Abstract
Background:
Dental caries is the most common infectious disease affecting humans and is the predominant cause of tooth loss in children. Although Candida's role in dental caries has been studied extensively, limited homogenous studies have been conducted and none have been found, that associate Candida with dental caries, while correlating it to different age groups.
Aim:
The study aimed to quantify oral Candida in school children and correlate candidal carriage to the caries index and further analyze an age association.
Subjects and Methods:
Decayed-Filled teeth/Decayed-Missing-Filled Teeth (dft/DMFT) index scores of 150 subjects were evaluated, and concentrated oral rinse samples were collected from each participant for mycologic investigation. Based on the age and caries activity, the participants were categorized into three groups consisting of 50 each such as Group-I (caries active participants of 6–12 years age), Group-II (caries active participants in 13–18 years age), and Group-III (caries-free participants in 6–18 years age); CHROMagar™ was used as a primary culture medium for candidal growth. The data was statistically analyzed using Unpaired t-test, Chi-square test and Spearman's rank order.
Results:
The results demonstrated that as age increases, the dft/DMFT scores as well as the candidal growth decreased. In addition, the oral candidal carriage levels were found to be low in caries-free group (Group-III) when compared to the study groups.
Conclusion:
The presence of Candida was directly related to the caries status and inversely proportional to the age.
Keywords: Dental caries, Decayed-Missing-Filled teeth, Oral Candida, Saliva
Introduction
Over 400 microbial species inhabit the oral cavity and cause various oral lesions.[1] According to the 1996 bulletin from the Centre for Disease Control and Prevention, dental caries is said to be the most prevalent of the infectious diseases that affect humans.[2] Dental caries is defined as a bacterial driven, generally chronic, site-specific, multifactorial, dynamic disease process that results from the imbalance in the physiologic equilibrium between tooth mineral and plaque fluid, that is, when the pH drops, it results in net mineral loss over time.[3]
As stated, the origin of dental caries is multifactorial with acidogenic bacterial biofilm an absolute requirement for bacterial acid generation. This dynamic status provides an interactive environment for the multiple bacterial species and the pH fluctuations results in large ecological changes. However, it was shown by Hara and Zero[4] that acidogenic biofilm is not the only factor which determines caries disease activity; factors such as saliva, pellicle, diet, location, morphology, composition, ultrastructure, and posteruptive age of the tooth are all critical. Research has also challenged the concept of mutans streptococci and lactobacilli as the only important microorganisms in caries disease,[3] and many investigators have shown the association between Candida species and dental caries.[5,6,7,8,9,10] This led to a new micro-ecological concept of caries research, isolating collagenases from Candida albicans that increased the interest in Candida species and their possible role in the etiopathogenesis of dental caries.
Oral Candida species can bind to a variety of host cell receptors via lectin-like and protein–protein type interactions and can also coaggregate with bacterial cells of a variety of genera, particularly with oral streptococci indicating that yeast cells contribute to the development, stabilization, and maintenance of oral mixed microbial populations, and play an important role in maintaining the balance of the oral micro environment.[1] Candida has been found to metabolize dietary sugars to acid, which leads to the dissolution of hydroxyapatite crystals in enamel and dentin.[11] In addition, it has been reported that the level of oral Candida species could be useful as an indicator of microbial risk in dental caries.[12,13] Hence, this study was undertaken to determine the frequency, quantity, and prevalent species of oral Candida in school going children aged 6–18 years and correlating with their dental caries index and age.
Subjects and Methods
The study was a case–control study conducted on 150 healthy male participants aged 6–18 years, who were randomly selected from five different government welfare hostels of the district. Although the subjects belonged to low socioeconomic status, they were nourished by a similar diet provided by the district authority. Fifty randomly selected, age-matched controlled individuals, consisted of the caries-free group (CFG), chosen from the same pool. The minimum sample size was derived by using the sample size calculator / formula for unmatched case control studies without continuity correction.[14] With a two-sided confidence level (1-alpha) of 95%, power of 80%, ratio of control to cases of 0.5, proportion of controls/cases with exposure of 42 and 75 percent respectively, where in the minimum sample size of 50 and 25, for cases and controls respectively, was required for each group, thus making a total of 100 cases and 50 controls”. Individuals, with adverse habits or on medication for any oral or systemic manifestations and intraoral prosthesis, were excluded from the study. Informed consent for participation was obtained from the legal guardians of the participants. Approval from the research and Institutional Ethical Committee was obtained before commencement of the study.
A purposefully designed form was used to record demographic data, past medical history, personal history, and caries status of individuals. Caries index was recorded using the decayed-filled teeth/decayed-missing-filled teeth (dft/DMFT) score. Depending on the values of these Indexes, four subgroups of children were created[6] such as 1 = caries-free, 2 = low (1–5 caries lesions), 3 = moderate (6–10 lesions), and 4 = high (>10 lesions).
The concentrated oral rinse technique was used for the quantification of Candida species.[12] Samples were collected, before brushing (5–6 am), in a universal container, with 10 ml sterile phosphate-buffered saline (PBS). All the samples were concentrated (10-fold) by centrifugation at 1700 g for 10 min. The residue was resuspended in 1 ml of PBS and vortexed for 30 s to obtain a concentrated oral rinse. A loop, full of concentrated oral rinse, was streaked on to a CHROMagar™ Candida medium, and incubated aerobically at 37°C for 48 h. Growth presented as colored colonies: C. albicans – green; Candida tropicalis – purple halo with a dark blue green color; Candida krusei-pale pink center with white edge, rough, spreading colony; and Candida parapsilosis – pale pink.
Their quantity was categorized as 0 – absent, 1 – very sparse (<10 CFU/ml), 2 – sparse (10–102 CFU/ml), 3 – moderate (102–103 CFU/ml), and 4 – rich (>103 CFU/ml).[13] Further confirmation of C. albicans was done using the germ tube test.
Results
Data pertaining to the size of the sample, the age range, with a mean (standard deviation) in each group are shown in Table 1.
Table 1.
Mean age distribution of subjects
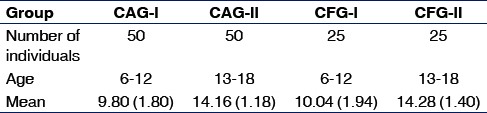
The dft/DMFT scores for the caries active group (CAG-I, CAG-II) were recorded with a minimum value of 1 and a maximum score of 12. An unpaired t-test compared the means of the dft/DMFT scores between the two study groups [Table 2] and found it to be statistically significant (P = 0.01).
Table 2.
Comparison of decayed-filled teeth/decayedmissing-filled teeth values between CAG-I and CAG-II

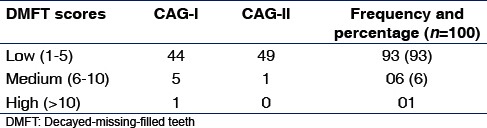
Depending on the values of these Indices, three subgroups of children were created and a frequency distribution plotted, where a higher score was noted in CAG-I than CAG-II [Table 3].
Table 3.
Dental caries scores and group distribution
Positive candidal growth was observed in 117 samples while quantification of Candida revealed that 27.4 percent of subjects had a score of 4, with the highest candidal carriage seen in CAG-I and CAG-II [Table 4].
Table 4.
Quantification of Candida colonies group-wise distribution
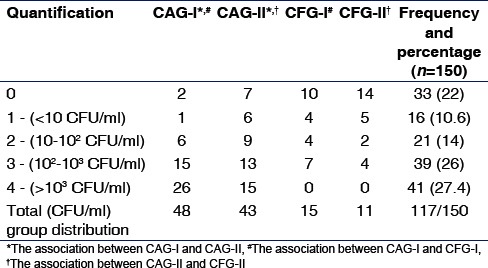
To analyze the carriage of oral Candida, the Pearson Chi-square test was performed, which revealed a statistical significance of P < 0.01 and P < 0.001 between the groups and their respective controls [Table 4].
Data analysis, using Spearman's rank order for linear relationship, showed a positive relation, between oral candidal carriage and DMFT scores (ρ =0.67) in both study groups (P < 0.001) [Graph 1].
Graph 1.
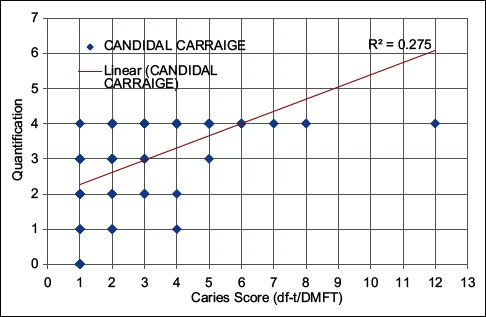
Correlation between quantification of oral candida and caries prevalence in CAG-I and II
While the linear relationship between oral candidal carriage and age showed a positive correlation between 6 and 10 years of age, a negative correlation was noted in the 11–18 years of age group [Graph 2].
Graph 2.
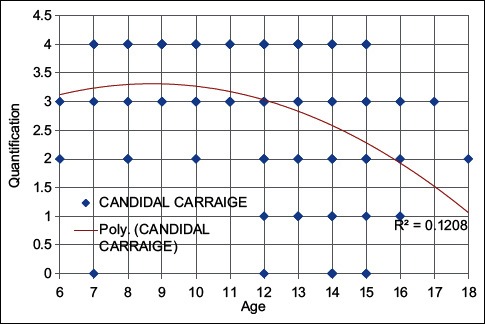
Correlation between age and candidal carriage
Further, on subtyping the species, an increased incidence of C. albicans (78%), followed by C. tropicalis (20.67%), C. parapsilosis (5.34%), and C. krusei (1.34%) was observed. Among the four species, C. albicans and C. tropicalis were isolated from all the groups studied while C. parapsilosis and C. krusei were absent in control group [Table 5].
Table 5.
Candida species isolated from the oral rinse samples of all the studied groups

Data analysis in relation to oral candidal species made known that there was significant difference (P < 0.001) observed for C. albicans while it was not significant (P > 0.001) for other Candida species isolated in the present study.
Discussion
Despite the presence of numerous antimicrobial factors in the oral cavity and saliva being one of the natural defense mechanisms in monitoring, regulating, and maintaining the integrity of the hard and soft tissues, the oral cavity is considered to be a unique environment by offering a variety of ecological niches for microbial colonization.
Studies[15] have shown that saliva is generally considered the most suitable sample to provide information on the microbial population as it represents all the sites of the oral cavity and is easily obtainable, noninvasive, and patient compliant.
Although many factors were taken into consideration for homogeneity between the individuals, it is important to mention that salivary flow rate, pH, and composition were not evaluated which may act as other predisposing colonization factors.
To control the confounding factors of the multifactorial nature of dental caries, individuals of similar diet, gender, demography, and socioeconomic status, aged between 6 and 18 years were selected.
Given the criteria for selection of participants, contrary to the assumption that only a minor variation would exist, the dft/DMFT showed a large score variation ranging from 1 to 12 among both caries active groups. The Student's t-test revealed a significant P value (0.014) where the younger participants (CAG-I) had a higher caries index [Table 2].
Similar to a study by Ugun-Can et al.,[6] the majority of participants in our study belonged to the low caries index group [Table 3]. Between the CFGs, the high and medium index shared significance, suggesting that a higher index of caries was present in the 6–12 years group compared to 13–18 years group, implying caries decreased with increase in age.
Hara and Zero[4] in their study attributed the age factor to the dynamic environment of repeated episodes of demineralization and remineralization, which render the enamel resistant to subsequent acid change over time, therefore suggesting that caries susceptibility on the enamel surface is greatest after eruption and tends to decrease with age.
Other probable reasons would be the presence of mixed dentition, allowing for increased retentive areas. Furthermore, the erupting teeth are said to be less mature with high levels of carbonate which renders the enamel less stable, thus, more prone for demineralization.[16] It has also been suggested that thickness of enamel in primary tooth is less favoring, resulting in a higher incidence of caries.
Salivary samples of all the subjects showed a prevalence of 78% of Candida [Table 4], similar to studies by Siahi-Benlarbi et al.[17] and Cortelli et al.[18] Further quantification by counting CFUS showed that 27.4% of the samples had a score of 4 (>103 CFU/ml) while few other studies had a lower percentage of distribution (33–60%).[5,6,9] This variation could be a result of the sample size, age distribution, gender considerations, sampling techniques, etc.
A group-wise distribution showed the highest oral candidal carriage, 96% in CAG I similar to the study by Raja et al.,[9] and was statistically significant, when compared with the CAG-II [Table 4], suggesting that the lower age group had a higher candidal carriage, with higher caries index too.
The Spearman rank order plotted between caries index and Candida carriage showed that higher the caries index, higher is the Candida colony count and vice versa [Graph 1]. Similarly, the Spearman rank order for linear relationship between Candida carriage and age showed a positive correlation existing in 6–10 years age group while negative in between 11 and 18 age group, suggesting a higher carriage in younger children similar to a study by Martin and Wilkinson[19] [Graph 2].
In fact, this led to some authors suggesting that Candida counts can be used as a caries risk indicator as yeasts were more efficient than lactobacilli in discriminating between high- and low-risk caries groups,[12,20,21] also the culture and counting of Candida proved to be more convenient and less technique sensitive than that of lactobacilli as colonies were more distinct and less critical.[13]
Subtyping of Candida isolated four strains with a 78% predominance of C. albicans [Table 5], similar to other studies[5,22] that found an 80.87% and 93.75% isolation of C. albicans, respectively. When the strains were correlated between the study groups and three controls, it was statistically significant only for C. albicans and not for C. tropicalis, C. parapsilosis, and C. krusei, thus suggesting that C. albicans are present in all the individuals irrespective of their caries status as found by many investigators.[5,9,17,22] The finding of other strains did not correlate with the caries experience of the studied population.
The results of the study conclude that the presence of Candida correlates to the caries status while both Candida and caries are inversely related to age. Hence, the possibility of multifactorial organisms, especially Candida, should be borne in mind when providing or developing dental caries treatment alternatives. However, more homogenous studies are warranted to have conclusive evidence as geography plays a role, so also do habits and cultural characteristics of individuals.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Qi QG, Hu T, Zhou XD. Frequency, species and molecular characterization of oral Candida in hosts of different age in China. J Oral Pathol Med. 2005;34:352–6. doi: 10.1111/j.1600-0714.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 2.Caufield PW, Griffen AL. Dental caries. An infectious and transmissible disease. Pediatr Clin North Am. 2000;47:1001–19. doi: 10.1016/s0031-3955(05)70255-8. v. [DOI] [PubMed] [Google Scholar]
- 3.Fontana M, Young DA, Wolff MS, Pitts NB, Longbottom C. Defining dental caries for 2010 and beyond. Dent Clin North Am. 2010;54:423–40. doi: 10.1016/j.cden.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Hara AT, Zero DT. The caries environment: Saliva, pellicle, diet, and hard tissue ultrastructure. Dent Clin North Am. 2010;54:455–67. doi: 10.1016/j.cden.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Moalic E, Gestalin A, Quinio D, Gest PE, Zerilli A, Le Flohic AM. The extent of oral fungal flora in 353 students and possible relationships with dental caries. Caries Res. 2001;35:149–55. doi: 10.1159/000047447. [DOI] [PubMed] [Google Scholar]
- 6.Ugun-Can B, Kadir T, Akyüz S. Oral candidal carriage in children with and without dental caries. Quintessence Int. 2007;38:45–9. [PubMed] [Google Scholar]
- 7.de Carvalho FG, Paristto TM, Hebling J, Spolidorio LC, Spolidorio DM. Presence of Candida spp. in infants oral cavity and its association with early childhood caries. Braz J Oral Sci. 2007;6:1249–53. [Google Scholar]
- 8.Rozkiewicz D, Daniluk T, Zaremba ML, Cylwik-Rokicka D, Stokowska W, Pawinska M, et al. Oral Candida albicans carriage in healthy preschool and school children. Adv Med Sci. 2006;51(Suppl 1):187–90. [PubMed] [Google Scholar]
- 9.Raja M, Hannan A, Ali K. Association of oral candidal carriage with dental caries in children. Caries Res. 2010;44:272–6. doi: 10.1159/000314675. [DOI] [PubMed] [Google Scholar]
- 10.Klinke T, Guggenheim B, Klimm W, Thurnheer T. Dental caries in rats associated with Candida albicans. Caries Res. 2011;45:100–6. doi: 10.1159/000324809. [DOI] [PubMed] [Google Scholar]
- 11.Nikawa H, Yamashiro H, Makihira S, Nishimura M, Egusa H, Furukawa M, et al. In vitro cariogenic potential of Candida albicans. Mycoses. 2003;46:471–8. doi: 10.1046/j.0933-7407.2003.00888.x. [DOI] [PubMed] [Google Scholar]
- 12.Pienihäkkinen K. Caries prediction through combined use of incipient caries lesions, salivary buffering capacity, lactobacilli and yeasts in Hungary [corrected] Community Dent Oral Epidemiol. 1987;15:325–8. doi: 10.1111/j.1600-0528.1987.tb01745.x. [DOI] [PubMed] [Google Scholar]
- 13.Coulter WA, Murray SD, Kinirons MJ. The use of a concentrated oral rinse culture technique to sample oral Candida and lactobacilli in children, and the relationship between Candida and lactobacilli levels and dental caries experience: A pilot study. Int J Paediatr Dent. 1993;3:17–21. doi: 10.1111/j.1365-263x.1993.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 14.Kelsey. Methods in Observational epidemiology. 2nd Edition. [Last accessed on 2016 Apr 25]. Table 12-15 Fleiss, Statistical Methods for Rates and Proportions, formulas 3.18 & 3.19 [Internet] Available from http://www.openepi.com/SampleSize/SSCC.htm .
- 15.Luo AH, Yang DQ, Xin BC, Paster BJ, Qin J. Microbial profiles in saliva from children with and without caries in mixed dentition. Oral Dis. 2012;18:595–601. doi: 10.1111/j.1601-0825.2012.01915.x. [DOI] [PubMed] [Google Scholar]
- 16.Peterson SN, Snesrud E, Schork NJ, Bretz WA. Dental caries pathogenicity: A genomic and metagenomic perspective. Int Dent J. 2011;61(Suppl 1):11–22. doi: 10.1111/j.1875-595X.2011.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siahi-Benlarbi R, Nies SM, Sziegoleit A, Bauer J, Schranz D, Wetzel WE. Caries-, Candida- and Candida antigen/antibody frequency in children after heart transplantation and children with congenital heart disease. Pediatr Transplant. 2010;14:715–21. doi: 10.1111/j.1399-3046.2008.01115.x. [DOI] [PubMed] [Google Scholar]
- 18.Cortelli SC, Junqueira JC, Faria Ida S, Koga Ito CY, Cortelli JR. Correlation between Candida spp. and DMFT index in a rural population. Braz J Oral Sci. 2006;5:1007–11. [Google Scholar]
- 19.Martin MV, Wilkinson GR. The oral yeast flora of 10-year-old schoolchildren. Sabouraudia. 1983;21:129–35. doi: 10.1080/00362178385380201. [DOI] [PubMed] [Google Scholar]
- 20.Pienihäkkinen K, Scheinin A, Bánóczy J. Screening of caries in children through salivary lactobacilli and yeasts. Scand J Dent Res. 1987;95:397–404. doi: 10.1111/j.1600-0722.1987.tb01630.x. [DOI] [PubMed] [Google Scholar]
- 21.Pienihäkkinen K. Salivary lactobacilli and yeasts in relation to caries increment. Annually repeated measurements versus a single determination. Acta Odontol Scand. 1988;46:57–62. doi: 10.3109/00016358809004747. [DOI] [PubMed] [Google Scholar]
- 22.Darwazeh AM, al-Bashir A. Oral candidal flora in healthy infants. J Oral Pathol Med. 1995;24:361–4. doi: 10.1111/j.1600-0714.1995.tb01200.x. [DOI] [PubMed] [Google Scholar]


