Abstract
Background and Aims Stomatal conductance has long been considered of key interest in the study of plant adaptation to water stress. The expected increase in extreme meteorological events under a climate change scenario may compromise survival in Eucalyptus globulus plantations established in south-western Spain. We investigated to what extent changes in stomatal conductance in response to high vapour pressure deficits and water shortage are mediated by hydraulic and chemical signals in greenhouse-grown E. globulus clones.
Methods Rooted cuttings were grown in pots and submitted to two watering regimes. Stomatal conductance, shoot water potential, sap pH and hydraulic conductance were measured consecutively in each plant over 4 weeks under vapour pressure deficits ranging 0·42 to 2·25 kPa. Evapotranspiration, growth in leaf area and shoot biomass were also determined.
Key Results There was a significant effect of both clone and watering regime in stomatal conductance and leaf-specific hydraulic conductance, but not in sap pH. Sap pH decreased as water potential and stomatal conductance decreased under increasing vapour pressure deficit. There was no significant relationship between stomatal conductance and leaf-specific hydraulic conductance. Stomata closure precluded shoot water potential from falling below −1·8 MPa. The percentage loss of hydraulic conductance ranged from 40 to 85 %. The highest and lowest leaf-specific hydraulic conductances were measured in clones from the same half-sib families. Water shortage reduced growth and evapotranspiration, decreases in evapotranspiration ranging from 14 to 32 % in the five clones tested.
Conclusions Changes in sap pH seemed to be a response to changes in atmospheric conditions rather than soil water in the species. Stomata closed after a considerable amount of hydraulic conductance was lost, although intraspecific differences in leaf-specific hydraulic conductance suggest the possibility of selection for improved productivity under water-limiting conditions combined with high temperatures in the early stages of growth.
Keywords: Eucalyptus globulus, stomatal conductance, leaf-specific hydraulic conductance, sap alkalization, acidification, vapour pressure deficit, percentage loss of hydraulic conductance, water potential, water stress
INTRODUCTION
Eucalyptus globulus is widely used for pulp production around the world and can be considered one of the most important eucalypt species given its high growth rate and pulping properties. Eucalyptus globulus plantations established in south-west Spain are subjected to both high temperatures and severe summer drought (Pita et al., 2001). The use of selected clones has improved both growth and survival under such limiting conditions (Supplementary Data Fig. S1). However, some of these clones might fail under extreme meteorological conditions, as shown by the effects of the exceptional drought of 2005.
Stomatal conductance has long been considered of key interest in the study of plant adaptation to drought and high temperatures (Pearce et al., 2005; Grossnickle and Russell, 2010). This is particularly true for E. globulus plantations established under Mediterranean climates, because (1) high vapour pressure deficits (VPDs) may result in water stress even when soil water is abundant, and (2) E. globulus was found to reach its highest productivities through lower water-use efficiency in field trials established in south-western Spain (Pita et al., 2001). After analysing the strong dependence of a wide range of photosynthetic parameters on stomatal conductance, Medrano et al. (2002) proposed the use of mid-morning, light-saturated stomatal conductance as a reference parameter to reflect the intensity of water stress. Stomata control several trade-offs that determine growth under water-limiting conditions. Minimizing water loss by stomatal closure under drought conditions reduces CO2 uptake and leaf cooling via transpiration but increases water use efficiency while allowing the plant to avoid low shoot water potentials. Stomata have long been recognized as an efficient means of controlling the risk of xylem embolism (Jones and Sutherland, 1991), at least under non-extreme soil water deficits (Meinzer et al., 2009). In some species, stomata may close at the incipience of xylem embolism, as in walnut (Juglans regia × nigra) (Cochard et al., 2002). In other species, stomatal conductance and transpiration are maximized at the expense of a certain degree of embolism (Manzoni et al., 2013).
Both hydraulic and chemical signals participate in the regulation of stomatal conductance. Stomata have been found to respond to, among other things, cavitation-induced changes in stem hydraulic conductance (Ripullone et al., 2007; Hölttä et al., 2012), the abscisic acid (ABA) concentration in the xylem sap (Heilmeier et al., 2007) and xylem sap alkalization (Sobeih et al., 2004; Wan et al., 2004). Although differences between species in hydraulic traits such as vulnerability to cavitation or leaf-specific hydraulic conductance have been widely documented (Fu et al., 2012; Tixier et al., 2014), studies comparing genotypes from a single species are less frequent.
Root-to-shoot signalling is often considered to be important in regulating shoot growth and water use when soil conditions change. Identifying signal molecules and their roles is seen as a potential way to modify crop water use (Dodd, 2005). In contrast, root signalling has been considered less effective for very tall species, in which signal transmission may be too slow for a feed-forward model of short-term stomatal response and thus other factors, such as ABA production or release within the leaves, may be more important (Heilmeier et al., 2007). However, it must be considered that changes in xylem sap may arise from root export of signalling substances and also from changes in sap composition during long-distance transport in the stem (Dodd, 2005).
The objective of this study was to investigate the combined effects of VPD and water shortage on stomatal conductance in E. globulus clones. More precisely, we aimed to (1) investigate the extent to which changes in stomatal conductance are mediated by changes in hydraulic conductance and/or xylem sap pH in the species and (2) identify differences between clones in the response to water shortage and VPD. We hypothesized that (1) xylem sap pH may increase with decreasing soil water, (2) xylem sap pH may respond to changes in VPD and (3) hydraulic traits may differ between clones.
To test these hypotheses, a greenhouse experiment was carried out with closely related E. globulus genotypes of contrasting drought resistance.
MATERIALS AND METHODS
Plant material and growing conditions
The experiment was carried out in a greenhouse (15–35 °C), from May to the end of June. Maximum photosynthetically active radiation (PAR) was 1600 μmol m−2 s−1. Air temperature and relative humidity (Rh, %) were recorded with a Lambrecht thermo-hygrograph. Saturation vapour pressure (Psat) was calculated at two-hour intervals from air temperature (Nobel, 2009) and VPD was derived from: VPD=Psat(1 − Rh/100). Values of VPD at any other time were calculated from these values by linear interpolation. Daily maximum VPD was typically reached around 1800 h (local time) and ranged from 1·46 to 3·84 kPa.
Seventy E. globulus Labill. rooted cuttings grown from scions less than 1 year old were transplanted to 50-litre pots filled with the same weight of lightly fertilized peat (Kekkilä B6 white 420, Vandaa, Finland) mixed with perlite (1 : 1 v/v). Five extra pots were used to draw the relationship between the volumetric soil water content (Hvol) measured with a TRIME TDR system (IMKO Gmbh, Ettlingen, Germany) and pot weight (W), covering the range of weight values in the experiment. The relationship between the two variables (Hvol= 0·0162W − 16·45, r2=0·96, n=25) was used to determine volumetric soil water content from the weight of the potted plants. Plant weight was considered negligible, as it was much lower than the pot weight.
Five clones were selected for the experiment. Clones T and OD are F1 clones that had been widely used in commercial plantations in south-west Spain. Interestingly, both clones differed in their response to the exceptional drought of 2005 (worst drought since 1947, Aemet, 2005). Clone T was most affected and therefore withdrawn from production from then on. Clone C14 is an F0 clone that shows an enhanced survival rate but lower growth rates than clones OD and T (Supplementary Data Table S1). Clones PI and SA are F1 clones that belong to the same half-sib families as OD and T, respectively, and were chosen for this study simply because of their shared affiliation with the others, clone C14 being the common progenitor.
After transplanting, plants were allowed to grow and acclimate for a period of 3 weeks. Plants were watered two to three times a week and fertilized twice with 1 g per plant of soluble Peters fertilizer (N/P/K 20 : 20 : 20) (Scotts International, Heerlen, The Netherlands) during this period. On 31 May [day 0 (d0)], two watering regimes (R1 and R2) were established. In R1, the plants were watered until a weight of 2600 g was reached, while for R2 plants the figure was 2300 g. These values corresponded to 90 and 73 % of the volumetric soil water content at field capacity for R1 and R2, respectively. Plants from the two watering regimes were watered up to these weights throughout the experiment. All plants were watered on Mondays, Wednesdays and Fridays except during the third week of measurements in which R2 plants were not watered on Wednesday to increase the level of water stress.
Measurements
The amount of water lost by evapotranspiration was calculated between irrigations from the weight of each potted plant before and after watering.
Synchronous measurements of stomatal conductance, sap pH, shoot water potential (Ψ) and hydraulic conductance (hereafter ‘physiological parameters’) were carried out plant by plant on d4, d8, d9, d10, d16, d18 d19, d23 and d26. From d4 to d10, six plants per day (three from each watering regime, from the same three clones) were measured and harvested. On d16–d19 the sample size was increased up to ten plants per day (one per clone and watering regime). Eight plants from one single watering regime (one to two per clone) were measured on d23 (R1) and d26 (R2) to establish whether the moment at which the measurements were taken had a significant effect on the physiological parameters.
Stomatal conductance to water vapour and net photosynthetic rate were measured in the youngest fully expanded leaf using a portable gas exchange chamber (Li-Cor 6400XT; Li-Cor, Lincoln, NB, USA). All measurements were made between 1045 and 1315 h (local time), under 300-W metal halide lamps to ensure a PAR above 1000 μmol m−2 s−1. We later verified that there was no significant relationship between the rate of photosynthesis and PAR values in the range 1000–1600 μmol m−2 s−1, meaning that light intensity could be considered saturating for all measurements.
Immediately after measuring gas exchange, each plant was taken to the laboratory (less than 5 min from the greenhouse), weighed with its container and cut under water just below the 6th–7th node. Time of harvesting was annotated for each plant. Water potential (Ψ) was determined in the shoot apex using a Scholander-type pressure chamber (Plant Moisture Systems, Santa Barbara, CA, USA). Prior to this, about 3 cm of bark was removed from the cut end of the apical portion of the stem. After recording Ψ, an over-pressure of 0·2–0·4 MPa was applied to the shoot to collect xylem sap. Xylem sap pH was measured immediately afterwards using a microelectrode (Model 5208, CRISON Instruments, Barcelona, Spain) interfaced with a pH meter (CRISON micropH 2002, CRISON Instruments). A similar procedure has been used previously in shoots (Dodd et al., 2003) and leaves (Rodrigues et al., 2008).
At the same time as the water potential and sap pH were measured, the basal portion of the plant was prepared for hydraulic conductance measurements: all leaves were cut off under water and the stem was fixed to a tubing system connected to a low-pressure water reservoir. Hydraulic conductance was determined before (Ki) and after (KM) removing xylem embolism as explained elsewhere (Pita et al., 2003). The percentage loss of hydraulic conductance (PLC) was calculated from PLC=100(KM − Ki)/KM. When measuring plant leaf area (WinFolia, Regent Instruments, Quebec, Canada), the surface of leaves in the apical portion (Lap) was determined separately from the basal leaves. Leaf-specific hydraulic conductance (LSC) was calculated from LSC=Ki/Lap. We found no significant relationship between LSC and the length of the stem. Therefore, LSC data were not corrected for stem length. Stems and leaves were oven dried at 60 °C and weighed.
Statistical analyses
Analysis of covariance (ANCOVA) was used to analyse the effect of clone and watering regime on all variables. Time (day number) was used as a continuous predictor when analysing differences in growth, whereas VPD was used as a continuous predictor when analysing differences in the physiological parameters.
ANCOVA was also used to verify that there was no significant effect of the time of harvesting on the physiological parameters, using the time of harvesting (hour:minutes) as a continuous predictor, and to compare regression lines. The effect of the clone or watering regime was also tested on a daily basis through one-way ANOVA. Relationships between variables were analysed through simple linear regression.
Percentage data were arcsin transformed prior to analyses. All variables were tested for normality and homogeneity of variance. Differences were considered statistically significant at P ≤ 0·05. Tukey’s honest significant difference method was used to separate the means. STATISTICA version 6·0 (StatSoft, Tulsa, OK, USA) was used for all tests.
RESULTS
Growth and evapotranspiration
Shoot biomass and leaf area increased linearly with time. Water shortage significantly decreased growth (Table 1). Figure 1 shows the time course of average shoot biomass for both watering regimes. Similar results were obtained for leaf area (not shown). There were no significant differences between clones in leaf area or shoot biomass (Table 1).
Table 1.
Results from the ANCOVA for the variables tested
| LA (cm2 per plant) | Wp (g per plant) | Ψ (MPa) | Sap pH | gs (mol m−2 s−1) | LSC (10−5 kg s−1 m−2 MPa−1) | PLC* | |
|---|---|---|---|---|---|---|---|
| P-values | |||||||
| Covariate | <0·0001 | <0·001 | <0·0001 | <0·0001 | <0·0001 | 0·026 | 0·002 |
| Clone | 0·120 | 0·063 | 0·948 | 0·282 | 0·045 | 0·008 | 0·104 |
| Watering | 0·002 | 0·016 | 0·002 | 0·987 | 0·013 | 0·025 | 0·771 |
| Clone × watering | 0·807 | 0·323 | 0·351 | 0·627 | 0·981 | 0·784 | 0·534 |
| Adjusted means±s.e. | |||||||
| Clone | |||||||
| C14 | 1851±146a | 10·39±0·90a | −1·32±0·05a | 6·6±0·05a | 0·34±0·049a | 35·2±3·7ab | 0·81±0·06a |
| OD | 2050±137a | 13·43±0·91a | −1·35±0·05a | 6·5±0·05a | 0·48±0·050ab | 40·2±3·9a | 0·71±0·06a |
| PI | 1865±141a | 11·49±0·90a | −1·35±0·05a | 6·7±0·05a | 0·40±0·051ab | 40·9±3·6a | 0·76±0·06a |
| SA | 2217±128a | 13·29±0·85a | −1·32±0·05a | 6·6±0·05a | 0·51±0·046b | 23·8±3·5b | 0·92±0·05a |
| T | 2270±140a | 13·36±0·94a | −1·30±0·05a | 6·6±0·05a | 0·53±0·052ab | 29·9±4·0ab | 0·78±0·06a |
| Watering regime | |||||||
| R1 | 2252±87a | 13·38±0·57a | −1·26±0·03a | 6·58±0·03a | 0·51±0·032a | 37·9±2·4a | 0·77±0·04a |
| R2 | 1849±87b | 11·4±0·56b | −1·40±0·03b | 6·59±0·03a | 0·39±0·032a | 30·1±2·4b | 0·81±0·04a |
Time was used as covariate to analyse leaf area (LA) and dry biomass (Wp). Vapour pressure deficit was used as covariate for shoot water potential (Ψ), sap pH, stomatal conductance (gs), leaf specific hydraulic conductance (LSC) and the percentage loss of hydraulic conductance (PLC). Different letters denote significant differences at P <0·05.
*Values were arcsin transformed for the analysis.
Fig. 1.
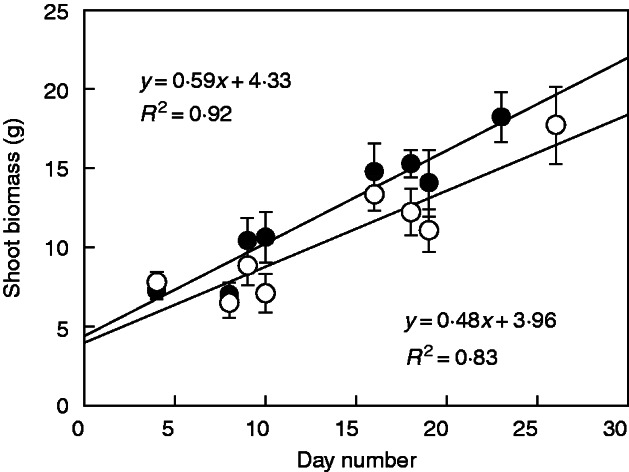
Linear growth functions fitted by least squares regression to the average shoot biomass (n = 3–10) of plants grown under two watering regimes, R1 (filled symbols) and R2 (empty symbols) (see text for further details)
The mean rate of evapotranspiration measured between irrigations in R1 plants increased from 212±7·5 g day−1 at the beginning of the experiment up to 397±20·8 g day−1 at the end. The total amount of water lost by evapotranspiration increased linearly with time. There were significant differences in the slope of the regression lines fitted to both watering regimes (ANCOVA, F=59·69, P<0·00001). We found no significant differences between clones in the rate of evapotranspiration measured at the beginning of the experiment, either in plants from treatment R1 (one-way ANOVA, F=1·47, P=0·23) or in plants from treatment R2 (one-way ANOVA, F=0·63, P=0·64). However, we found a significant effect of the clone on the slope of the regression lines fitted to evapotranspiration vs. time. These slopes estimate the average rates of evapotranspiration measured throughout the experiment for each clone–watering regime combination. Under the more favourable watering regime, the highest rate of evapotranspiration corresponded to clone SA and the lowest to clone C14 (Table 2). For treatment R2, the highest evapotranspiration rates were measured in clones SA and T and the lowest in clones OD and PI. Water shortage resulted in decreases in the rate of evapotranspiration that ranged from 14 to 32 % (Table 2).
Table 2.
Average rates of evapotranspiration (g d−1) calculated by watering regime as the slope of the relationship between time elapsed from the beginning of the experiment and the total amount of water lost by evapotranspiration
| Clone |
||||||
|---|---|---|---|---|---|---|
| C14 | SA | T | OD | PI | P | |
| Watering regime | ||||||
| R1 | 258·86a | 326·02b | 295·59c | 315·15bc | 294·36c | 0·006 |
| R2 | 217·12bc | 224·1b | 254·95a | 214·36b | 199·83c | <0·001 |
The data were measured in plants from five E. globulus clones submitted to two watering regimes (R2 > 0·9 for all the lines). Different letters denote significant differences between clones for each watering regime.
Physiological parameters
Increasing VPD inside the greenhouse led to a decrease in both Ψ and mid-morning stomatal conductance in plants from both watering regimes for the last days of the study. Stomatal conductance and Ψ remained above 0·5 mol m−2 s−1 and −1·4 MPa, respectively, from d4 to d16 and fell below these threshold values from d18 onwards (Fig. 2C, D). Xylem sap pH peaked on d8–d16 and then decreased as stomatal conductance decreased (Fig. 2C, E), whereas PLC remained high and stable throughout the experiment (except for plants from treatment R2 on d4).
Fig. 2.
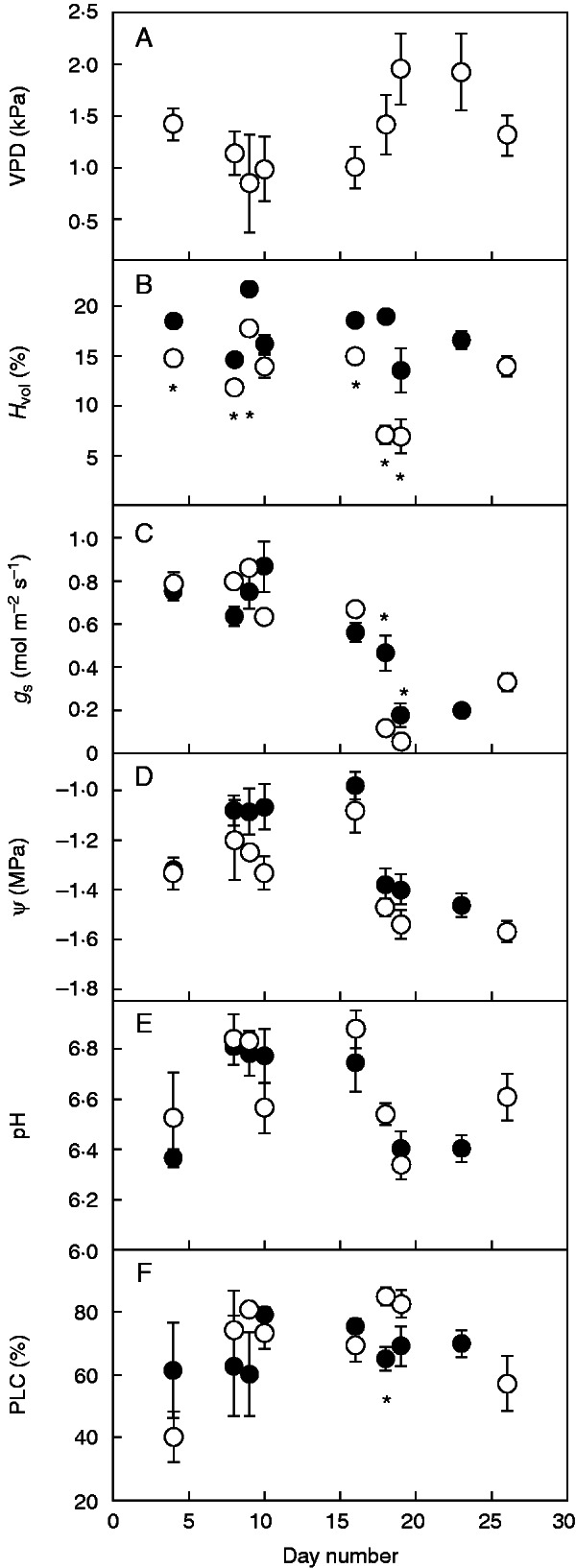
Daily mean±s.e. values of (A) average air vapour pressure deficit inside the greenhouse at the time of measuring, (B) mean volumetric soil water content for the plants measured each day, (C) light-saturated mid-morning stomatal conductance, (D) shoot water potential, (E) xylem sap pH and (F) percentage loss of stem hydraulic conductance. Data are means of 3–10 observations for two watering regimes, R1 (filled symbols) or R2 (empty symbols). An asterisk denotes significant differences between watering regimes.
No significant effect of the watering regime on xylem sap pH was found on any of the measurement occasions (Fig. 2E). This result was particularly striking for d18 and d19, when R2 plants were submitted to a 4-d drought cycle, while R1 plants were watered every 2 d. Stomatal conductance was significantly higher in R1 plants than in R2 plants on d18 and d19 (Fig. 2C). Despite the differences in soil water (Fig. 2B) and stomatal conductance, xylem sap pH values were almost identical for both watering regimes on both days (Fig. 2E).
Results from the ANCOVA revealed a significant effect of VPD on all variables tested (Table 1). There was a tight coordination in the response of stomatal conductance, xylem sap pH and Ψ to VPD (Fig. 3). Stomata closure precluded Ψ from falling below −1·8 MPa (Fig 3B, C). Xylem sap pH decreased as VPD increased (Fig. 3A) but there were no significant differences between either clones or watering regimes in xylem sap pH, nor was there a significant clone × watering regime interaction (Table 1). There was a significant effect of watering regime on stomatal conductance, water potential and LSC. We found significant differences between clones in stomatal conductance and LSC (Table 1). Interestingly, clonal ranking was exactly the same for stomatal conductance and leaf area. The highest stomatal conductance and leaf area values were measured in clones from the same half-sib family (T and SA), and the lowest in the F0 clone (Table 1).
Fig. 3.
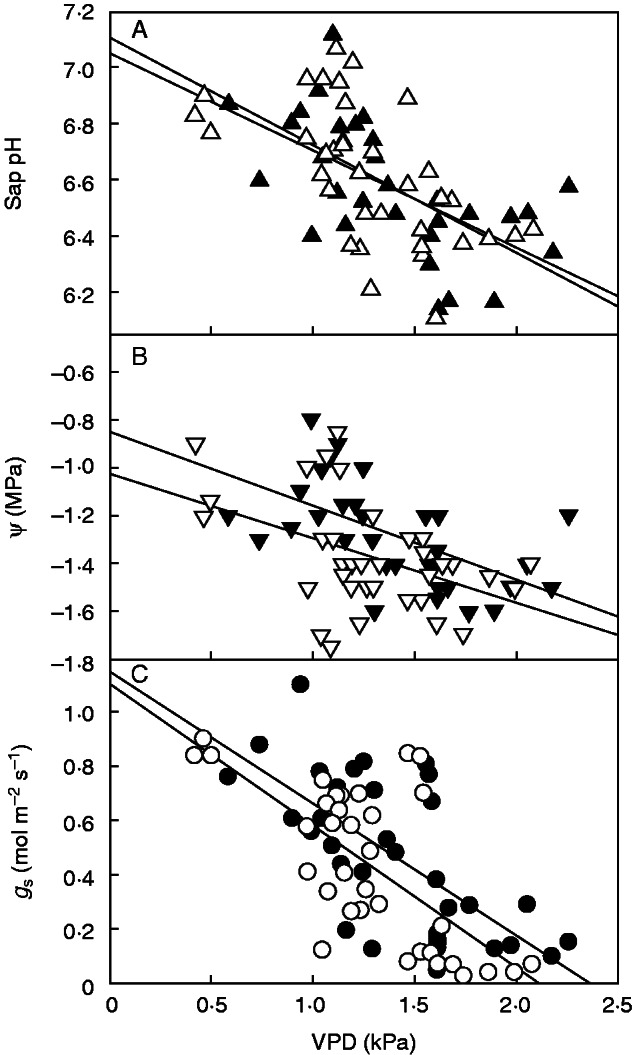
Relationships between air vapour pressure deficit inside the greenhouse at the time of measuring and (A) sap pH, (B) shoot water potential and (C) stomatal conductance. Every point within each graph corresponds to data measured on one single plant. Filled symbols correspond to plants under the more favourable watering regime (R1) and empty symbols to the less favourable R2 watering. Regression lines were fitted to both watering regimes.
Differences between clones in LSC were highly significant. However, if KM instead of Ki was used to calculate LSCmax, no significant differences were found between clones (ANCOVA, F=1·04, P = 0·40) or watering regimes (ANCOVA, F=1·22, P=0·27). Therefore, differences in LSC appear to be related to the loss of hydraulic conductance rather than to anatomical traits such as vessel size. In accordance with these results, there was a strong and negative relationship between LSC and PLC (r2=0·58, P<0·0001). The lowest PLC values were measured in the same clones in which the highest LSC values were measured (OD and PI) and the highest PLC and lowest LSC were measured in clone SA (Table 1).
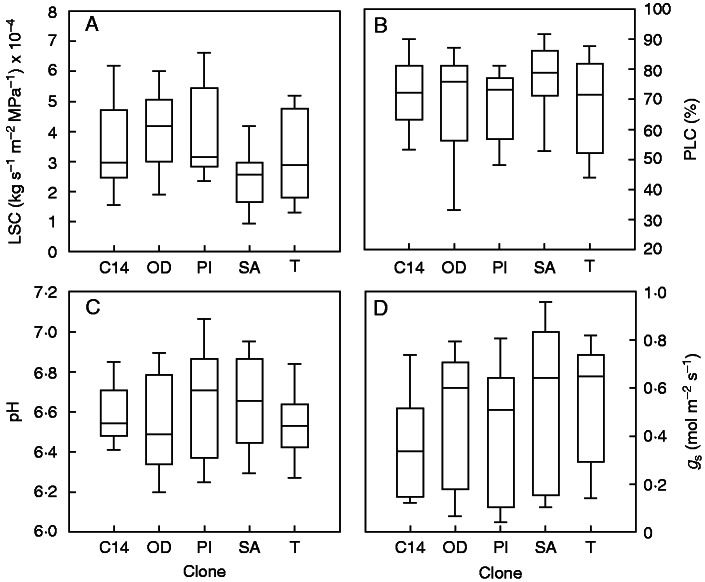
Qualitative analysis of stomatal conductance showed that both the lowest median and the lowest 75th percentile were measured in clone C14 (Fig. 4D). The highest stomatal conductance for the 25th percentile was measured in clone T (Fig. 4D). The latter result shows that under the most stressful conditions plants from clone T did not close stomata as efficiently as the others. The lowest pH value for the 75th percentile was measured in clone T, for which a pH higher than 6·6 was measured only in 25 % of the plants (Fig. 4C). Median PLC values were between 70 and 80 % for all the clones (Fig. 4B). The lowest LSC values for the 25th percentile were measured in clones T and SA, whereas the highest LSC median was measured in clone OD (Fig. 4A). Interestingly, LSC values measured in clones OD and PI were significantly different from the remaining clones (Table 1). These clones belong to the same half-sib family.
Fig. 4.
Box and whisker plots of (A) leaf specific hydraulic conductance, (B) the percentage loss of hydraulic conductance, (C) xylem sap pH and (D) stomatal conductance for the five clones tested. The boundaries of the box represent the 25th and 75th percentiles, the mid-line within the box indicates the median, and whisker caps show the 10th and 90th percentiles.
For all clones and treatments combined, there was a highly significant relationship between stomatal conductance and values of Ψ (r2=0·34, P<0·0001) or sap pH (r2=0·21, P=0·0004) and no relationship with either PLC (r2=0·04, P=0·08) or LSC (r2=0·007, P=0·51). Stomatal conductance decreased significantly as Ψ became more negative (Fig. 5). The highest values of sap pH were measured under concurrent comparatively high stomatal conductance (gs > 0·4 mol m−2 s−1) and high Ψ (Fig. 5).
Fig. 5.
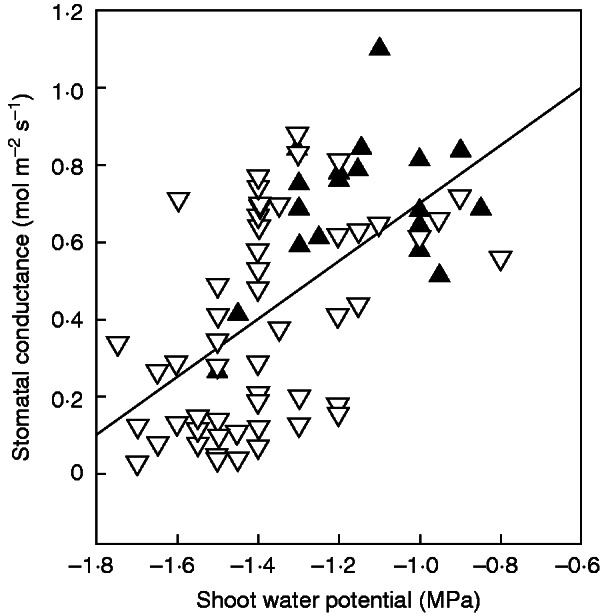
Relationship between stomatal conductance and shoot water potential. Each pair of values was measured consecutively on a single plant. Filled symbols correspond to data measured in plants with a xylem sap pH higher than 6·77, which was the average 75th percentile for pH data. Data from all clones and treatments are pooled together. See text for further details.
DISCUSSION
Stomatal conductance and hydraulic conductance of the stem
High values of stomatal conductance, such as those measured in the present study (Fig. 3), have been associated with low water-use efficiency in fast-growing pioneer species (Pearce et al., 2005) and may be advantageous to compete for available soil water. The adaptive advantage of comparatively high stomatal conductance for eucalypt plantations will depend on the ability to keep a tight control of stomata, to avoid a catastrophic xylem failure. Previous studies have reported high values of native embolism in this (Pita et al., 2003) and other woody species. For example, a PLC as high as 76·7 % was measured in less than 2-year-old twigs from field-grown Eucalyptus crebra and Eucalyptus xanthoclada (Rice et al., 2004). It has been suggested that the formation of embolism may have some positive side effects, such as increasing the hydraulic capacitance (Vergeynst et al., 2015). Although the common occurrence of xylem cavitation remains controversial (Cochard and Delzon, 2013), the differences in stomatal regulation between coexisting ferns and angiosperms reported by Brodribb and Holbrook (2004) suggest that the evolution of a more specialized stomatal physiology may allow gas exchange to be maximized by forcing the xylem to achieve its highest flow rate, in a riskier but more successful water-use strategy (Sperry, 2004). Tolerance of a certain degree of embolism may depend, although not exclusively, on the ability of some species to refill embolized vessels (Trifilò et al., 2014). The suggestion that the hydraulic conductance of the stem is overbuilt (Sterck et al., 2011) must be taken into consideration. In addition, growth of xylem tissue may allow a significant recovery of lost hydraulic function in some tree species (Urli et al., 2013). Furthermore, tree species may lose all their leaves when reaching a PLC higher than 80 %, but still be capable of resprouting after watering (Urli et al., 2013). Therefore, not only the loss of hydraulic conductance but also the amount of remaining hydraulic conductance must be considered when analysing the vulnerability to cavitation (Hacke et al., 2015) or stem hydraulic constraints to gas exchange. LSC is a measure of the hydraulic sufficiency of the stem to supply water to the leaves (Tyree and Zimmermann, 2002). Decreases in LSC under drought conditions have been previously reported in other tree species (Domec et al., 2009; Sellin et al., 2014) and are considered a plastic response to water stress that may compromise water use, and therefore growth, even after the water supply is restored (Eamus et al., 2000). The effect of decreasing LSC on growth was inconclusive in our study, as both LSC and growth decreased in R2 plants in relation to R1 plants but the significant effect of the clone on LSC values did not result in significant differences in growth between clones.
We found no significant relationship between values of stomatal conductance and either PLC or LSC. Moreover, values of stomatal conductance as high as 0·81 mol m−2 s−1 were measured in plants with a PLC close to 80 % in the present study (Fig. 2). These results suggest that loss of hydraulic conductance had little effect on limiting stomatal conductance under our experimental conditions. Similarly, PLC values as high as 50–60 % were found to be compatible with a relatively high stomatal conductance in Ceratonia siliqua, Laurus nobilis and Olea europea trees (Trifilò et al., 2014). By contrast, stomata closure precluded shoot water potential from dropping below −1·8 MPa (Fig. 3) while average PLC remained below 85 % throughout the experiment (Fig. 2F). Interestingly, the PLC corresponding to − 1·8 MPa ranged from 67 to 82 % in field-grown E. globulus clones, as calculated from the vulnerability curves constructed by Pammenter and Vander Willigen (1998). This range of values is quite similar to ours, despite differences in the age of the plants and techniques used to dehydrate the shoots in the two studies. These results suggest that stomata closure was not acting to prevent the onset of cavitation, but rather was an attempt to prevent stem hydraulic conductance from decreasing any further. In accordance with these results, Urli et al. (2013) found that the embolism threshold leading to irreversible drought damage was close to 88 % in five angiosperm tree species. As four of these species reached water potentials close to their minimum recoverable potential under drought conditions, they concluded that safety margins calculated for an 88 % loss of hydraulic conductance would be much more meaningful in angiosperms than the P50 safety margin traditionally used.
Xylem sap pH
Water shortage reduced shoot growth, evapotranspiration, water potential, stomatal conductance and LSC, but had no significant effect on sap pH. Under our experimental conditions, the values for xylem sap pH ranged from 6·1 to 7·0. These values are similar to those reported for Populus deltoides (Aubrey et al., 2011) and Populus nigra (Secchi and Zwieniecki, 2012) but are higher than those measured in stems of field-grown E. globulus by Cerasoli et al. (2009). Despite this wide range of values, we found no significant variation in xylem sap pH between watering regimes. This result suggests that sap alkalization did not act as a mechanism of root to shoot signalling of soil water deficit under our experimental conditions. This is particularly interesting given the shortness of the plants used, as the effect of path length on signal transmission is negligible in seedlings compared with tall trees.
Sap alkalization can be triggered by environmental conditions that stimulate transpiration, such as VPD (Chaves and Oliveira, 2004). In a recent study, Aubrey et al. (2011) observed that xylem sap pH derived from stems and twigs of Populus deltoides L. increased when VPD was lowest, and concluded that sap pH may increase under environmental conditions that result in low transpiration rates. We found a negative correlation between sap pH and VPD (Fig. 3), but measured the highest values of sap pH in plants that showed no strong stomatal limitations to transpiration (Fig. 5).
Whereas xylem sap alkalization has been reported as a common effect of several kinds of stress, the response pattern of stomatal conductance to elevated xylem pH remains unclear. Despite the decrease in stomatal conductance observed in response to modification of xylem sap pH by alkaline foliar sprays, Sharp and Davies (2009) only found concurrent sap alkalization and stomatal closure in four out of 22 perennial species receiving mild and severe soil water deficits. By contrast, Secchi and Zwieniecki (2012) observed that severe water stress resulted in a sudden drop of xylem sap pH in Populus nigra and found that sap pH decreased in embolized vessels undergoing osmotically driven refilling. In accordance with this, we measured a decrease of about 0·4 pH units from d16 to d19 (Fig. 2). The lowest values of stomatal conductance were measured on d18–d23. It has been suggested that stomata closure may favour embolism repair (Tombesi et al., 2015). Interestingly, PLC values measured on d26 in R2 plants were lower than those measured in plants from the more favourable R1 watering regime 3 d before (Fig. 2). Changes in sap pH due to different factors may add to those arising in response to soil drying either antagonistically or synergistically (Sharp and Davies, 2009). The lowering of sap pH under osmotically driven refilling may contribute to explain why sap alkalization is considered a common response to soil drying in herbaceous species but remains elusive in woody species (Sharp and Davies, 2009), as woody species (and particularly tree species) cannot rely on root pressure as a mechanism of embolism repair.
Clonal effects
The lowest evapotranspiration rate was measured under the more favourable watering regime in the F0 clone (C14). Stomatal conductance was also lowest in this clone (Table 1). Therefore, F1 clones were able to profit from extra soil water to a greater extent than the F0 clone. In a previous study we obtained a strong positive correlation between carbon isotope discrimination and the average diameter at breast height in E. gobulus plantations established in south-western Spain (Pita et al., 2001). This result highlights the relevance of reaching high values of stomatal conductance to achieve enhanced growth under a dry Mediterranean climate. In the present study we found a tight relationship between leaf area and stomatal conductance that further supports these findings and is in accordance with the poor growth shown by clone C14 if compared with T or OD in the field (Supplementary Data Table S1). The highest rate of evapotranspiration was measured in clone T under the less favourable R2 treatment. Accordingly, values of stomatal conductance measured under the most stressful conditions were higher in clone T than in the remaining clones (Fig. 4). These results could contribute to explain why clone T was severely affected by the exceptional drought of 2005, particularly if we consider that the lowest values of LSC were measured in clones T and SA (Table 1). In a previous study, Vilagrosa et al. (2003) suggested the existence of an LSC threshold for early leaf shedding. This is a common response to drought in E. globulus plantations established in south-west Spain (Supplementary Fig. S2). It is also a costly response in terms of growth and productivity. Early leaf shedding can also be considered the last line of plant defence against the effects of drought. Clones with a low LSC therefore seem less capable of achieving reasonable growth and survival under Mediterranean climates. This seems particularly relevant under a climate change scenario with a predicted increase in extreme meteorological events.
In conclusion, xylem sap alkalization did not show a clear relationship with soil water status, contrary to our first hypothesis, but sap pH decreased significantly as VPD increased, in agreement with our second hypothesis. Stomata closed after a considerable amount of hydraulic conductance was lost, although the clone effect for LSC was significant, suggesting the possibility of selection for improved productivity under water-limiting conditions combined with high temperatures in the early stages of growth.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following: Table S1: survival and growth at age 3 years in two field trials established in south-west Spain prior to the exceptional drought of 2005. Figure S1: photo showing the enhanced growth of clone OD under the most water-limiting conditions. Figure S2: hemispherical photos taken in June and September at the same eucalypt plantation established in south-west Spain, showing the effect of early leaf shedding.
ACKNOWLEDGEMENTS
We thank Professor Jose A. Pardos and Dr Gabriel Toval, former Research Managing Director of ENCE S.A., for their support and encouragement. We also thank ENCE S.A. for providing the plant material and useful information about the clones tested and Adam B. Collins for language correction of the manuscript. We are grateful for the comments of our colleagues and anonymous reviewers that helped us to improve the manuscript. This work was funded by the Ministerio de Ciencia e Innovacion, Spain (AGL200607886).
LITERATURE CITED
- Aemet . 2005. Año hidrometeorológico. Siete meses de extrema sequía. Spanish Meteorological Agency Web. http://www.aemet.es/es/noticias/2005/20050905 (last accessed 1 April 2015).
- Aubrey DP, Boyles JG, Krynsky LS, Teskey RO. 2011. Spatial and temporal patterns of xylem sap pH derived from stems and twigs of Populus deltoides L. Environmental and Experimental Botany 71: 376–381. [Google Scholar]
- Brodribb TJ, Holbrook NM. 2004. Stomatal protection against hydraulic failure: a comparison of coexisting ferns and angiosperms. New Phytologist 162: 663–670. [DOI] [PubMed] [Google Scholar]
- Cerasoli S, McGuire MA, Faria J. et al. 2009. CO2 efflux, CO2 concentration and photosynthetic refixation in stems of Eucalyptus globulus (Labill.). Journal of Experimental Botany 60: 99–105. [DOI] [PubMed] [Google Scholar]
- Chaves MM, Oliveira MM. 2004. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. Journal of Experimental Botany 55: 2365–2384. [DOI] [PubMed] [Google Scholar]
- Cochard H, Delzon S. 2013. Hydraulic failure and repair are not routine in trees. Annals of Forest Science 70: 659–661. [Google Scholar]
- Cochard H, Coll L, Le Roux X, Ameglio T. 2002. Unravelling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiology 128: 282–290. [PMC free article] [PubMed] [Google Scholar]
- Dodd IC. 2005. Root-to-shoot signalling: assessing the roles of “up” in the up and down world of long-distance signalling in planta. Plant and Soil 274: 251–270. [Google Scholar]
- Dodd IC, Tan LP, He J. 2003. Do increases in xylem sap pH and/or ABA concentration mediate stomatal closure following nitrate deprivation? Journal of Experimental Botany 54: 1281–1288. [DOI] [PubMed] [Google Scholar]
- Domec J-C, Noormets A, King JS. et al. 2009. Decoupling the influence of leaf and root hydraulic conductances on stomatal conductance and its sensitivity to vapour pressure deficit as soil dries in a loblolly pine plantation. Plant Cell and Environment 32: 980–991. [DOI] [PubMed] [Google Scholar]
- Eamus D, O’Grady AP, Hutley L. 2000. Dry season conditions determine wet season water use in the wet–dry tropical savannas of northern Australia. Tree Physiology 20: 1219–1226. [DOI] [PubMed] [Google Scholar]
- Fu P-L, Jiang Y-J, Wang A-Y. et al. 2012. Stem hydraulic traits and leaf water-stress tolerance are co-ordinated with the leaf phenology of angiosperm trees in an Asian tropical dry karst forest. Annals of Botany 110: 189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossnickle SC, Russell JH. 2010. Physiological variation among western red cedar (Thuja plicata Donn ex D. Don) populations in response to short-term drought. Annals of Forest Science 67: 506–517. [Google Scholar]
- Hacke UG, Venturas MD, MacKinnon ED, Jacobsen AL, Sperry JS, Pratt RB. 2015. The standard centrifuge method accurately measures vulnerability curves of long-veselled olive stems. New Phytologist 205: 116–127. [DOI] [PubMed] [Google Scholar]
- Heilmeier H, Schulze ED, Fan J, Hartung W. 2007. General relations of stomatal responses to xylem sap abscisic acid under stress in the rooting zone – A global perspective. Flora 202: 624–636. [Google Scholar]
- Hölttä T, Juurola E, Lindfors L, Porcar-Castell A. 2012. Cavitation induced by a surfactant leads to transient release of water stress and subsequent ‘run away’ embolism in Scots pine (Pinus sylvestris) seedlings. Journal of Experimental Botany 63: 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG, Sutherland RA. 1991. Stomatal control of xylem embolism. Plant Cell and Environment 14: 607–612. [Google Scholar]
- Manzoni S, Vico G, Katul G, Palmroth S, Jackson RB, Porporato A. 2013. Hydraulic limits on maximum plant transpiration and the emergence of the safety–efficiency trade-off. New Phytologist 198: 169–178. [DOI] [PubMed] [Google Scholar]
- Medrano H, Escalona JM, Bota J, Gulías J, Flexas J. 2002. Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Annals of Botany 89: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer FC, Johnson DM, Lachenbruch B, McCulloh KA, Woodruff DR. 2009. Xylem hydraulic safety margins in woody plants: coordination of stomatal control of xylem tension with hydraulic capacitance. Functional Ecology 23: 922–930. [Google Scholar]
- Nobel PS. 2009. Physicochemical and environmental plant physiology, 4th edn. Oxford: Academic Press-Elsevier Inc. [Google Scholar]
- Pammenter NW, Vander Willigen C. 1998. A mathematical and statistical analysis of the curves illustrating vulnerability to cavitation. Tree Physiology 18: 589–593. [DOI] [PubMed] [Google Scholar]
- Pearce DW, Millard S, Bray DF, Rood SB. 2005. Stomatal characteristic of riparian poplar species in a semi-arid environment. Tree Physiology 26: 211–218. [DOI] [PubMed] [Google Scholar]
- Pita P, Soria F, Cañas I, Toval G, Pardos JA. 2001. Carbon isotope discrimination and its relationship to drought resistance under field conditions in genotypes of Eucalyptus globulus Labill. Forest Ecology and Management 141: 211–221. [Google Scholar]
- Pita P, Gascó A, Pardos JA. 2003. Xylem cavitation and leaf water potential in Eucalyptus globulus clones under well-watered and drought conditions. Functional Plant Biology 30: 891–899. [DOI] [PubMed] [Google Scholar]
- Ripullone F, Guerrieri MR, Nole A, Magnani F, Borghetti M. 2007. Stomatal conductance and leaf water potential responses to hydraulic conductance variation in Pinus pinaster seedlings. Tree Structure and Function 21: 371–378. [Google Scholar]
- Rice KJ, Matzner SL, Byer W, Brown JR. 2004. Patterns of tree dieback in Queensland, Australia: the importance of drought stress and the role of resistance to cavitation. Oecologia 139: 190–198. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Santos TP, Rodrigues AP. et al. 2008. Hydraulic and chemical signalling in the regulation of stomatal conductance and plant water use in field grapevines growing under deficit irrigation. Functional Plant Biology 35: 565–579. [DOI] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. 2012. Analysis of xylem sap from functional (non-embolized) and non-functional (embolized) vessels of Populus nigra: chemistry of refilling. Plant Physiology 160: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellin A, Niglas A, Öunapuu-Pikas E, Kupper P. 2014. Rapid and long-term effects of water deficit on gas exchange and hydraulic conductance of silver birch trees grown under varying atmospheric humidity. BMC Plant Biology 14: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RG, Davies WJ. 2009. Variability among species in the apoplastic pH signalling response to drying soils. Journal of Experimental Botany 60: 4363–4370. [DOI] [PubMed] [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. 2004. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. Journal of Experimental Botany 55: 2353–2363. [DOI] [PubMed] [Google Scholar]
- Sperry JS. 2004. Coordinating stomatal and xylem functioning – an evolutionary perspective. New Phytologist 162: 568–570. [DOI] [PubMed] [Google Scholar]
- Sterck F, Markesteijn L, Schieving F, Poorter L. 2011. Functional traits determine trade-offs and niches in a tropical forest community. Proceedings of the National Academy of Sciences of the United States of America 108: 20627–20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tixier A, Herbette S, Jansen S. et al. 2014. Modelling the mechanical behaviour of pit membranes in bordered pits with respect to cavitation resistance in angiosperms. Annals of Botany 114: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombesi S, Nardini A, Frioni T. et al. 2015. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Scientific Reports 5: 12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilò P, Barbera PM, Raimondo F, Nardini A, LoGullo MA. 2014. Coping with drought-induced xylem cavitation: coordination of embolism repair and ionic effects in three Mediterranean evergreens. Tree Physiology 34: 109–122. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Zimmermann MH. 2002. Xylem structure and the ascent of sap, 2nd edn. Berlin: Springer. [Google Scholar]
- Urli M, Portè AJ, Cochard H, Guengant Y, Burlett R, Delzon S. 2013. Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiology 33: 672–683. [DOI] [PubMed] [Google Scholar]
- Vergeynst LL, Dierick M, Bogaers J, Cnudde V, Steppe K. 2015. Cavitation: a blessing in disguise? New method to establish vulnerability curves and assess hydraulic capacitance of woody tissues. Tree Physiology 35: 400–409. [DOI] [PubMed] [Google Scholar]
- Vilagrosa A, Bellot J, Vallejo R, Gil-Pelegrin E. 2003. Cavitation, stomatal conductance and leaf dieback in seedlings of two co-occurring Mediterranean shrubs during an intense drought. Journal of Experimental Botany 54: 2015–2024. [DOI] [PubMed] [Google Scholar]
- Wan X, Landhäusser SM, Zwiazek JJ, Lieffers VJ. 2004. Stomatal conductance and xylem sap properties of aspen (Populus tremuloides) in response to low soil temperature. Physiologia Plantarum 122: 79–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.