Abstract
Background and Aims Leaf mass per area (LMA) is an important leaf trait; however, correlations between LMA and leaf anatomical features and photosynthesis have not been fully investigated, especially in cereal crops. The objectives of this study were (a) to investigate the correlations between LMA and leaf anatomical traits; and (b) to clarify the response of LMA to nitrogen supply and its effect on photosynthetic nitrogen use efficiency (PNUE).
Methods In the present study, 11 rice varieties were pot grown under sufficient nitrogen (SN) conditions, and four selected rice cultivars were grown under low nitrogen (LN) conditions. Leaf anatomical traits, gas exchange and leaf N content were measured.
Key Results There was large variation in LMA across selected rice varieties. Regression analysis showed that the variation in LMA was more closely related to leaf density (LD) than to leaf thickness (LT). LMA was positively related to the percentage of mesophyll tissue area (%mesophyll), negatively related to the percentage of epidermis tissue area (%epidermis) and unrelated to the percentage of vascular tissue area (%vascular). The response of LMA to N supplementation was dependent on the variety and was also mainly determined by the response of LD to N. Compared with SN, photosynthesis was significantly decreased under LN, while PNUE was increased. The increase in PNUE was more critical in rice cultivars with a higher LMA under SN supply.
Conclusions Leaf density is the major cause of the variation in LMA across rice varieties and N treatments, and an increase in LMA under high N conditions would aggravate the decrease in PNUE.
Keywords: Leaf density, leaf mass per area, leaf photosynthesis, leaf thickness, photosynthetic N use efficiency, rice
INTRODUCTION
The leaf is the primary organ for photosynthesis and mediates resources and energy fluxes in the ecosphere. Leaf mass per area (LMA) is an important leaf trait that is strongly correlated with leaf functional, biochemical and structural traits. Recent work has demonstrated that LMA together with other traits, such as vein length per leaf area (VLA), mass-based leaf nitrogen (N) content (Nmass), leaf photosynthetic rate (A) and leaf hydraulic conductance (Kleaf), scale with each other (Wright et al., 2004; Sack et al., 2013). A well-known generalization is that fast-growing species tend to have a lower LMA, a higher mass-based leaf photosynthesis (Amass) and a higher Nmass, but a shorter leaf life span relative to slow-growing species (Wright et al., 2004).
Leaf mass per area can be considered as the product of two physical properties: leaf density (LD) and leaf thickness (LT). The LT ranges between 100 and 700 μm, and LD ranges between 0·1 and 0·6 g cm–3 across a range of species (Poorter et al., 2009). However, it is not known what the dominant factor that drives the variation in LMA across species and/or genotypes is. An early study proposed that variation in LMA among species is mainly driven by variation in LT (Vile et al., 2005). In contrast, Poorter et al. (2009) claimed that LD and LT explained 80 and 20 % of the differences in LMA, respectively, across a wide range of plant species, and they also found that LMA did not correlate with LT, but was closely related to LD within grasses, herbaceous dicots, deciduous or evergreen woody species groups. More details are needed to reveal the contribution of LT and LD to LMA.
Variation in LMA among species and genotypes can also be obtained by considering LMA as the sum of the dry mass per unit area of different leaf tissues (Roderick et al., 1999). The leaf vein is an important type of anatomical feature; leaves from different cultivars and species exhibit enormous diversity in VLA (Sack et al., 2012). There are two contradictory opinions on the contribution of VLA to LMA. Castro-Díez et al. (2000) reported a tight correlation between LMA and the proportion of leaf vein tissue among 52 European woody species. Furthermore, Blonder et al. (2011) recently showed that a high VLA can result in a high LMA, and proposed that VLA was the ‘origin’ of LMA. In contrast, Sack et al. (2013) re-analysed previous studies by building a large database and showed no correlation between LMA and VLA. There is a debate on the hypothesis that VLA is the origin of LMA (Blonder et al., 2014; Sack et al., 2014). However, most of these studies were conducted on woody species (Witkowski and Lamont, 1991; Castro-Díez et al., 2000) rather than on cereal crops such as rice, which have a faster growth rate.
Leaf functional attributes are closely linked to their anatomical characteristics. It is widely considered that leaf anatomy and structure can subsequently impact A; for instance, cell wall thickness and chloroplast surface exposed to the intercellular space significantly impact CO2 diffusion inside leaves. Unfortunately, few studies (Giuliani et al., 2013; Xiong et al., 2015a) have as yet combined leaf anatomical (e.g. LMA and LT) and biochemical (e.g. leaf N) attributes to highlight the determinants of A. For instance, the close relationship between A and area-based leaf nitrogen content (Narea) is determined by mesophyll volume, but it is not clear whether this relationship is affected by LMA.
Nitrogen is the most important nutrient for plant growth and development. Under low N (LN), the depression of plant growth is more severe than that of photosynthesis (Muller et al., 2011). This would result in accumulation of non-structural carbohydrates (NSCs) in leaves (Chatterton and Silvius, 1981; Poorter et al., 2009) and a subsequent increase in LMA. Leaf protein content is closely related to N supply; therefore, decreased protein content under LN would potentially lead to a decrease in LMA. After carrying out a meta-analysis, Poorter et al. (2009) concluded that LMA would be slightly increased under LN conditions. Again, the response of LMA to N supply and its potential effects on A and photosynthetic N use efficiency (PNUE) are not fully exploited in cereal crops such as rice plants.
In the present study, 11 rice genotypes, comprising six cultivars and five wild types, were pot grown under sufficient N (SN) and LN conditions in four selected rice cultivars. The objectives of this study were (a) to clarify the contribution of LT and LD to LMA variation among rice genotypes, (b) to investigate the correlations between LMA and leaf anatomical traits; and (c) to clarify the response of LMA to N supply and its effect on PNUE.
MATERIALS AND METHODS
Plant materials
Based on our trial test (data not shown), 11 rice varieties (listed in Table 1) with a large range of LMA were grown in a pot experiment during May and July in 2013 at Huazhong Agricultural University (114·37°E, 30·48°N), Wuhan, China. Rice plants were grown in 15·0 L pots, which were filled with 13·0 kg of soil. Three pots each containing three plants were grown per variety. N, phosphorus (P) and potassium (K) were applied as basal fertilizers at a rate of 3·0, 1·95 and 1·95 g per pot, respectively. To investigate the effect of N supplementation on LMA and leaf functions, four rice cultivars with a similar LMA under SN (Table 2) were selected to be grown under LN (0·5 g per pot). Plants were grown outdoors and watered daily to maintain a minimum 2 cm water layer to avoid drought stress. Pests were intensively controlled using chemical pesticides.
Table 1.
Species and subspecies of 11 rice varieties used in this study
| No. | Species | Variety | Subspecies | Abbreviation |
|---|---|---|---|---|
| 1 | Oryza punctata L. | I90 | – | – |
| 2 | Oryza rufipogon L. | I08 | – | – |
| 3 | Oryza rufipogon L. | Ruf | – | – |
| 4 | Oryza australiansis L. | Aus | – | – |
| 5 | Oryza latifolia L. | Lat | – | – |
| 6 | Oryza sativa L. | Nipponbare | Japonica | Nip |
| 7 | Oryza sativa L. | Shanyou 63 | Indica | SY |
| 8 | Oryza sativa L. | Huanghuazhan | Indica | HHZ |
| 9 | Oryza sativa L. | N22 | Indica | N22 |
| 10 | Oryza sativa L. | Gangyou 118 | Indica | GY |
| 11 | Oryza sativa L. | Zhenshan 97B | Indica | ZS |
Table 2.
Leaf anatomical features in different rice varieties
| Variety | N | LMA | LD | LT | VTmajor | VTminor | %epidermis | %mesophyll | %vascular | IVD | VLA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g m–2) | (mg mm–3) | (mm) | (mm) | (mm) | (mm) | (mm mm–2) | |||||
| Sufficient N (SN) | |||||||||||
| I90 | SN | 26·9 | 0·039 | 0·234 | 0·173 | 0·087 | 17·4 | 50·6 | 32·1 | 0·178 | 4·45 |
| I08 | SN | 39·9 | 0·115 | 0·216 | 0·123 | 0·094 | 7·3 | 66·4 | 26·3 | 0·197 | 4·90 |
| Ruf | SN | 33·4 | 0·069 | 0·176 | 0·158 | 0·111 | 20·8 | 53·6 | 25·6 | 0·178 | 4·44 |
| Aus | SN | 38·6 | 0·088 | 0·200 | 0·191 | 0·152 | 13·0 | 66·3 | 20·7 | 0·188 | 3·95 |
| Lat | SN | 45·3 | 0·120 | 0·271 | 0·255 | 0·156 | 5·8 | 68·2 | 26·0 | 0·280 | 3·50 |
| Nip | SN | 40·8 | 0·136 | 0·257 | 0·205 | 0·080 | 16·0 | 62·7 | 21·3 | 0·245 | 2·98 |
| SY | SN | 36·1 | 0·133 | 0·228 | 0·223 | 0·103 | 24·1 | 47·2 | 29·7 | 0·196 | 3·91 |
| HHZ | SN | 43·8 | 0·153 | 0·294 | 0·251 | 0·108 | 13·3 | 64·0 | 22·7 | 0·261 | 4·23 |
| N22 | SN | 40·5 | 0·132 | 0·265 | 0·208 | 0·084 | 16·7 | 62·0 | 21·3 | 0·234 | 5·64 |
| GY | SN | 43·2 | 0·144 | 0·204 | 0·211 | 0·086 | 19·3 | 58·7 | 22·0 | 0·208 | 4·12 |
| ZS | SN | 42·9 | 0·140 | 0·205 | 0·184 | 0·089 | 10·7 | 65·0 | 24·3 | 0·210 | 3·99 |
| Low N (LN) | |||||||||||
| HHZ | LN | 51·4 | 0·173 | 0·306 | 0·265 | 0·116 | 11·0 | 64·0 | 25·0 | 0·221 | 4·48 |
| N22 | LN | 34·8 | 0·113 | 0·191 | 0·148 | 0·084 | 15·3 | 63·0 | 21·7 | 0·212 | 6·02 |
| GY | LN | 41·7 | 0·137 | 0·206 | 0·195 | 0·092 | 8·0 | 65·3 | 26·7 | 0·195 | 4·20 |
| ZS | LN | 42·2 | 0·140 | 0·213 | 0·193 | 0·090 | 12·1 | 63·2 | 24·7 | 0·196 | 4·17 |
| ANOVA | |||||||||||
| V | *** | *** | ** | ** | *** | *** | * | * | ** | *** | |
| N | ** | ** | * | * | ns | *** | * | ** | *** | *** | |
| V × N | *** | ** | ** | * | ns | * | * | ** | ** | ** | |
n = 3 plants.
V, variety; N, nitrogen; SN, sufficient N treatment; LN, low N treatment; LMA, leaf mass per area; LT, leaf thickness; VTmajor, major vein thickness; VTminor, minor vein thickness; LD, leaf density; %epidermis, percentage of epidermis area at leaf cross-section; %mesophyll, percentage of mesophyll tissue area at leaf cross-section; %vascular, percentage of vascular tissue area at leaf cross-section; IVD, intervein distance; VLA, leaf vein length per area.
*P < 0·05; **P < 0·01; ***P < 0·001; ns (non-significant), P > 0·05.
Measurements were made starting 40 d after transplanting. Three newly expanded leaves from each variety under both treatments were detached and the leaf area measured using a leaf area meter (LI-Cor 3000C, Li-Cor, NE, USA). Leaves were then oven-dried at 80 °C to reach a constant weight. The LMA was calculated as the ratio of dry mass to leaf area (g m–2).
Gas exchange measurements
Steady-state gas exchange and chlorophyll fluorescence parameters were simultaneously measured using a portable gas exchange system equipped with a 6400-40 leaf chamber (LI-6400 XT; Li-Cor) between 0900 and 1500 h. There was no significant midday depression in our measurement conditions (data not shown). Measurements were performed on the middle of the youngest fully expanded leaves of three different plants in each cultivar. Leaf temperature during measurements was maintained at 28 °C. In the leaf chamber, the photosynthetic photon flux density (PPFD) was maintained at 1500 μmol m–2 s–1 (in the present study, the saturation light for selected genotypes ranges from 900 to 1300 μmol m–2 s–1 under SN), leaf to air vapour pressure deficit (VPD) at 1·0–1·5 kPa and CO2 concentration at 400 μmol m–2 s–1. After equilibration to a steady state, the data were recorded. The actual photochemical efficiency of photosystem II (ΦPSII) was calculated as follows:
| (1) |
where Fs represents steady-state fluorescence and Fm′ represents maximum fluorescence. Electron transport rates (ETRs) were computed as follows:
| (2) |
where α is the leaf absorptance and β represents the distribution of electrons between PSI and PSII.
After gas exchange and chlorophyll fluorescence measurements, the gas exchange system was immediately switched to a low O2 concentration (<2 %) by supplying high purity N without removing the leaves from the chamber, and then a light response curve was determined to estimate α and β. During these measurements, chamber conditions were identical to those described above, except that the PPFD was controlled across a gradient of 800, 600, 400, 200, 100 and 0 μmol m–2 s–1. After reaching a steady state, the parameters of gas exchange and chlorophyll fluorescence were simultaneously recorded. The slope of the relationship between 1/4 ΦPSII and the quantum efficiency of CO2 uptake (ΦCO2) is considered to be the value of α·β (Valentini et al., 1995; Long and Bernacchi, 2003).
The variable J method was used to calculate Cc and gm (Harley et al., 1992). Cc was calculated as follows:
| (3) |
where Γ* represents the CO2 compensation point in the absence of respiration. In the present study, a Г* value of 40 μmol mol–1 and an Rd value of 1 μmol m–2 s–1 typical for rice plants were used (Yamori et al., 2011). Then, gm was calculated as follows:
| (4) |
where Ci represents the intercellular CO2 concentration.
Leaf N and Rubisco contents
After gas exchange measurement, leaves were oven-dried at 80 °C to a constant weight, and then ground using a mixer mill homogenizer (MM400, Retsch, Germany). The N concentration was measured using an NC analyzer (IsoPrime100 IRMS, Isoprime Ltd, UK). The PNUE was calculated as: PNUE = A/Narea.
The ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) content of newly expanded leaves was determined according to Makino et al. (1985, 1986). Briefly, leaves were sampled and immersed in liquid N and then stored at –70 °C until extraction. A 0·5 g aliquot of material was ground using a mortar and pestle on ice with addition of buffer solution containing 50 mmol L–1 Tris–HCl (pH 8·0), 5 mmol L–1 β-mercaptoethanol and 12·5 % (v/v) glycerol, and the extracts were centrifuged for 15 min at 1500 g at 2 °C. Supernatant solution was mixed with dissolving solution, containing 2 % (w/v) SDS, 4 % (v/v) β-mercaptoethanol and 10 % (v/v) glycerol, and the mixture was boiled in water for 5 min for the assay of protein electrophoresis. The samples were loaded onto SDS––PAGE containing a 12·5 % (w/v) polyacrylamide gel and then electrophoresis was performed. Afterwards, the gels were washed with deionized water several times and then stained with 0·25 % Coomassie Blue staining solution for 12 h and destained until the background was clear. Both large subunits and small subunits of Rubisco were cut from the gel and transfererd into a 10 mL cuvette with 2 mL of formamide, and washed in a 50 °C water bath under room temperature for 8 h. The washed solutions were measured at 595 nm (Infinite M200, Tecan U.S., Inc.) using background glue as the blank and bovine serum albumin (BSA) as standard protein.
Vein length per area and leaf thickness
Three leaves per variety or treatment were cleared in 20 % aqueous NaOH after measurements of leaf width with a ruler. Then, three sections approx. 5·0 mm long were cut from the middle of each leaf, followed by staining and mounting in glycerol to determine the leaf vein density. Rice leaf veins can be categorized into three types based on their size: midrib, major veins and minor veins (Scarpella et al., 2003; Smillie et al., 2012). In the present study, major and minor vein numbers and the intervein distance (IVD; distance between two minor veins) were recorded using a microscope (SA3300, Tech Instrument Co. Ltd., Beijing, China) at ×40 magnification. The LT was measured in situ at the middle of the leaves (avoiding midribs) using a DTG03 digital thickness gauge (Digital Micrometers Ltd, Sheffield, UK). For each leaf, ten points were measured and then averaged.
Light microscopy
Small leaf sections of about 4·0 × 1·2 mm were cut from the middle of new fully expanded leaves (avoiding the midribs) near tillers on which gas exchange measurements were performed. These leaves were almost of the same developmental age as leaves used for gas exchange experiments. The leaf sections were infiltrated with fixative (2·5 % glutaric aldehyde in 0·1 m phosphate buffer pH 7·6) at 4 °C with a syringe, and post-fixed in 2 % buffered osmium tetroxide at 20 °C for 2 h. Leaf cross-sections were cut using a fully automated rotary microtome (Leica RM2265, Leica Microsystems, Milton Keynes, UK). The leaf sections were stained with 1 % (w/v) toluidine blue in 1 % (w/v) Na2B4O7, and they were examined at ×100 magnification with an Olympus IX71 light microscope (Olympus Optical, Tokyo, Japan). Three leaves in each variety or treatment were analysed.
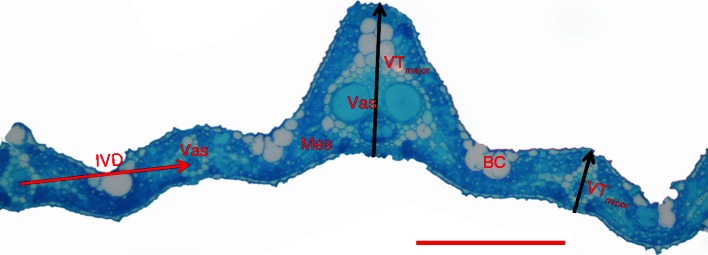
Major vein thickness (VTmajor), minor vein thickness (VTminor) and the width of cross-sections were measured from light microscopy images with Image J software (National Institutes of Health, USA). In addition, the area of mesophyll tissue (including bulliform cells and intercell air space), epidermis tissue, vascular tissue and the entire leaf cross-section were also measured from light microscopy images. Details of leaf anatomy and how VTmajor, VTminor and IVD were measured are given in the legend of Fig. 1; LD was calculated as:
| (5) |
Fig. 1.
Diagram illustrating details of the leaf anatomical traits measured. Black arrows show major (VTmajor) and minor vein thicknesses (VTminor), and red arrow shows the intervein distance between two minor veins (IVD). BC, bulliform cells; Mes, mesophyll cells; Vas, vascular tissue. Scale bar = 100 μm.
The percentage areas of mesophyll tissue (%mesophyll), epidermis tissue (%epidermis) and vascular tissue (%vascular) were calculated according to eqns (6), (7) and (8), respectively.
| (6) |
| (7) |
| (8) |
Statistical analysis
One- and two-way analyses of variance (ANOVAs) were performed in SAS9·2 (SAS Institute Inc., Cary, NC, USA). The means of three replications were compared using the least significant difference (Fisher’s LSD) multiple comparison test (P < 0·05). Linear regression analysis was used to test for correlation between anatomical traits (Sigmaplot; SPSS Inc., Chicago, IL, USA). Regressions were considered to be significant at P < 0·05.
RESULTS
Leaf thickness and leaf density
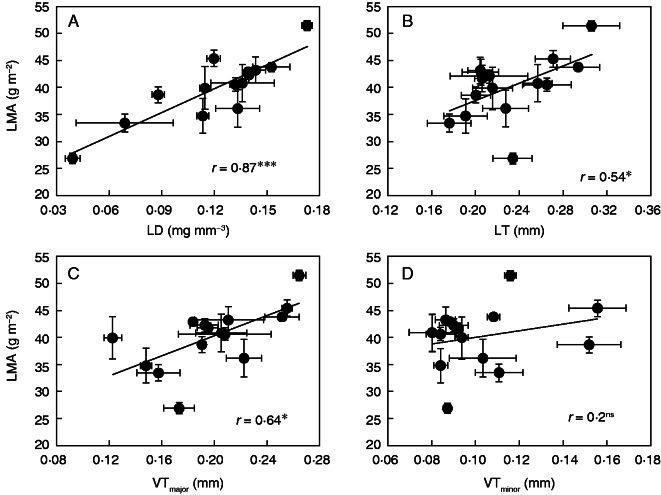
The LMA, LD and LT varied considerably between the rice cultivars that were studied (Table 2). LMA varied between 26·9 g m–2 in genotype I90 at SN to 51·4 g m–2 in HHZ at LN, with an average of 39·7 g m–2. LD values varied from 0·039 mg mm–3 in I90 to 0·173 mg mm–3 in HHZ across SN and LN, with an average of 0·122 mg mm–3. LMA was closely and positively correlated with LD (Fig. 2A, r = 0·87, P < 0·001).
Fig. 2.
Relationships between leaf mass per area (LMA) and (A) leaf density (LD), (B) leaf thickness (LT), (C) major vein thickness (VTmajor) and (D) minor vein thickness (VTminor) in different rice varieties. Data points are means ± s.d. of three replicates. The lines are the linear regressions; * and *** indicate significance at P < 0·05 and P < 0·001, respectively, and ns indicates non-significance at P < 0·05.
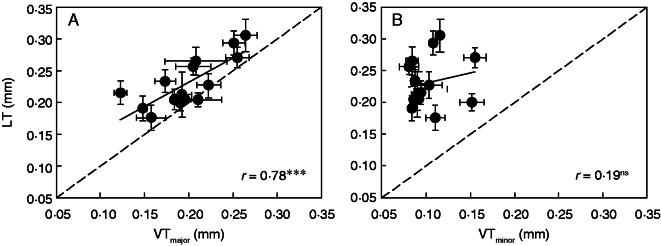
The average LT was 0·231 mm, with a minimum of 0·176 mm in Ruf and a maximum of 0·306 mm in HHZ (Table 2). Across the selected genotypes, VTmajor was similar to LT (range from 0·176 to 0·306 mm), with an average of 0·200 mm, while VTminor (range from 0·080 to 0·156 mm) was lower than LT. LMA was positively correlated with both LT (Fig. 2B, r = 54, P < 0·05) and VTmajor (Fig. 2C, r = 64, P < 0·05), but not correlated with VTminor (Fig. 2D, r = 0·25, P = 0·25). Compared with the correlation between LMA and LD, the weak correlation between LMA and LT might have resulted from I90, which was an outlier from the LMA–LT regression (Fig. 2B). The LT was strongly correlated with VTmajor (Fig. 3A, r = 0·78, P < 0·001), but not with VTminor (Fig. 3B, r = 0·19, P = 0·31).
Fig. 3.
Relationships between leaf thickness (LT) and (A) major vein thickness (VTmajor) and (B) minor vein thickness (VTminor) in rice plants. Data points are the means ± s.d. of three replicates. The solid lines represent line regressions, *** indicates significance at P < 0·001, and ns indicates non-significance at P < 0·05. The dotted lines show the slope of a 1:1 relationship.
Leaf vein and mesophyll
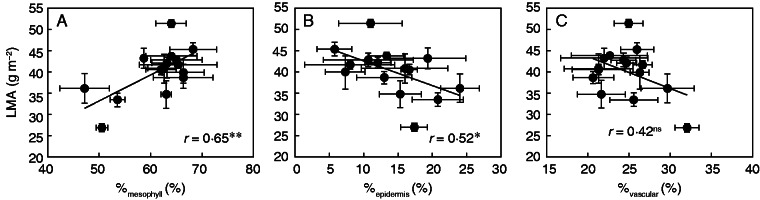
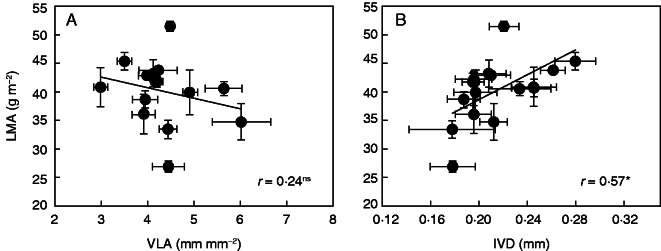
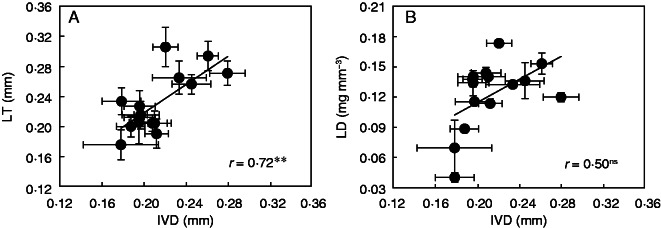
By analysing the area of anatomical features from the section images, it was found that mesophyll occupied, on average, 61·3 % of the leaf section area, with a range from 47·2 to 68·2 % (Table 2). The vasculature area ranged from 20·7 to 32·1 % and the epidermis from 5·8 to 24·1 %. LMA was positively related to %mesophyll (Fig. 4A, r = 0·65, P < 0·01) and negatively related to %epidermis (Fig. 4B, r = 0·52, P < 0·05). However, LMA was not correlated with %vascular (Fig. 4C). Across all cultivars, IVD and VLA varied 1·7- and 2·2-fold, respectively (Table 2), and LMA was not correlated with VLA (Fig. 5A), but was positively related to IVD (Fig. 5B, r = 57, P < 0·05). A positive relationship was found between LT and IVD (Fig. 6A, r = 0·72, P < 0·01) but there was no relationship between LD and IVD (Fig. 6B, r = 0·50, P = 0·07).
Fig. 4.
Relationships between leaf mass per area (LMA) and (A) percentage of mesophyll tissue area (including bulliform cells and intercellular air space) of leaf cross-section (%mesophyll), (B) percentage of epidermis area of leaf cross-section (%epidermis) and (C) percentage of vascular tissue area of leaf cross-section (%vascular). Data points are the means ± s.d. of three replications. The solid lines represent line regressions; * and ** indicate significance at P < 0·05 and P < 0·01, respectively, and ns indicates non-significance at P < 0·05.
Fig. 5.
Relationships between leaf mass per area (LMA) and (A) leaf vein length per area (VLA) and (B) the intervein distance (IVD) in different rice varieties. Data points are means ± s.d. of three replications. The solid lines represent line regressions, * indicates significance at P < 0·05, and ns indicates non-significance at P < 0·05.
Fig. 6.
Relationships between intervein distance (IVD) and (A) leaf thickness (LT) and (B) leaf density (LD) in rice plants. Data points are means ± s.d. of three replications. The solid lines represent linear regressions, ** indicates significance at P < 0·05, and ns indicates non-significance at P < 0·05.
Responses of LMA and A to N supply
The response of LMA to N supply showed a large variation across the selected rice cultivars (Table 2). LMA in LN increased in HHZ, but decreased in N22. Nitrogen supply had no significant effect on LMA in the other two rice cultivars. The response of LD to N supply was similar to that of LMA. Nitrogen supply had no significant effect on either VTmajor or VTminor, except for a significant decrease in VTmajor under LN treatment in N22. In general, N supply had a significant effect on %mesophyll, %epidermis or %vascular (Table 2).
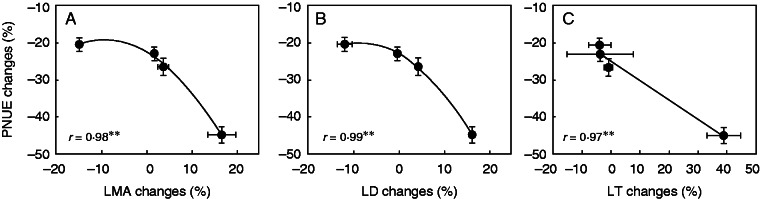
The response of A to N limitation varied within cultivars. A decreased from 26 % in ZS to 53 % in GY (Table 3). Narea and Rubisco contents were, to a greater extent, decreased under LN supply. Narea of LN decreased from 63 % in ZS to 139 % in N22, and Rubisco content decreased from 36 % in GY to 100 % in N22. Consequently, PNUE was increased from 23 % in ZS to 41 % in N22. Both stomatal conductance (gs) and mesophyll conductance (gm) were decreased by N limitation. Changes of PNUE with N supplementation were significantly and negatively related to changes of LMA, LD and LT (Fig. 7).
Table 3.
Gas exchange parameters, leaf N and Rubisco content
| Parameters | T | Variety |
|||
|---|---|---|---|---|---|
| HHZ | N22 | GY | ZS | ||
| A (μmol m–2 s–1) | LN | 23·9 ± 0.6b | 16·9 ± 1.1b | 19.1 ± 0·9b | 22·9 ± 1.5b |
| SN | 31·0 ± 1.1a | 23·9 ± 0.4a | 29.4 ± 0.7a | 28.8 ± 0.4a | |
| gs (mol m–2 s–1) | LN | 0.28 ± 0·07b | 0.20 ± 0·03b | 0.23 ± 0·03b | 0.21 ± 0·02b |
| SN | 0.36 ± 0·04a | 0.29 ± 0·01a | 0.39 ± 0·03a | 0.35 ± 0·06a | |
| Ci (μmol mol–1) | LN | 288.3 ± 20.6a | 295·9 ± 8.2a | 293.8 ± 13.3a | 269.7 ± 6.2b |
| SN | 279.8 ± 20.6a | 291.1 ± 3·0a | 291.2 ± 5.3a | 285.6 ± 11.5a | |
| Cc (μmol mol–1) | LN | 153.3 ± 30.3a | 217.8 ± 8.7a | 160.6 ± 5·9a | 132.2 ± 3.6b |
| SN | 150.7 ± 8.4a | 190.2 ± 2·9b | 159.7 ± 8.8a | 188.6 ± 8·9a | |
| gm (mol m–2 s–1) | LN | 0.18 ± 0·01b | 0.22 ± 0·02b | 0.14 ± 0·01b | 0.17 ± 0·01b |
| SN | 0.24 ± 0·01a | 0.24 ± 0·01a | 0.22 ± 0·06a | 0.30 ± 0·01a | |
| Narea (g m–2) | LN | 1.10 ± 0·08b | 0.69 ± 0·02b | 0.83 ± 0·03b | 1.22 ± 0·02b |
| SN | 2·00 ± 0·06a | 1.65 ± 0·03a | 1.73 ± 0·07a | 1·98 ± 0·06a | |
| Rubisco (mg cm–2) | LN | 0.18 ± 0·02b | 0.10 ± 0·01b | 0.22 ± 0·03b | 0.18 ± 0·02b |
| SN | 0.34 ± 0·01a | 0.20 ± 0·01a | 0.30 ± 0·01a | 0.33 ± 0·05a | |
| PNUE (μmol g–1 s–1) | LN | 21.7 ± 2.1a | 24.5 ± 1.7a | 23.2 ± 1.7a | 18·9 ± 1.2a |
| SN | 15.5 ± 0.6b | 14.5 ± 0.4b | 17·0 ± 0.7b | 14.6 ± 0.6b | |
A, photosynthetic rate; gs, stomatal conductance, Ci, intercellular CO2 concentration; Cc, CO2 concentration in the chloroplasts; gm, mesophyll conductance, Narea, area-based leaf nitrogen content; PNUE, photosynthetic N use efficiency; T, treatment.
Mean differences between two N treatments were tested using one-way analysis of variance with a Fisher’s LSD test. Values followed by different superscript letters are significantly different at P < 0·05.
Fig. 7.
Response of PNUE changes to changes in LMA (A), LD (B) and LT (C). The X changes from LN to SN conditions were defined as ln(XSN/XLN), and the bars represent the 95 % confidence interval. ** indicates significance at P < 0·01.
DISCUSSION
Effects of LD vs. LT on LMA
Identifying the contributions of LT and LD to interspecific and/or intraspecific variations of LMA is important in plant science. However, their contributions are still unclear although they have been widely studied, especially in cereal crops. Choong et al. (1992) reported that the variation in LMA was mainly driven by the variation in LT. Conversely, Castro-Díez et al. (2000) showed that LMA was correlated with LD but not with LT across 52 European woody species, and Villar et al. (2013) reported that LD and LT contributed equally to the variation in LMA. However, other studies have suggested that variation in LMA is related more to LD than to LT (Witkowski and Lamont, 1991; Poorter et al., 2009). The different results may be caused by the selection of species in these studies; therefore, an investigation within a single species will be useful. Here, we demonstrated that the variation of LMA across cultivars was more related to, and may have been mainly driven by, LD rather than LT.
Leaf mass per area can also be broken down into dry mass per area of different leaf anatomical tissues: mesophyll, epidermis and vascular. Leaf density might relate to the combination of anatomical tissues, due to the variation of density across tissues. In the present study, we showed that the mesophyll accounted for >60 % of the leaf cross-sectional area (Table 2). Mesophyll cells contain a considerable amount of non-structural chemical components, such as NSCs and proteins, which aggregated in the symplast and particularly in the chloroplast. The starch mass comprises 28 % (Chatterton and Silvius, 1981) or >40 % (Poorter et al., 2009) of total leaf dry mass across the wide range of species, which means that mesophyll cells are denser than epidermal cells (Poorter et al., 2009), where a larger volume is comprised of vacuoles (Winter et al., 1993). Thus, leaves would be denser and LMA would be higher when there is a larger volume of mesophyll cells; conversely, leaves would have a lower density and LMA when a larger proportion of leaf section is taken up by epidermal cells. Our data support this hypothesis; a positive correlation was found between LMA and %mesophyll (Fig. 4A) and a negative correlation between LMA and %epidermis (Fig. 4B).
In addition, structural carbohydrates (SCs) and lignin (present mainly in cell walls and vascular bundles) also account for a large proportion of leaf dry mass (Poorter et al., 2009). High-LMA leaves may contain higher concentrations of these two components and have a higher vascular density than low-LMA leaves (Poorter et al., 2009). A positive correlation between LMA and %vascular has been reported in previous studies (Poorter et al., 2009; Villar et al., 2013). However, no correlation was observed between LMA and %vascular across the selected rice genotypes in this study (Fig. 4C). This is probably because air spaces in vascular tissue are much more numerous in grass leaves than in other species, which offsets the positive effects of SCs and lignin on the vascular density and LMA.
As previously discussed, there is still no consensus on whether VLA is related to LMA or not (Blonder et al., 2011; Sack et al., 2013). In the present study, no significant relationship between VLA and LMA was found in rice (Fig. 5A), supporting the results of our previous study (Xiong et al., 2015a). However, IVD was positively related to LMA (Fig. 5B), because LT rather than LD was higher in high-LMA leaves (Fig. 6).
Effect of N supplements on LMA
Leaf N status can substantially affect leaf anatomical structure and functions in rice plants (Lee et al., 2011; Xiong et al., 2015b). Under LN supply, inhibition of plant growth will be more severe than that of leaf photosynthesis (Muller et al., 2011), which will potentially lead to the accumulation of NSCs and a higher LMA (Pan et al., 2011). Conversely, leaf protein content will be lower under LN supplementation (Poorter and Pothmann, 1992; Poorter et al., 2009). In the present study, we did not determine total protein content; however, the Rubisco content, which accounts for about 50 % of total soluble leaf protein (Makino et al., 1994), was significantly decreased under LN. This might, to some extent, offset the benefit of the enhanced NSC concentration on LMA increase.
In addition to the variations in chemical composition, leaf anatomical traits can also be significantly affected by N supply (Xiong et al., 2015b). Chloroplast size, which will potentially increase LD and LMA because chloroplasts are much denser than vacuoles, can be enlarged by an increase in N supplementation (Poorter et al., 2009; Xiong et al., 2015b). However, these increase in cell size during leaf expansion are usually accompanied by increases in the size/area of the vacuole and intercellular air space, which will potentially decrease LD and LMA (Volkenburgh, 1999). Therefore, LMA can be either increased or decreased with an improved N supply. In the present study, the response of LMA to N supply was dependent on the variety examined. LMA decreased in HHZ under SN supply (Table 2) and increased in N22, but was unchanged in GY and ZS. VTminor was unresponsive to N supply in all selected genotypes. Variations in LD and VTmajor in response to N supply in N22, GY and ZS were similar to that in LMA; however, the decreased LMA in HHZ under SN supply was accompanied by decreased LD, but not VTmajor. Therefore, variation in response of LMA to N supply was again more related to changes of LD than LT.
Impacts of LMA on A and PNUE
In the present study, PNUE increased under LN supply in all varieties, but the magnitude of the change in PNUE varied with varieties. Based on the widely used photosynthetic model (Farquhar et al., 1980), under the current atmospheric CO2 (approx. 380 ppm) and temperature (25 °C) conditions, A is mainly limited by the carboxylation capacity of Rubisco and the CO2 concentration in chloroplasts (Cc). The former is determined by both Rubisco content and activity status. In the present study, it was found that Rubisco content increased with increasing N supply in all cultivars, and the Cc was decreased in N22 whereas it was increased in ZS.
In C3 plants, gm is a major determinant of Cc and A. The relationships between gm and anatomical traits, such as mesophyll cell packing, shape and cell wall thickness, have been well documented (Tomas et al., 2013; Xiong et al., 2015b). Mesophyll cell wall thickness, mesophyll cell wall surface area exposed to intercellular air spaces per leaf area (Sm), and chloroplast surface area exposed to intercellular air spaces (Sc) are the most important structural components of a leaf that are related to gm. The variation of gm across the selected genotypes in the present study may relate to the leaf anatomical features. Recently, we demonstrated that increased gm under the supplemented SN condition is related to the increases in Sm and Sc (Xiong et al., 2015b). Here, we found that the response of gm to N supply varied with cultivars, and the response seems to be related to the changes in the LMA. If the LMA, which here appears to be closely related to LD, is increased by high N supply (e.g. N22), mesophyll cells should be more closely packed, resulting in a lower fraction of intercellular air spaces. This will potentially decrease the increasing percentages of Sm and Sc, resulting in a relatively insensitive response of gm to N supply (e.g. N22). Due to insufficient CO2, the increased Rubisco in high N leaves will function more for N storage rather than as an enzyme, and consequently result in more severely decreased PNUE (Fig. 7).
Leaf photosynthesis is strongly correlated with LMA, and exploiting the natural variation of LMA and its plasticity to the growth environment is very important to improve leaf photosynthesis. This study not only highlights the importance of LD in the plasticity of LMA, but also shows the influence of LMA on leaf photosynthesis, especially PNUE. Therefore, optimizing leaf structure through decreasing LD can result in a benefit in terms of leaf photosynthesis and NUE at the leaf scale in Oryza. Further research, focusing on the influences of structural components (e.g. the fraction of intercellular air spaces) and chemical components (e.g. NSCs, lignin and protein contents) on LMA and LD, should be conducted to investigate the determination of LD and the influence on leaf photosynthesis.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31301840), A Foundation for the Author of National Excellent Doctoral Dissertation of PR China (201465), Hubei provincial Natural Science Foundation (2013CFB201), and Fundamental Research Funds for the Central Universities (2013PY107 and 2012SC13).
LITERATURE CITED
- Blonder B, Violle C, Bentley LP, Enquist BJ. 2011. Venation networks and the origin of the leaf economics spectrum. Ecology Letters 14: 91–100. [DOI] [PubMed] [Google Scholar]
- Blonder B, Violle C, Bentley LP, Enquist BJ. 2014. Inclusion of vein traits improves predictive power for the leaf economic spectrum: a response to Sack et al. (2013). Journal of Experimental Botany 65: 5109–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Díez P, Puyravaud JP, Cornelissen JHC. 2000. Leaf structure and anatomy as related to leaf mass per area variation in seedlings of a wide range of woody plant species and types. Oecologia 124: 476–486. [DOI] [PubMed] [Google Scholar]
- Chatterton NJ, Silvius JE. 1981. Photosynthate partitioning into starch in soybean leaves: II. irradiance level and daily photosynthetic period duration effects. Plant Physiology 67: 257–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong MF, Lucas PW, Ong JSY, Pereira B, Tan HTW, Turner IM. 1992. Leaf fracture toughness and sclerophylly: their correlations and ecological implications. New Phytologist 121: 597–610. [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- Giuliani R, Koteyeva N, Voznesenskaya E, Evans MA, Cousins AB, Edwards GE. 2013. Coordination of leaf photosynthesis, transpiration, and structural traits in rice and wild relatives (Genus Oryza). Plant Physiology 162: 1632–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. 1992. Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiology 98: 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TD, Barrott SH, Reich PB. 2011. Photosynthetic responses of 13 grassland species across 11 years of free-air CO2 enrichment is modest, consistent and independent of N supply. Global Change Biology 17: 2893–2904. [Google Scholar]
- Long SP, Bernacchi CJ. 2003. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany 54: 2393–2401. [DOI] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohira K. 1985. Enzymic properties of ribulose-1,5-bisphosphate carboxylase/oxygenase purified from rice leaves. Plant Physiology 79: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Mae T, Ohira K. 1986. Colorimetric measurement of protein stained with Coomassie Brilliant Blue R on sodium dodecyl sulfate–polyacrylamide gel electrophoresis by eluting with formamide. Agricultural Biological Chemistry 50: 1911–1912. [Google Scholar]
- Makino A, Nakano H, Mae T. 1994. Responses of ribulose-1, 5-bisphosphate carboxylase, cytochrome f, and sucrose synthesis enzymes in rice leaves to leaf nitrogen and their relationships to photosynthesis. Plant Physiology 105: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Pantin F, Génard M, et al. 2011. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. Journal of Experimental Botany 62: 1715–1729. [DOI] [PubMed] [Google Scholar]
- Pan J, Cui K, Wei D, Huang J, Xiang J, Nie L. 2011. Relationships of non-structural carbohydrates accumulation and translocation with yield formation in rice recombinant inbred lines under two nitrogen levels. Physiologia Plantarum 141: 321–331. [DOI] [PubMed] [Google Scholar]
- Poorter H, Pothmann P. 1992. Growth and carbon economy of a fast-growing and a slow-growing grass species as dependent on ontogeny. New Phytologist 120: 159–166. [Google Scholar]
- Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R. 2009. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182: 565–588. [DOI] [PubMed] [Google Scholar]
- Roderick ML, Berry SL, Saunders AR, Noble IR. 1999. On the relationship between the composition, morphology and function of leaves. Functional Ecology 13: 696–710. [Google Scholar]
- Sack L, Scoffoni C, McKown AD, et al. 2012. Developmentally based scaling of leaf venation architecture explains global ecological patterns. Nature Communications 3: 837. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C, John GP, et al. 2013. How do leaf veins influence the worldwide leaf economic spectrum? Review and synthesis. Journal of Experimental Botany 64: 4053–4080. [DOI] [PubMed] [Google Scholar]
- Sack L, Scoffoni C, John GP, et al. 2014. Leaf mass per area is independent of vein length per area: avoiding pitfalls when modelling phenotypic integration (reply to Blonder et al. 2014). Journal of Experimental Botany 65: 5115–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Rueb S, Meijer AH. 2003. The RADICLELESS1 gene is required for vascular pattern formation in rice. Development 130: 645–658. [DOI] [PubMed] [Google Scholar]
- Smillie IRA, Pyke KA, Murchie EH. 2012. Variation in vein density and mesophyll cell architecture in a rice deletion mutant population. Journal of Experimental Botany 63: 4563–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas M, Flexas J, Copolovici L, et al. 2013. Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. Journal of Experimental Botany 64: 2269–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini R, Epron D, De Angelis P, Matteucci G, Dreyer E. 1995. In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water supply. Plant, Cell and Environment 18: 631–640. [Google Scholar]
- Vile D, Garnier E, Shipley B, et al. 2005. Specific leaf area and dry matter content estimate thickness in laminar leaves. Annals of Botany 96: 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar R, Ruiz-Robleto J, Ubera JL, Poorter H. 2013. Exploring variation in leaf mass per area (LMA) from leaf to cell: an anatomical analysis of 26 woody species. American Journal of Botany 100: 1969–1980. [DOI] [PubMed] [Google Scholar]
- Volkenburgh EV. 1999. Leaf expansion – an integrating plant behaviour. Plant, Cell and Environment 22: 1463–1473. [Google Scholar]
- Winter H, Robinson D G, Heldt HW. 1993. Subcellular volumes and metabolite concentrations in barley leaves. Planta 191: 180–190. [Google Scholar]
- Witkowski ETF, Lamont BB. 1991. Leaf specific mass confounds leaf density and thickness. Oecologia 88: 486–493. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. 2004. The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- Xiong D, Yu T, Zhang T, Li Y, Peng S, Huang J. 2015a. Leaf hydraulic conductance is coordinated with leaf morpho-anatomical traits and nitrogen status in the genus Oryza. Journal of Experimental Botany 66: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D, Liu X, Liu L, et al. 2015b. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant, Cell and Environment 38: 2541–2550. [DOI] [PubMed] [Google Scholar]
- Yamori W, Nagai T, Makino A. 2011. The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant, Cell and Environment 34: 764–777. [DOI] [PubMed] [Google Scholar]