelihood ratio tests with a χ2 distribution with 1 degree of freedom based on maximum likelihood values for fixed factors (F) and restricted maximum likelihood values for random factors (R). P-values are reported in parentheses and significant effects are in bold. Shoot and root biomass data were log-transformed prior to analysis
| Factors | Shoot biomass (g) | Root biomass (g) |
|---|---|---|
| (F) Damage | 14·5 (0·00014) | 9·6 (0·0019) |
| (F) Harvest time | 22·8 (<0·0001) | 7·9 (0·0049) |
| (F) Damage × harvest time | 0·5 (0·4795) | 2·3 (0·1293) |
| (R) Species (family) | 17·6 (<0·0001) | 8·7 (0·0031) |
| (R) Species × damage | 2·9 (0·0885) | 0·0 (1·0000) |
| (R) Species × harvest time | 43·1 (<0·0001) | 73·5 (<0·0001) |
| (R) Species × damage × harvest time | 0·3 (0·5838) | 0·5 (0·4795) |
Fig. 1.
Biomass allocation patterns for control and damaged (receiving 50 % defoliation with simultaneous jasmonic acid application) plants from early (1·5 weeks) and late (5 weeks) harvest times. Species differ in the numerical range for biomass (y-axis) due to interspecific variation in plant size. Bars are means ± 1 s.e.
Fig 2.
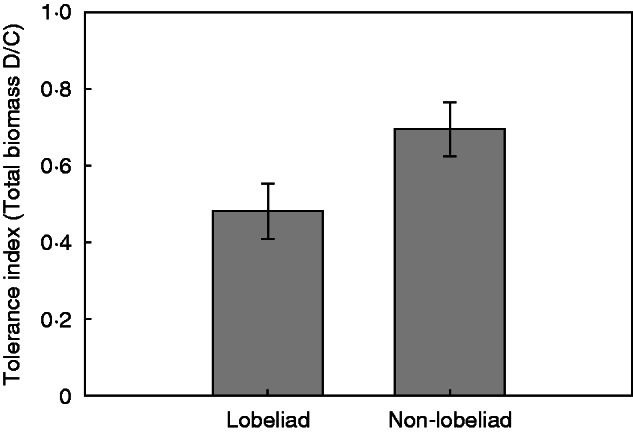
Mean levels of tolerance, quantified as the ratio of damaged/control plant means, for lobeliad species in the Campanulaceae family compared with all other species. The data are species-level means, with n = 4 for lobeliads and n = 5 for non-lobeliads. Bars are means ± 1 s.e.
Although species did not vary significantly in damage tolerance, a trend appeared suggesting that lobeliads have lower tolerance than the other species examined (Figure 1). To test this, a univariate ANOVA with phylogeny (lobeliad versus other families) as the main factor with species-level means for the tolerance index as the unit of replication was conducted. Indeed, lobeliad species did have a lower tolerance index on average than do the other species examined (Fig. 2), a difference that was marginally significant (F1,7 = 4·36, P = 0·0751).
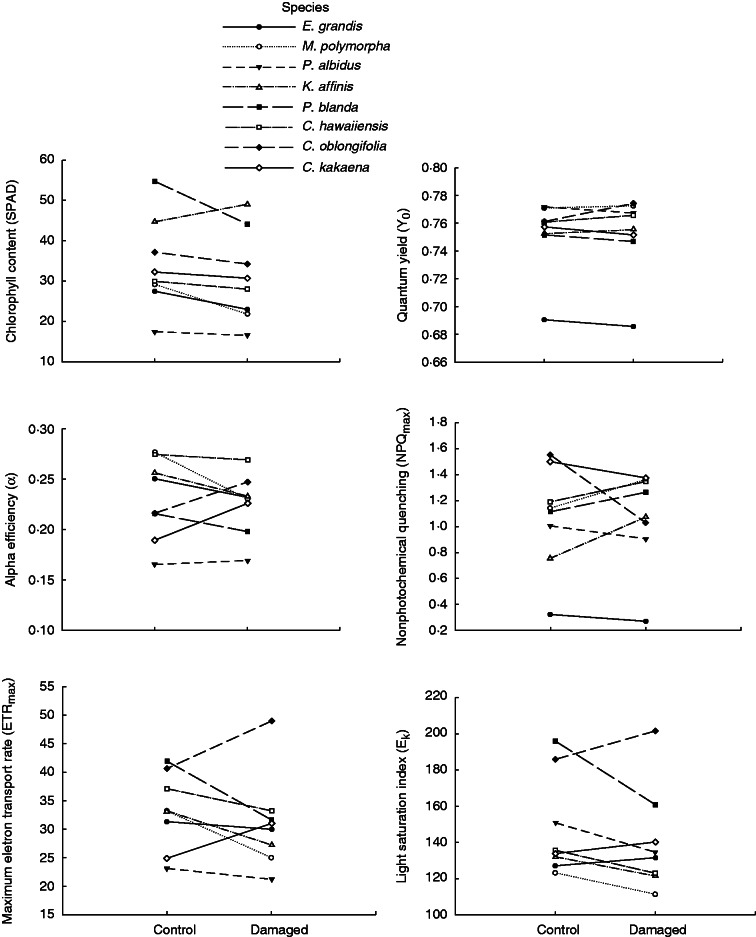
Overall, damage had no significant effect on the photosynthetic variables examined (Table 3). However, considerable variation was observed among species, and also in how species adjusted photosynthesis following damage (significant effects of species and species × damage interaction; Table 3, Fig. 3). For most variables, including the maximum electron transport rate, light saturation index, α efficiency, and non-photochemical quenching, some species had induced increases (positive slopes in norms of reaction) while other species had induced decreases (negative slopes), revealing a clear lack of uniformity in how Hawaiian seedlings respond to damage.
Table 3.
Summary of mixed model ANOVA results for photosynthetic parameters measured 2 d following damage treatments to simulate herbivory (50 % defoliation with simultaneous jasmonic acid application). Damage was treated as a fixed factor (F) assessed using maximum likelihood ratio tests, and species (and its interaction with damage) were treated as random effects (R) assessed using restricted maximum likelihood ratio tests. Significance for all factors was assessed with a χ2 distribution with 1 degree of freedom. P-values are reported in parentheses and significant effects are in bold
| Photosynthetic variables | Damage (F) | Species (family) (R) | Species × damage (R) |
|---|---|---|---|
| Chlorophyll content (SPAD) | 2·2 (0·1380) | 17·3 (<0·0001) | 23·5 (<0·0001) |
| Quantum yield (Y0) | 0·4 (0·5270) | 111·4 (<0·0001) | 0·5 (0·4795) |
| α | 0·1 (0·7518) | 1·0 (0·3173) | 6·5 (0·0107) |
| Maximum electron transport rate (ETRmax) | 1·5 (0·2206) | 0·8 (0·3710) | 10·1 (0·0014) |
| Light saturation index (Ek) | 1·3 (0·2542) | 75·5 (<0·0001) | 3·1 (0·0782) |
| Maximum non-photochemical quenching (NPQmax) | 0·1 (0·7518) | 7·9 (0·0049) | 6·7 (0·0096) |
Fig. 3.
Norm of reaction for induced responses in six photosynthetic parameters. Each line connects means for control and damaged plants for a single species.
Despite significant variation among species in their biomass and photosynthesis, only a single trait was significantly related to tolerance, as revealed by the regression analyses (Supplementary Data Table 1). The constitutive (pre-damage) level of non-photochemical quenching was significantly negatively related to tolerance (R2 = 0·5297, P = 0·0262), suggesting that plants that utilize more of the absorbed light, thereby dissipating less as heat, tolerate damage better than those with high levels of heat dissipation (Fig. 4).
Fig. 4.
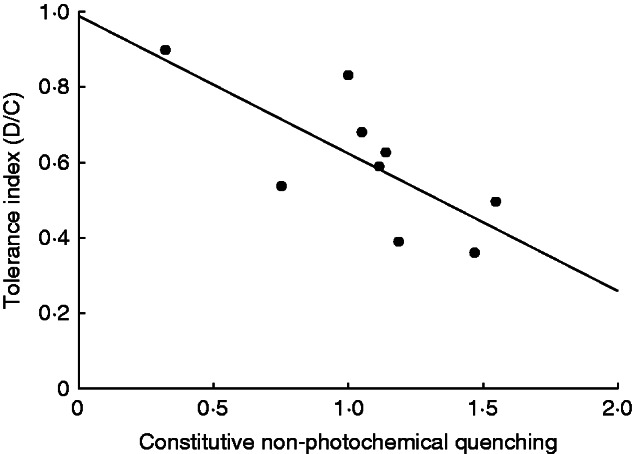
Significant negative relationship between constitutive (pre-damage) levels of non-photochemical quenching and the tolerance index, revealing that low rates of heat dissipation are associated with high herbivory tolerance. Species is the unit of replication; n = 9.
Because damage consistently reduced root biomass relative to controls, I had predicted that this shift in biomass allocation might enhance tolerance by allowing damaged plants to prioritize shoot growth and maximize replacement of lost leaf tissue. However, while the relationship was in the predicted negative direction (a greater decrease in root growth was associated with higher tolerance), it was not significant (R2 = 0·2500, P = 0·1705).
DISCUSSION
Native Hawaiian plants expressed low tolerance to simulated seedling herbivory, despite induced changes to biomass allocation and photosynthesis. Damaged seedlings displayed not only reduced shoot biomass but also reduced root biomass, consistent with reductions in root biomass found in other studies and likely associated with a decrease in carbohydrate transfer to roots following defoliation (Barton, 2008; Yoshizuka and Roach, 2011; Lucas et al., 2013; Schmidt et al., 2015). Biomass allocation did not enhance tolerance, as revealed by the genotypic regression analyses, suggesting that it is a constraint and not a mechanism of seedling tolerance. Moreover, the decrease in root biomass was persistent, in contrast with the ephemeral reductions in root biomass of other species (Barton, 2013), and damaged seedlings were still significantly smaller in terms of both shoot and root biomass 5 weeks after the simulated herbivory treatments were applied. Although it is feasible that seedlings might demonstrate full tolerance if given additional time for compensatory growth, it is likely that asymmetrical competition during a 5-week window would only magnify the differences in size of damaged and undamaged plants under field conditions. Moreover, several species showed stronger damage effects at the late harvest than they did at the early harvest. For example, damaged seedlings of Cyanea angustifolia were 13 % smaller than control plants at the early harvest, but were 62 % smaller at the late harvest. This increasing loss of vigour reveals how seedling damage can have delayed negative effects on plant fitness. Because applied damage levels were relatively low in this study at 50 % defoliation, it is likely that these results are conservative if anything. Herbivores can easily damage plants at 90–100 % defoliation (Hanley et al., 1996a, b; Shiels et al., 2014), and so the tolerance indices observed in this study may be significant overestimates compared with realized tolerance to higher levels of natural herbivory.
Seedling stress tolerance is generally thought to depend on a conservative strategy in which plants grow slowly and invest in storage tissues (Grime, 1977; Chapin et al., 1993). The accumulation of total non-structural carbohydrates (TNCs) is posited as the functional trait underlying this stress tolerance strategy, with evidence supporting the prediction that species with relatively high levels of TNCs tolerate stressors such as shade and defoliation better than species with low levels of TNCs (Myers and Kitajima, 2007; Willaume and Pages, 2011). Thus, seedlings with relatively large cotyledons and/or roots tend to tolerate herbivory (Rivera-Solis et al., 2012; Barton, 2013; Lucas et al., 2013), presumably due to storage of TNCs in these structures. Following damage, stored TNCs are remobilized to enhance shoot compensation (Myers and Kitajima, 2007; Willaume and Pages, 2011), and carbohydrate transport to roots decreases (Schmidt et al., 2015), induced responses that underlie observed decreases in root biomass following defoliation (Barton, 2008; Yoshizuka and Roach, 2011). While substantial evidence from other studies supports the conclusion that investment in storage enhances stress tolerance in seedlings, it is unclear why neither pre-damage root biomass nor induced changes in root biomass were found to enhance tolerance in the Hawaiian seedlings examined here. The ten Hawaiian species demonstrated a range of growth rates, as evidenced by their significant variation in size, suggesting that the species probably span the gradient from fast-growing/low stress tolerance to slow-growing/high stress tolerance (Grime, 1977). Yet neither plant size (e.g. growth rate) nor storage tissues relate to damage tolerance. It may be that the unique environment of the Hawaiian Islands, with the depauperate plant species diversity and disharmonic herbivore community, has decoupled the growth–tolerance trade-off found in continental species. Alternatively, it may be that the experimental conditions simply were not conducive to detecting this trade-off and stress tolerance. Seedlings were grown in a covered lanai that had relatively low light availability (130 μmol photosynthetically active radiation m−2 s−2) compared with surrounding open areas (1994 μmol photosynthetically active radiation m−2 s−2). Although shade is consistent with the field conditions where these plants grow (McDaniel and Ostertag, 2010) and other studies have nonetheless detected strong roles of storage in defoliation tolerance under very low light availability (Myers and Kitajima, 2007), it may have contributed to the results here. Future studies explicitly measuring TNCs would clarify the role, or lack thereof, of storage for Hawaiian seedling tolerance.
Variation among studies in the ages of plants examined could also explain why damage tolerance differs here from other studies on seedling–herbivore interactions. In fact, many previous studies were most likely not explicitly testing seedlings as plants were as old as 8 weeks at the time of damage (Myers and Kitajima, 2007; Lucas et al., 2013), and tolerance has been shown to increase during early ontogeny in some species (Hanley and Fegan, 2007). Technically, the seedling stage ends once seed reserves, in either cotyledons or endosperm, are depleted (Hanley et al., 2004). Because both biomass allocation patterns to roots versus shoots and plasticity in allocation patterns shift across plant ontogeny (Gedroc et al., 1996), it is almost certain that seedling tolerance and the role of biomass allocation will also shift across ontogeny, even between early ontogenetic stages (Barton, 2013). This developmental variation is likely to make it difficult to generalize among studies on seedling–herbivore interactions and tolerance unless scientists more carefully define the stage under examination. Reporting ontogenetic stage with respect to size (i.e. two-leaf stage) is likely to be more informative and more easily comparable across studies than reporting stage with respect to chronological age (i.e. 8 weeks old).
While plant size and biomass allocation were not significantly related to seedling tolerance, one photosynthetic parameter was – the constitutive level of NPQ. High levels of heat dissipation were related to weaker tolerance while those species using more of their absorbed light energy (thereby dissipating less as heat; low NPQ) had higher tolerance. This result adds to the growing body of literature demonstrating an important role for light use efficiency in herbivory tolerance. In particular, NPQ has previously been found to decrease in response to damage in seedlings of Plantago lanceolata (Barton, 2013) and Cucumis sativus (Thomson et al., 2003). However, induced responses were different in slightly older juvenile plants of Plantago major, in which an increase in NPQ following damage was significantly related to higher tolerance (Barton, 2013). While a decrease in NPQ has been interpreted to reflect greater light use efficiency in damaged plants (via a decrease in heat dissipation), the increase in NPQ for older plants may relate to a generalized stress response. These studies highlight the value in measuring photosynthetic light reactions in the context of herbivory, traits that have generally been overlooked by a focus on the photosynthetic dynamics of gas exchange (Nabity et al., 2009).
Low tolerance in native Hawaiian plants supports evidence from field studies that invasive herbivores have negative effects on native seedling regeneration in Hawaiian forests (Joe and Daehler, 2008; Drake and Hunt, 2009; Nogueira-Filho et al., 2009; Thaxton et al., 2010). Although these field studies cannot differentiate between resistance and tolerance as the factors underlying native seedling vulnerability, this study reveals that low tolerance is likely a key component of this vulnerability. Combined with other studies showing that invasive slugs and snails prefer native over non-native seedlings in experimental arenas (Shiels et al., 2014), the emerging picture is one in which Hawaiian seedlings have weak seedling defence in terms of both resistance and tolerance. Although statistically similar responses for the ten species examined here suggest that low tolerance may be a general trait for Hawaiian plants, perhaps due to the historically weak selection pressure for seedling defence (Carlquist, 1970), there is clear evidence for some variation among species. Particularly low tolerance was observed for the lobeliad species, Hawaii's most diverse plant lineage (Givnish et al., 2009), but also a group with exceptionally high rates of endangerment and extinction in Hawaii. The low tolerance revealed by this study is consistent with the high rates of mortality caused by invasive slugs to lobeliad seedlings in the field (Joe and Daehler, 2008), highlighting the importance of plant defence (or lack thereof) for the persistence of native island plants under threat from invasive herbivores (Caujapé-Castells et al., 2010). However, in another study tolerance was found to be higher in seedlings and juvenile plants than in reproductively mature plants for the endemic Hawaiian prickly poppy Argemone glauca (Barton, 2014), emphasizing that seedling defences are well developed in some island plants.
Although this study corroborates earlier evidence that Hawaiian seedlings are vulnerable to herbivory, it remains unclear whether low seedling tolerance to damage is unique to Hawaiian plants. It has been generally hypothesized that island plants should have weaker defences than continental plants due to the disharmony in island versus continental herbivore communities and predicted weaker herbivore selection pressure on islands (Bowen and VanVuren, 1997; Ziegler, 2002). However, seedling defence and tolerance to herbivory have not been examined within the context of island versus continental comparisons and are beyond the scope of this experiment. In general, evidence for whether seedlings are more or less tolerant to herbivory than older ontogenetic stages is mixed (Barton and Koricheva, 2010), with some reviewers concluding that young plants are less tolerant than older plants (Nykänen and Koricheva, 2004; Hanley and Fegan, 2007; Massad, 2013). For those species showing clear patterns of high seedling tolerance, the plants tend to be ruderal and fast-growing, like P. lanceolata (Barton, 2008, 2013; Hanley, 2012) and Embothrium coccineum (Salgado-Luarte and Gianoli, 2012). In this study, the only species with a ruderal life history was the lovegrass Eragrostis grandis, which also expressed the highest level of tolerance. Thus, weak seedling tolerance may be a fairly general trait in non-ruderal plants, and not one associated particularly with native island plants. Future studies measuring herbivory and tolerance under field conditions in island and continental species would provide insights into the generalizability of the patterns and underlying mechanisms observed here.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: regression analyses to test relationships between tolerance index (total biomass damaged/control plants at late harvest) and traits potentially driving tolerance.
ACKNOWLEDGEMENTS
The author thanks K. Coleman and T. Pave for greenhouse assistance, and A. Yoshinaga at Lyon Arboretum for contributing seeds. Mick Hanley, Juan Fornoni and one anonymous reviewer provided important insights and suggestions that improved the manuscript. This work was supported by the College of Natural Sciences and the Native Hawaiian Science and Engineering Mentorship Program at the University of Hawai'i at Mānoa.
LITERATURE CITED
- Armstrong DP, Westoby M. 1993. Seedlings from large seeds tolerate defoliation better – a test using phylogenetically independent contrasts. Ecology 74: 1092–1100. [Google Scholar]
- Baker NR. 2008. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annual Review of Plant Biology 59: 89–113. [DOI] [PubMed] [Google Scholar]
- Barton KE. 2008. Phenotypic plasticity in seedling defense strategies: compensatory growth and chemical induction. Oikos 117: 917–925. [Google Scholar]
- Barton KE. 2013. Ontogenetic patterns in the mechanisms of tolerance to herbivory in Plantago. Annals of Botany 112: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE. 2014. Prickles, latex and tolerance in the endemic Hawaiian prickly poppy (Argemone glauca): variation between populations, across ontogeny and due to phenotypic plasticity. Oecologia 174: 1273–1281. [DOI] [PubMed] [Google Scholar]
- Barton KE. 2016. Tougher and thornier: general patterns in the induction of physical defence traits. Functional Ecology; 30: 181–187. [Google Scholar]
- Barton KE, Hanley ME. 2013. Seedling-herbivore interactions: insights into plant defence and regeneration patterns. Annals of Botany 112: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE, Koricheva J. 2010. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. American Naturalist 175: 481–493. [DOI] [PubMed] [Google Scholar]
- Beer S, Axelsson L. 2004. Limitations in the use of PAM fluorometry for measuring photosynthetic rates of macroalgae at high irradiances. European Journal of Phycology 39: 1–7. [Google Scholar]
- Bloor JMG, Grubb PJ. 2003. Growth and mortality in high and low light: trends among 15 shade-tolerant tropical rain forest tree species. Journal of Ecology 91: 77–85. [Google Scholar]
- Boege K, Marquis RJ. 2005. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution 20: 441–448. [DOI] [PubMed] [Google Scholar]
- Bossdorf O, Schroder S, Prati D, Auge H. 2004. Palatability and tolerance to simulated herbivory in native and introduced populations of Alliaria petiolata (Brassicaceae). American Journal of Botany 91: 856–862. [DOI] [PubMed] [Google Scholar]
- Bowen L, VanVuren D. 1997. Insular endemic plants lack defenses against herbivores. Conservation Biology 11: 1249–1254. [Google Scholar]
- Carlquist SJ. 1970. Hawai'i: a natural history, 2nd edn. Lawai, Kaua’i, Hawai’i: Pacific Tropical Botanical Garden. [Google Scholar]
- Carrillo J, McDermott D, Siemann E. 2014. Loss of specificity: native but not invasive populations of Triadica sebifera vary in tolerance to different herbivores. Oecologia 174: 863–871. [DOI] [PubMed] [Google Scholar]
- Caujapé-Castells J, Tye A, Crawford DJ, et al. 2010. Conservation of oceanic island floras: present and future global challenges. Perspectives in Plant Ecology Evolution and Systematics 12: 107–129. [Google Scholar]
- Chapin FS, Autumn K, Pugnaire F. 1993. Evolution of suites of traits in response to environmental stress. American Naturalist 142: S78–S92. [Google Scholar]
- Connolly J, Wayne P. 1996. Asymmetric competition between plant species. Oecologia 108: 311–320. [DOI] [PubMed] [Google Scholar]
- Drake DR, Hunt TL. 2009. Invasive rodents on islands: integrating historical and contemporary ecology. Biological Invasions 11: 1483–1487. [Google Scholar]
- Fang XW, Yuan JL, Wang G, Zhao ZG. 2006. Fruit production of shrub, Caragana korshinskii, following above-ground partial shoot removal: mechanisms underlying compensation. Plant Ecology 187: 213–225. [Google Scholar]
- Fornoni J. 2011. Ecological and evolutionary implications of plant tolerance to herbivory. Functional Ecology 25: 399–407. [Google Scholar]
- Freeman RS, Brody AK, Neefus CD. 2003. Flowering phenology and compensation for herbivory in Ipomopsis aggregata. Oecologia 136: 394–401. [DOI] [PubMed] [Google Scholar]
- Gedroc JJ, McConnaughay KDM, Coleman JS. 1996. Plasticity in root/shoot partitioning: optimal, ontogenetic, or both? Functional Ecology 10: 44–50. [Google Scholar]
- Geiger DR, Servaites JC. 1994. Diurnal regulation of photosynthetic carbon metabolism in C3 plants. Annual Review of Plant Physiology and Plant Molecular Biology 45: 235–256. [Google Scholar]
- Genty B, Briantais JM, Baker NR. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- Givnish TJ, Millam KC, Mast AR, et al. 2009. Origin, adaptive radiation and diversification of the Hawaiian lobeliads (Asterales: Campanulaceae). Proceedings of the Royal Society of London Series B, Biological Sciences 276: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grime JP. 1977. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist 111: 1169–1194. [Google Scholar]
- Gruntman M, Novoplansky A. 2011. Ontogenetic contingency of tolerance mechanisms in response to apical damage. Annals of Botany 108: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME. 2012. Seedling defoliation, plant growth and flowering potential in native- and invasive-range Plantago lanceolata populations. Weed Research 52: 252–259. [Google Scholar]
- Hanley ME, Fegan EL. 2007. Timing of cotyledon damage affects growth and flowering in mature plants. Plant, Cell and Environment 30: 812–819. [DOI] [PubMed] [Google Scholar]
- Hanley ME, May OC. 2006. Cotyledon damage at the seedling stage affects growth and flowering potential in mature plants. New Phytologist 169: 243–250. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Sykes RJ. 2009. Impacts of seedling herbivory on plant competition and implications for species coexistence. Annals of Botany 103: 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. 1995. An experimental field study of the effects of mollusc grazing on seedling recruitment and survival in grassland. Journal of Ecology 83: 621–627. [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. 1996a. The effect of mollusc grazing on seedling recruitment in artificially created grassland gaps. Oecologia 106: 240–246. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Fenner M, Edwards PJ. 1996b. Mollusc grazing and seedling survivorship of four common grassland plant species: the role of gap size, species and season. Acta Oecologica 17: 331–341. [Google Scholar]
- Hanley ME, Fenner M, Whibley H, Darvill B. 2004. Early plant growth: identifying the end point of the seedling phase. New Phytologist 163: 61–66. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Girling RD, Felix AE, Olliff ED, Newland PL, Poppy GM. 2013. Olfactory selection of Plantago lanceolata by snails declines with seedling age. Annals of Botany 112: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukioja E, Koricheva J. 2000. Tolerance to herbivory in woody vs. herbaceous plants. Evolutionary Ecology 14: 551–562. [Google Scholar]
- Hoan R, Ormond R, Barton KE. 2014. Prickly poppies can get pricklier: ontogenetic patterns in the induction of physical defense traits. PLoS One 9: E96796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwender CG, Cha DH, Czesak ME, et al. 2012. Protein storage and root:shoot reallocation provide tolerance to damage in a hybrid willow system. Oecologia 169: 49–60. [DOI] [PubMed] [Google Scholar]
- Hoque S, Avila-Sakar G. 2015. Trade-offs and ontogenetic changes in resistance and tolerance to insect herbivory in Arabidopsis. International Journal of Plant Sciences 176: 150–158. [Google Scholar]
- Joe SM, Daehler CC. 2008. Invasive slugs as under-appreciated obstacles to rare plant restoration: evidence from the Hawaiian Islands. Biological Invasions 10: 245–255. [Google Scholar]
- Kenward MG, Roger JH. 1997. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53: 983–997. [PubMed] [Google Scholar]
- van Kleunen M, Ramponi G, Schmid B. 2004. Effects of herbivory simulated by clipping and jasmonic acid on Solidago canadensis. Basic and Applied Ecology 5: 173–181. [Google Scholar]
- Korpita T, Gomez S, Orians CM. 2013. Cues from a specialist herbivore increase tolerance to defoliation in tomato. Functional Ecology 28: 395–401. [Google Scholar]
- Latzel V, Malikova L, Klimesova J. 2011. Compensatory growth of Euphorbia peplus regenerating from a bud bank. Botany 89: 313–321. [Google Scholar]
- Lucas CM, Bruna EM, Nascimento CMN. 2013. Seedling co-tolerance of multiple stressors in a disturbed tropical floodplain forest. Ecosphere 4: 20. [Google Scholar]
- Massad TJ. 2013. Ontogenetic differences of herbivory on woody and herbaceous plants: a meta-analysis demonstrating unique effects of herbivory on the young and the old, the slow and the fast. Oecologia 172: 1–10. [DOI] [PubMed] [Google Scholar]
- McDaniel S, Ostertag R. 2010. Strategic light manipulation as a restoration strategy to reduce alien grasses and encourage native regeneration in Hawaiian mesic forests. Applied Vegetation Science 13: 280–290. [Google Scholar]
- Moreira X, Abdala-Roberts L, Hernandez-Cumplido J, Cuny MAC, Glauser G, Benrey B. 2015. Specificity of induced defenses, growth, and reproduction in lima bean (Phaseolus lunatus) in response to multispecies herbivory. American Journal of Botany 102: 1300–1308. [DOI] [PubMed] [Google Scholar]
- Muola A, Mutikainen P, Laukkanen L, Lilley M, Leimu R. 2010. Genetic variation in herbivore resistance and tolerance: the role of plant life-history stage and type of damage. Journal of Evolutionary Biology 23: 2185–2196. [DOI] [PubMed] [Google Scholar]
- Myers JA, Kitajima K. 2007. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. Journal of Ecology 95: 383–395. [Google Scholar]
- Nabity PD, Zavala JA, DeLucia EH. 2009. Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Annals of Botany 103: 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Filho SLG, Nogueira SSC, Fragoso JMV. 2009. Ecological impacts of feral pigs in the Hawaiian islands. Biodiversity and Conservation 18: 3677–3683. [Google Scholar]
- Nykänen H, Koricheva J. 2004. Damage-induced changes in woody plants and their effects on insect herbivore performance: a meta-analysis. Oikos 104: 247–268. [Google Scholar]
- Orians CM, Thorn A, Gomez S. 2011. Herbivore-induced resource sequestration in plants: why bother? Oecologia 167: 1–9. [DOI] [PubMed] [Google Scholar]
- Pasquini SC, Santiago LS. 2012. Nutrients limit photosynthesis in seedlings of a lowland tropical forest tree species. Oecologia 168: 311–319. [DOI] [PubMed] [Google Scholar]
- Rasmann S, Johnson MD, Agrawal AA. 2009. Induced responses to herbivory and jasmonate in three milkweed species. Journal of Chemical Ecology 35: 1326–1334. [DOI] [PubMed] [Google Scholar]
- Rivera-Solis G, Abdala-Roberts L, Cervera JC, Parra-Tabla V, Ruiz-Ruiz J, Betancur-Ancona D. 2012. Mechanisms and traits associated with compensation for defoliation in Ruellia nudiflora. Plant Ecology 213: 303–314. [Google Scholar]
- Salgado-Luarte C, Gianoli E. 2012. herbivores modify selection on plant functional traits in a temperate rainforest understory. American Naturalist 180: E42–E53. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Hummel GM, Thiele B, Schurr U, Thorpe MR. 2015. Leaf wounding or simulated herbivory in young N. attenuata plants reduces carbon delivery to roots and root tips. Planta 241: 917–928. [DOI] [PubMed] [Google Scholar]
- Schwachtje J, Minchin PEH, Jahnke S, van Dongen JT, Schittko U, Baldwin IT. 2006. SNF1-related kinases allow plants to tolerate herbivory by allocating carbon to roots. Proceedings of the National Academy of Sciences of the USA 103: 12935–12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels AB, Ennis MK, Shiels L. 2014. Trait-based plant mortality and preference for native versus non-native seedlings by invasive slug and snail herbivores in Hawaii. Biological Invasions 16: 1929–1940. [Google Scholar]
- Silvertown J, Franco M, Pisanty I, Mendoza A. 1993. Comparative plant demography – relative importance of life-cycle components to the finite rate of increase in increase of woody and herbaceous perennials. Journal of Ecology 81: 465–476. [Google Scholar]
- Simms EL. 2000. Defining tolerance as a norm of reaction. Evolutionary Ecology 14: 563–570. [Google Scholar]
- Strauss SY, Agrawal AA. 1999. The ecology and evolution of plant tolerance to herbivory. Trends in Ecology and Evolution 14: 179–185. [DOI] [PubMed] [Google Scholar]
- Thaxton JM, Cole TC, Cordell S, Cabin RJ, Sandquist DR, Litton CM. 2010. Native species regeneration following ungulate exclusion and nonnative grass removal in a remnant Hawaiian dry forest. Pacific Science 64: 533–544. [Google Scholar]
- Thomson VP, Cunningham SA, Ball MC, Nicotra AB. 2003. Compensation for herbivory by Cucumis sativus through increased photosynthetic capacity and efficiency. Oecologia 134: 167–175. [DOI] [PubMed] [Google Scholar]
- Tiffin P. 2000. Mechanisms of tolerance to herbivore damage: what do we know? Evolutionary Ecology 14: 523–536. [Google Scholar]
- Weltzin JF, Archer SR, Heitschmidt RK. 1998. Defoliation and woody plant (Prosopis glandulosa) seedling regeneration: potential vs realized herbivory tolerance. Plant Ecology 138: 127–135. [Google Scholar]
- West BT, Welch KB, Galecki AT. 2014. Linear mixed models: a practical guide using statistical software, 2nd edn. Chapman and Hall. [Google Scholar]
- Willaume M, Pages L. 2011. Correlated responses of root growth and sugar concentrations to various defoliation treatments and rhythmic shoot growth in oak tree seedlings (Quercus pubescens). Annals of Botany 107: 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizuka EM, Roach DA. 2011. Plastic growth responses to simulated herbivory. International Journal of Plant Sciences 172: 521–529. [Google Scholar]
- Ziegler AC. 2002. Hawaiian natural history, ecology, and evolution. Honolulu: University of Hawai'i Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.