Abstract
Context:
There is scant research on premenstrual syndrome (PMS) and its more severe counterpart, premenstrual dysphoric disorder (PMDD) in Indian females. This study aimed to evaluate symptoms of PMS in medical students and to find the association of sociodemographic variables and lifestyle factors with PMDD.
Subjects and Methods:
A total of 179 medical students residing in the hostel of an Indian medical college and its affiliated teaching hospital were approached, of which 100 (55.8%) returned the completed questionnaires. Data related to lifestyle factors was collected. Self-screening quiz for Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision PMDD and Shortened Premenstrual Assessment Form were used for diagnosis of PMDD and detection of symptomatology, respectively.
Results:
PMDD was present in 37% of the respondents. It was found at a higher rate in older and postgraduate students. PMDD was significantly associated with lifestyle factors, namely, sleep, physical activity, total tea/coffee intake, and change in tea/coffee and food intake under stress. The most common physical and psychological symptoms were body ache/joint pain and feeling depressed/blue, respectively.
Conclusions:
PMDD is fairly common in Indian medical students residing in hostel although cultural factors may influence symptom expression. This study suggests that PMDD is associated with lifestyle factors in young, professional, urban women. Modification in lifestyle may thus be an important approach for management of PMS/PMDD. Prospective studies with larger representative samples are needed to validate these findings.
Keywords: India, lifestyle factors, medical students, premenstrual dysphoric disorder
Premenstrual dysphoric disorder (PMDD) is considered a severe form of premenstrual syndrome (PMS) that impairs quality of life as much as other depressive and anxiety disorders do.[1] In addition, the burden of PMS/PMDD as well as the disability-adjusted life years lost due to this repeated-cyclic disorder is in the same magnitude as major psychiatric disorders, yet it remains largely underrecognized.[2] Based on the strong scientific evidence, PMDD has been moved from Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) Appendix B, “Criteria Sets and Axes Provided for Further Study,” to the main body of DSM-5.[3]
Although the precise etiology remains unknown, neurotransmitter abnormalities, particularly those of serotonin have most commonly been implicated,[4] partly because of observations made in preclinical studies, and partly because of the antidepressant and anxiolytic effects exerted by serotonergic drugs, and their proven efficacy in managing PMDD.[5,6] A neurobiological model of enhanced negative emotional processing and diminished positive emotional processing in PMDD during the premenstrual phase has recently been proposed.[7] Lifestyle factors may putatively aggravate symptoms of PMS/PMDD. Thus, lifestyle modifications such as reduced consumption of caffeine, salt, chocolate and refined sugars, increased daily moderate aerobic exercise and use of stress reduction techniques, and relaxation have been advocated as nonpharmacological measures for managing PMS, although definitive evidence for the same is lacking at this time.[1]
For long, PMS has been considered a western culture-bound syndrome. Cross-cultural clinical presentation and variation of PMS/PMDD have not been systematically studied, although available data does suggest that it occurs across cultures at comparable rates.[1] Even though PMDD is a biological phenomenon, sociocultural factors seem to determine perception, explanation, handling, and even help seeking related to it.[8,9] There is scant literature available on PMS/PMDD from low- and middle-income countries, including India. The objectives of this preliminary work were to evaluate symptoms of PMS in Indian medical students residing in hostel and to find the association between lifestyle factors and symptom severity.
SUBJECTS AND METHODS
The study protocol was approved by the Institutional Review Board of Smt. N. H. L. Municipal Medical College, Ahmedabad, India.
A total of 179 female medical students were residing in the hostel of the medical college and tertiary care teaching hospital where the study was conducted, and all of them were approached for inclusion in the study. Out of them, 96 (53.6%) were undergraduate (UG) students and 83 (46.3%) were postgraduate (PG) students. To encourage participation, each potential subject was approached individually by the first author and requested to participate.
The participant information sheet and consent form mentioned the rationale of the study in light of the dearth of data on PMDD from Indian population. It further stated that the study would help generate data and add to the understanding of PMDD in Indian women.
For the final analysis, we were left with 50 (51.5%) completed forms from UGs and 50 (60.2%) completed forms from PG medical students, as shown in Figure 1. Written informed consent was obtained from all the participants while anonymity was ensured.
Figure 1.
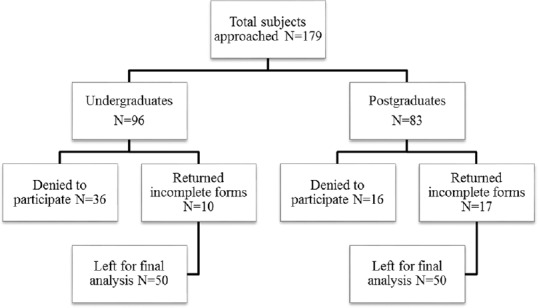
Subjects enrolled in the study
Data were collected on a cross-sectional basis using a self-administered questionnaire, consisting of three parts.
The first part of the questionnaire comprised questions about sociodemographic factors (age, marital status, and educational status) and lifestyle factors that included hours of sleep per day, hours spent in active physical activity (e.g., gym, yoga, aerobics, and swimming, etc.) per day, amount of tea/coffee intake (cups per day), along with effect of stress on tea/coffee intake and the food intake (whether increases, decreases, or does not change).
The second part had the Shortened Premenstrual Assessment Form (SPAF), a valid and reliable instrument which comprises 10 items related to physiological and psychological symptoms occurring about a week before menstruation.[10] The items were to be rated according to the intensity of symptoms during the last menstrual cycle from 1 to 6. The symptom was regarded as present only if the respondent rated four or above on that particular item (1 meaning “not present or no change from usual” and 6 meaning “extreme change, perhaps noticeable even to casual acquaintances”). The list included four behavioral/psychological symptoms and six physical symptoms.
The final portion was composed of a self-screening quiz (SSQ) for PMDD as per the DSM-IV-Text Revision (TR) criteria,[11] based on which the respondents were diagnosed as having PMDD [Appendix 1]. The SSQ consists of two lists of symptoms, all contained in the diagnostic criteria for DSM-IV-TR PMDD. The participants were asked to note all symptoms from both the lists that they experienced premenstrually at least during two menstrual cycles, followed by four questions with a “yes” or “no” response. An affirmative response on all the four questions was considered as PMDD to be present.
Statistical analysis
The data thus collected were analyzed using SPSS version 20 (IBM Corporation). The standard methods of descriptive statistics were used to describe the data (i.e., frequencies and percentages for categorical variables and mean values with standard deviations for continuous variables). Kolmogorov–Smirnov test was used to assess the normality of distribution of the continuous variables. Violations of assumptions needed for parametric tests necessitated nonparametric tests. Chi-square test and Mann–Whitney test were applied for assessing categorical and continuous variables, respectively. Significance was set at P < 0.05 (two-tailed).
RESULTS
In this study, a total of 179 hostel inmates were approached, 127 (70.9%) medical students consented to participate, of which 100 (55.8%) returned the completely filled questionnaire. The ratio of UG to PG students for the final analysis was 1:1. The mean age of the participants was 22.9 (standard deviation 2.9) years (range 19–28 years), and 12% of them were married. All the respondents reported, at least, one symptom of PMS present in a significant intensity on the SPAF. As per the SSQ, 37% were screened as having PMDD.
As shown in Table 1, PMDD was found at a higher rate in older and PG students, but was not associated with marital status. Students who had PMDD were sleeping less and were indulging less in physical activity as against those without the diagnosis. PMDD was also significantly associated with greater tea/coffee intake as well as increase in tea/coffee and food intake under stress. Students with PMDD scored higher on both psychological as well as physiological symptoms, as measured by SPAF.
Table 1.
Premenstrual dysphoric disorder and its association with different variables
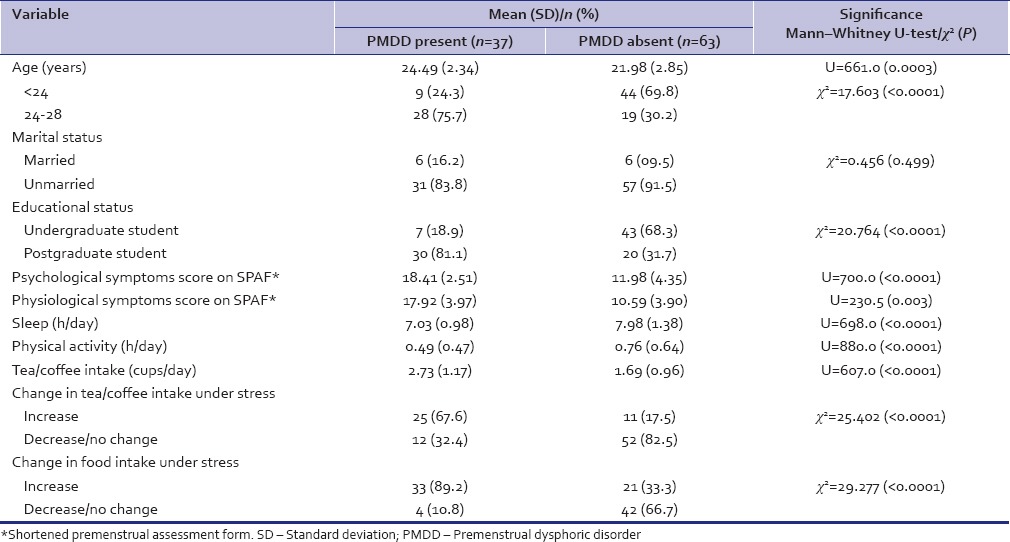
Figure 2 shows the frequency of physical and psychological symptoms reported by the respondents on SPAF. The most common physical and psychological symptoms were body ache/joint pain and feeling depressed/blue, respectively.
Figure 2.
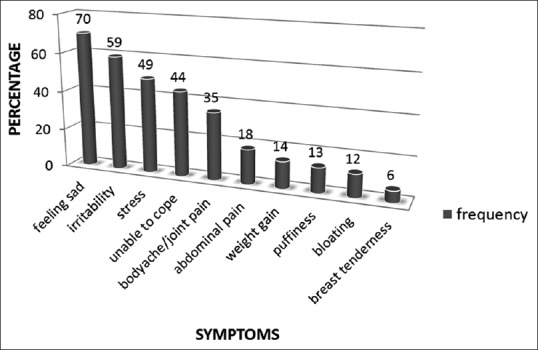
Frequency of physical and psychological symptoms reported by the subjects on shortened premenstrual assessment form
DISCUSSION
Frequency of premenstrual dysphoric disorder
The results of this work indicate that PMS is fairly common among medical students residing in hostel. 37% of the students had the severe form of PMS, namely PMDD. PMS estimates vary substantially in literature because of the differences in instruments, symptom patterns, and the use of prospective or retrospective protocols. Our findings are comparable with a study performed in Nigerian medical students, in which 36.1% of the respondents had DSM-IV-TR PMDD.[12] Another study in medical students in Saudi Arabia found PMS in 35.6% of the cases, based on the American College of Obstetrics and Gynecology (ACOG) criteria.[13] However, the rate of PMDD in our study is clearly high as compared to reports in other studies on medical and nonmedical students that reported PMDD in 6–18% of the students in their samples.[14,15,16,17,18] Assuming that living in the hostel setting is in itself stressful, this could have contributed to our finding of such high frequency of PMDD in the participants. Psychosocial stress levels are reportedly associated with severity of PMS.[19,20] Inclusion of a control group comprising medical students not residing in hostel could eliminate the effect of stress contributed by staying in a hostel. Delara et al. found in their work that PMDD was present in 37.2% of boarding high school students, a rate similar to that in our study.[21] Studying and staying away from home in a boarding school setting is comparable to staying in a hostel in terms of the stress generated by the situation.
Age, educational status, marital status, and premenstrual dysphoric disorder
The variance in age in our sample was relatively small as the age of participants ranged from 19 to 28 years. Studies that recruit participants of a wider range of age, preferably the entire reproductive age group (15–49 years) may be better equipped to establish the relationship between age and PMDD. A population-based research found PMS to be higher in women aged 35–44 years.[9] Conversely, other studies found older age to be associated with less severe PMS symptoms.[22,23,24] In keeping with our finding, another study on medical students found older student age to be a risk factor for PMS.[13] However, being a PG student rather than a UG student could be a potential confounder for the positive association between older age and PMDD in this work, and vice versa.
In agreement with our work, marital status was not found to affect PMS symptom severity in Thai[23] and Iranian[15] female university students, and a population-based sample of Swiss women.[25] Tschudin et al. reported a higher occurrence of PMDD in unmarried women.[9] There is no plausible explanation for the direct influence of marital status on PMDD, although indirect factors may have a role to play.
PG students are full-time resident doctors working in their respective departments and have work-related stress apart from studying. UG students supposedly have just study-related stress. They do have clinical postings, but no formal assigned duties or responsibilities. Overall, PG students are far more stressed than UGs. Women with PMS perceive having more work pressure, less autonomy on the job, and less variety in their work than controls.[26] This partly explains why a higher number of PG students screened as having PMDD as against UG students. Medical school interns working in 24-h shifts had a higher rate of PMS when compared to 3rd-year medical students who did not have shift work responsibilities,[27] which is in support of our finding.
Physical and psychological symptoms and premenstrual dysphoric disorder
A cross-sectional study across four continents concluded that there was a great deal of similarity in women's experiences of PMS symptoms across countries.[28] Abdominal bloating, cramps or abdominal pain, irritability, mastalgia, and joint/muscle/back pains were the most common symptoms reported across 14 nations.[28] Both physical and psychological symptoms were commonly reported by students in this study, the most common physical symptom being body ache and joint pain, and feeling depressed/blue being the most common behavioral complaint. All these symptoms were reported on the 10-item SPAF,[10] which is based on the most common symptoms of PMS. Hence, the symptoms reported in our work are more or less concordant with common symptoms reported in most other studies.
Certain differences are however striking. First, breast tenderness was reported by only 6% of the respondents, which is very low compared to most other studies. It cannot be discerned whether the students actually experienced it less often or experienced it significantly but underreported. It is apprehended that India is a relatively conservative society, where women may feel embarrassed reporting symptoms pertaining to breasts or genitals. Second, irritability as a symptom was less commonly reported than feeling depressed in this study. An Indian epidemiological study, which looked at self-reported premenstrual symptoms in noncomplaining women also found sadness to be more common than irritability.[29] This is in contrast with recent research reports, which found irritability to be more common than depressed mood.[13,30,31,32] Cross-cultural variations probably explain these findings.
The mean score for psychological symptoms was higher than that for physical symptoms for those with or without PMDD, which defies the widespread belief that Indian women tend to somatize psychological distress more often than women from developed countries. This study was conducted in an urban setting among highly educated women, which could be the reason for the predominance of psychological symptoms. Urban women tend to report more severe psychological symptoms than rural women.[33,34]
Lifestyle factors and premenstrual dysphoric disorder
Our research finds a robust association of PMDD with various lifestyle factors which included sleep, daily physical activity, caffeine intake, and food intake.
Sleep and premenstrual dysphoric disorder
Females with PMDD in our study reported less sleeping hours as against those with mild symptoms. Both insomnia and excessive sleepiness have been reported in women with severe PMS.[35] However, in a group of 23 patients with PMDD, Parry et al. found no differences between the late luteal phase and follicular phase in sleep time or time spent awake, as recorded in daily sleep logs.[36] Unpleasant dreams, awakenings, failure to wake at the expected time and tiredness in the morning, and heightened mental activity during the night and on awakening are sleep disturbances reported to be found in women with severe PMS,[37] all which may be perceived as decreased sleep. As concluded by Baker et al., subjectively perceived poor quality sleep is a characteristic of severe PMS, but sleep composition based on objective measures (polysomnography and quantitative electroencephalographic analysis) does not differ in association with premenstrual symptom expression in the late luteal phase.[38] A structured sleep schedule with consistent sleep and wake times is recommended, especially during the luteal phase as a part of the nonpharmacological management of PMS.[39]
Physical activity and premenstrual dysphoric disorder
Even in the absence of high-quality research and strong evidence base, a recent comprehensive review on exercise interventions in PMS[40] supports the findings of our work which shows a significant association between PMDD and fewer hours spent in physical activities, as also reported by a study evaluating PMS in college-aged women.[41] ACOG has advised that regular aerobic exercise may help relieve PMS, and it is substantiated by preliminary data.[42]
Tea/coffee intake and premenstrual dysphoric disorder
Students with PMDD were more likely to take caffeine and tea compared with those having mild symptoms. The association is strongly supported by a host of studies, which pointed out that consumption of caffeinated beverages including tea and coffee was strongly related to the presence of PMS and also established that the effect is clearly dose-dependent; the effect being only slightly reduced when daily total fluid consumption was controlled for.[41,43,44,45] However, Caan et al. could not find any significant relation between caffeine intake and PMS.[46] Like in our work, all these studies could only take into account the relative amount of caffeine consumed and not the absolute quantity, as respondents could not possibly and expectedly be having the same source for consumption of these caffeinated beverages. Hence, this problem of equivalence of exposure cannot entirely be done away with, and needs to be accepted as a limitation.
Change in food intake under stress and premenstrual dysphoric disorder
Another significant finding of our research was the association of PMDD with a self-reported increase in food intake under stress. This is in congruence with previous reports that consumption of foods and beverages that are high in sugar content or taste sweet is associated with severe PMS.[41,47] However, the inference that restricting sugar may relieve symptoms of PMS does not find research support. Rather, studies of diets that increase the relative intake of complex carbohydrates suggest benefit, which might be due to an enhanced transport of the serotonin precursor tryptophan into the brain, leading to a transient increase in the synthesis of this transmitter.[48,49] Because synthesis of brain serotonin increases after carbohydrate intake, it has been postulated that PMS subjects may over consume carbohydrates in an attempt to improve their dysphoric mood state.[50] Intake of carbohydrates, fats, proteins, vitamins, and minerals has been found to increase in women premenstrually,[51,52] and significantly so in women with PMS.[53]
Limitations
First, the study is conducted in a small and selective sample of medical students in an urban Indian setting, which restricts the generalizability of the results. Second, it is known that the use of retrospective questionnaires is not the best method for data collection of premenstrual symptoms. Prospective daily logging of symptoms over at least two cycles is the most preferred way as retrospective ratings tend to amplify the severity of premenstrual symptoms. However, there were practical difficulties in the application of prospective approaches, given the busy, and erratic work schedules of resident doctors. Retrospective rating does provide a good indication of respondents’ overall premenstrual experiences.[54] Moreover, findings of studies on the prevalence of premenstrual complaints based on retrospective reports are consistent with those from epidemiological studies that used prospective symptom ratings.[6] Third, we did not screen for psychiatric comorbidity which could have influenced the findings. Finally, a nonresponse rate of almost 30% is an important limitation of the study. More UG students denied to participate as compared to PG students, which may have confounded the findings.
CONCLUSION
Inherent limitations notwithstanding, this exploratory research concludes that PMDD is fairly common in medical students studying and working in an urban setting. Our findings refute the long-held belief that PMS is a western phenomenon,[55,56] and also the notion that PMS is generally mild and bearable in Indian women.[29] Rather, it is emphasized that PMS may be a relevant clinical construct even in the Indian context although cultural factors may influence symptom expression. Furthermore, the study suggests that PMDD is associated with lifestyle factors in young, professional, urban women. Thus, modifications in lifestyle may be an important approach for dealing with problematic PMS symptoms. Appropriately designed, prospective studies with larger representative samples are needed to replicate and validate these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Appendix 1: Self-screening quiz for premenstrual dysphoric disorder
First: Note all the symptoms from both the A-list and the B-list that you experienced during the 1-2 weeks before your menstrual period started (at least during two menstrual cycles)
A- List symptoms
□ I feel much more depressed, down, tearful, sad or hopeless
□ I feel anxious, tense, nervous, “keyed up” or “on edge”
□ I feel hypersensitive to rejection, or, my emotions feel very unstable and unpredictable
□ I feel much more irritable, or I get angry easily
B- List symptoms
□ I am much less interested than usual in my hobbies and daily activities
□ I find it much harder to concentrate
□ I feel much more tired and low in energy
□ I tend to crave carbohydrates, feel hungry all the time, or eat more than usual
□ I find myself tired, oversleeping or taking naps, or, I’m not sleeping well at night
□ I feel very overwhelmed or out of control like things are too much for me
-
□ I am bothered by any of the following physical symptoms
- Breast tenderness or swelling
- Increased headaches
- Joint or muscle pain
- Bloating or water retention
- Weight gain
Second: Answer “yes” or “no” to the following four questions
Does the number of “A-list” symptoms “plus” the number “B-list” symptoms you noted add up to 5 or more?
Is, at least, one of the symptoms you noted on the “A-list”?
Do most of the symptoms you noted disappear by the end of your period?
When you are having these symptoms, do they interfere or cause problems in your day-to-day activities or relationships?
REFERENCES
- 1.Freeman EW, Sondheimer SJ. Premenstrual dysphoric disorder: Recognition and treatment. Prim Care Companion J Clin Psychiatry. 2003;5:30–39. doi: 10.4088/pcc.v05n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28(Suppl 3):1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 3.Highlight of Changes from DSM-IV-TR to DSM-5. [Last accessed on 2013 Aug 17]. Available from: http://www.dsm5.org/Pages/Default.aspx .
- 4.Cunningham J, Yonkers KA, O’Brien S, Eriksson E. Update on research and treatment of premenstrual dysphoric disorder. Harv Rev Psychiatry. 2009;17:120–37. doi: 10.1080/10673220902891836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J, O’ Brien PM, Marjoribanks J, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2009:CD001396. doi: 10.1002/14651858.CD001396.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Yonkers KA, O’Brien PM, Eriksson E. Premenstrual syndrome. Lancet. 2008;371:1200–10. doi: 10.1016/S0140-6736(08)60527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Protopopescu X, Tuescher O, Pan H, Epstein J, Root J, Chang L, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord. 2008;108:87–94. doi: 10.1016/j.jad.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Chandra PS. The interface between psychiatry and women's reproductive and sexual health. Indian J Psychiatry. 2001;43:295–305. [PMC free article] [PubMed] [Google Scholar]
- 9.Tschudin S, Bertea PC, Zemp E. Prevalence and predictors of premenstrual syndrome and premenstrual dysphoric disorder in a population-based sample. Arch Womens Ment Health. 2010;13:485–94. doi: 10.1007/s00737-010-0165-3. [DOI] [PubMed] [Google Scholar]
- 10.Allen SS, McBride CM, Pirie PL. The shortened premenstrual assessment form. J Reprod Med. 1991;36:769–72. [PubMed] [Google Scholar]
- 11.4th ed. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. [Google Scholar]
- 12.Issa BA, Yussuf AD, Olatinwo AW, Ighodalo M. Premenstrual dysphoric disorder among medical students of a Nigerian university. Ann Afr Med. 2010;9:118–22. doi: 10.4103/1596-3519.68354. [DOI] [PubMed] [Google Scholar]
- 13.Balaha MH, Amr MA, Saleh Al Moghannum M, Saab Al Muhaidab N. The phenomenology of premenstrual syndrome in female medical students: A cross sectional study. Pan Afr Med J. 2010;5:4. doi: 10.4314/pamj.v5i1.56194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adewuya AO, Loto OM, Adewumi TA. Premenstrual dysphoric disorder amongst Nigerian university students: Prevalence, comorbid conditions, and correlates. Arch Womens Ment Health. 2008;11:13–8. doi: 10.1007/s00737-008-0213-4. [DOI] [PubMed] [Google Scholar]
- 15.Bakhshani NM, Mousavi MN, Khodabandeh G. Prevalence and severity of premenstrual symptoms among Iranian female university students. J Pak Med Assoc. 2009;59:205–8. [PubMed] [Google Scholar]
- 16.Nisar N, Zehra N, Haider G, Munir AA, Sohoo NA. Frequency, intensity and impact of premenstrual syndrome in medical students. J Coll Physicians Surg Pak. 2008;18:481–4. [PubMed] [Google Scholar]
- 17.Rojnic Kuzman M, Hotujac L. Premenstrual dysphoric disorder – A neglected diagnosis?. Preliminary study on a sample of Croatian students. Coll Antropol. 2007;31:131–7. [PubMed] [Google Scholar]
- 18.Tabassum S, Afridi B, Aman Z, Tabassum W, Durrani R. Premenstrual syndrome: Frequency and severity in young college girls. J Pak Med Assoc. 2005;55:546–9. [PubMed] [Google Scholar]
- 19.Deuster PA, Adera T, South-Paul J. Biological, social, and behavioral factors associated with premenstrual syndrome. Arch Fam Med. 1999;8:122–8. doi: 10.1001/archfami.8.2.122. [DOI] [PubMed] [Google Scholar]
- 20.Warner P, Bancroft J. Factors related to self-reporting of the pre-menstrual syndrome. Br J Psychiatry. 1990;157:249–60. doi: 10.1192/bjp.157.2.249. [DOI] [PubMed] [Google Scholar]
- 21.Delara M, Ghofranipour F, Azadfallah P, Tavafian SS, Kazemnejad A, Montazeri A. Health related quality of life among adolescents with premenstrual disorders: A cross sectional study. Health Qual Life Outcomes. 2012;10:1. doi: 10.1186/1477-7525-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman EW, Rickels K, Schweizer E, Ting T. Relationships between age and symptom severity among women seeking medical treatment for premenstrual symptoms. Psychol Med. 1995;25:309–15. doi: 10.1017/s0033291700036205. [DOI] [PubMed] [Google Scholar]
- 23.Thu M, Diaz EO, Sawhsarkapaw Premenstrual syndrome among female university students in Thailand. AU J Technol. 2006;9:158–62. [Google Scholar]
- 24.Sternfeld B, Swindle R, Chawla A, Long S, Kennedy S. Severity of premenstrual symptoms in a health maintenance organization population. Obstet Gynecol. 2002;99:1014–24. doi: 10.1016/s0029-7844(02)01958-0. [DOI] [PubMed] [Google Scholar]
- 25.Forrester-Knauss C, Zemp Stutz E, Weiss C, Tschudin S. The interrelation between premenstrual syndrome and major depression: Results from a population-based sample. BMC Public Health. 2011;11:795. doi: 10.1186/1471-2458-11-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuczmierczyk AR, Labrum AH, Johnson CC. Perception of family and work environments in women with premenstrual syndrome. J Psychosom Res. 1992;36:787–95. doi: 10.1016/0022-3999(92)90137-q. [DOI] [PubMed] [Google Scholar]
- 27.Namavar Jahromi B, Pakmehr S, Hagh-Shenas H. Work stress, premenstrual syndrome and dysphoric disorder: Are there any associations? Iran Red Crescent Med J. 2011;13:199–202. [PMC free article] [PubMed] [Google Scholar]
- 28.Dennerstein L, Lehert P, Heinemann K. Global study of women's experiences of premenstrual symptoms and their effects on daily life. Menopause Int. 2011;17:88–95. doi: 10.1258/mi.2011.011027. [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi SK, Chandra PS, Gururaj G, Beena MB, Pandian RD. Prevalence of premenstrual symptoms and syndromes: Preliminary observations. NIMHANS J. 1994;12:9–14. [Google Scholar]
- 30.Choi D, Lee DY, Lehert P, Lee IS, Kim SH, Dennerstein L. The impact of premenstrual symptoms on activities of daily life in Korean women. J Psychosom Obstet Gynaecol. 2010;31:10–5. doi: 10.3109/01674820903573920. [DOI] [PubMed] [Google Scholar]
- 31.Hartlage SA, Arduino KE. Toward the content validity of premenstrual dysphoric disorder: Do anger and irritability more than depressed mood represent treatment-seekers’ experiences? Psychol Rep. 2002;90:189–202. doi: 10.2466/pr0.2002.90.1.189. [DOI] [PubMed] [Google Scholar]
- 32.Lee AM, Wei R, Chung KF, Hui KT, Ip SK, Leung HL, et al. Premenstrual symptoms among Chinese female undergraduates: Relationship with stress and mental health. Hong Kong J Gynaecol Obstet Midwifery. 2005;5:10–21. [Google Scholar]
- 33.Marván ML, Díaz Eroza MC. Premenstrual symptomatology in rural and urban zones. Acta Psiquiatr Psicol Am Lat. 1995;41:316–21. [PubMed] [Google Scholar]
- 34.Marván ML, Díaz-Erosa M, Montesinos A. Premenstrual symptoms in Mexican women with different educational levels. J Psychol. 1998;132:517–26. doi: 10.1080/00223989809599284. [DOI] [PubMed] [Google Scholar]
- 35.Strine TW, Chapman DP, Ahluwalia IB. Menstrual-related problems and psychological distress among women in the United States. J Womens Health (Larchmt) 2005;14:316–23. doi: 10.1089/jwh.2005.14.316. [DOI] [PubMed] [Google Scholar]
- 36.Parry BL, Cover H, Mostofi N, LeVeau B, Sependa PA, Resnick A, et al. Early versus late partial sleep deprivation in patients with premenstrual dysphoric disorder and normal comparison subjects. Am J Psychiatry. 1995;152:404–12. doi: 10.1176/ajp.152.3.404. [DOI] [PubMed] [Google Scholar]
- 37.Mauri M, Reid RL, MacLean AW. Sleep in the premenstrual phase: A self-report study of PMS patients and normal controls. Acta Psychiatr Scand. 1988;78:82–6. doi: 10.1111/j.1600-0447.1988.tb06304.x. [DOI] [PubMed] [Google Scholar]
- 38.Baker FC, Kahan TL, Trinder J, Colrain IM. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 2007;30:1283–91. doi: 10.1093/sleep/30.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moline ML, Zendell SM. Evaluating and managing premenstrual syndrome. Medscape Womens Health. 2000;5:1. [PubMed] [Google Scholar]
- 40.Daley A. Exercise and premenstrual symptomatology: A comprehensive review. J Womens Health (Larchmt) 2009;18:895–9. doi: 10.1089/jwh.2008.1098. [DOI] [PubMed] [Google Scholar]
- 41.Rasheed P, Al-Sowielem LS. Prevalence and predictors of premenstrual syndrome among college-aged women in Saudi Arabia. Ann Saudi Med. 2003;23:381–7. doi: 10.5144/0256-4947.2003.381. [DOI] [PubMed] [Google Scholar]
- 42.Steege JF, Blumenthal JA. The effects of aerobic exercise on premenstrual symptoms in middle-aged women: A preliminary study. J Psychosom Res. 1993;37:127–33. doi: 10.1016/0022-3999(93)90079-u. [DOI] [PubMed] [Google Scholar]
- 43.Rossignol AM. Caffeine-containing beverages and premenstrual syndrome in young women. Am J Public Health. 1985;75:1335–7. doi: 10.2105/ajph.75.11.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossignol AM, Bonnlander H. Caffeine-containing beverages, total fluid consumption, and premenstrual syndrome. Am J Public Health. 1990;80:1106–10. doi: 10.2105/ajph.80.9.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossignol AM, Zhang JY, Chen YZ, Xiang Z. Tea and premenstrual syndrome in the People's Republic of China. Am J Public Health. 1989;79:67–9. doi: 10.2105/ajph.79.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caan B, Duncan D, Hiatt R, Lewis J, Chapman J, Armstrong MA. Association between alcoholic and caffeinated beverages and premenstrual syndrome. J Reprod Med. 1993;38:630–6. [PubMed] [Google Scholar]
- 47.Rossignol AM, Bonnlander H. Prevalence and severity of the premenstrual syndrome. Effects of foods and beverages that are sweet or high in sugar content. J Reprod Med. 1991;36:131–6. [PubMed] [Google Scholar]
- 48.Freeman EW, Stout AL, Endicott J, Spiers P. Treatment of premenstrual syndrome with a carbohydrate-rich beverage. Int J Gynaecol Obstet. 2002;77:253–4. doi: 10.1016/s0020-7292(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 49.Sayegh R, Schiff I, Wurtman J, Spiers P, McDermott J, Wurtman R. The effect of a carbohydrate-rich beverage on mood, appetite, and cognitive function in women with premenstrual syndrome. Obstet Gynecol. 1995;86(4 Pt 1):520–8. doi: 10.1016/0029-7844(95)00246-n. [DOI] [PubMed] [Google Scholar]
- 50.Wurtman JJ, Brzezinski A, Wurtman RJ, Laferrere B. Effect of nutrient intake on premenstrual depression. Am J Obstet Gynecol. 1989;161:1228–34. doi: 10.1016/0002-9378(89)90671-6. [DOI] [PubMed] [Google Scholar]
- 51.Cheikh Ismail LI, Al-Hourani H, Lightowler HJ, Aldhaheri AS, Henry CJ. Energy and nutrient intakes during different phases of the menstrual cycle in females in the United Arab Emirates. Ann Nutr Metab. 2009;54:124–8. doi: 10.1159/000209395. [DOI] [PubMed] [Google Scholar]
- 52.Martini MC, Lampe JW, Slavin JL, Kurzer MS. Effect of the menstrual cycle on energy and nutrient intake. Am J Clin Nutr. 1994;60:895–9. doi: 10.1093/ajcn/60.6.895. [DOI] [PubMed] [Google Scholar]
- 53.Cross GB, Marley J, Miles H, Willson K. Changes in nutrient intake during the menstrual cycle of overweight women with premenstrual syndrome. Br J Nutr. 2001;85:475–82. doi: 10.1079/bjn2000283. [DOI] [PubMed] [Google Scholar]
- 54.Haywood A, Slade P, King H. Assessing the assessment measures for menstrual cycle symptoms: A guide for researchers and clinicians. J Psychosom Res. 2002;52:223–37. doi: 10.1016/s0022-3999(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 55.Johnson TM. Premenstrual syndrome as a western culture-specific disorder. Cult Med Psychiatry. 1987;11:337–56. doi: 10.1007/BF00048518. [DOI] [PubMed] [Google Scholar]
- 56.Chrisler JC, Caplan P. The strange case of Dr. Jekyll and Ms. Hyde: How PMS became a cultural phenomenon and a psychiatric disorder. Annu Rev Sex Res. 2002;13:274–306. [PubMed] [Google Scholar]


