Abstract
Objective
Sphingosine-1-phosphate (S1P) was found to protect the endothelial surface glycocalyx (ESG) by inhibiting matrix metalloproteinase (MMP) activity-dependent shedding of ESG in cultured endothelial cell studies. We aimed to further test that S1P contributes to the maintenance of normal vascular permeability by protecting the ESG in intact microvessels.
Methods
We quantified the ESG in post-capillary venules of rat mesentery and measured the vascular permeability to albumin in the presence and absence of 1 μM S1P. We also measured permeability to albumin in the presence of MMP inhibitors and compared the measured permeability with those predicted by a transport model for the inter-endothelial cleft.
Results
We found that in the absence of S1P, the fluorescence intensity of the FITC-anti-heparan sulfate labeled ESG was ~10% of that in the presence of S1P, while the measured permeability to albumin was ~6.5 fold that in the presence of S1P. Similar results were observed with MMP inhibition. The predictions by the mathematical model further confirmed that S1P maintains microvascular permeability by preserving ESG.
Conclusions
Our results show that S1P contributes to the maintenance of normal vascular permeability by protecting the ESG in intact microvessels, consistent with parallel observation in cultured endothelial monolayers.
Keywords: heparan sulphate, in situ immunostaining, matrix metalloproteinase, transport model for the inter-endothelial cleft, rat mesentery
INTRODUCTION
Sphingosine-1-phosphate (S1P), is a sphingolipid in plasma that plays a critical role in the cardiovascular and immune systems. Red blood cells (RBCs) are a major source of S1P in plasma, which acts continuously to maintain normal vascular permeability under physiological conditions [7,8,11,19]. Serum albumin and high-density lipoprotein (HDL) carry ~90% of the S1P and both elicit the release of S1P from RBCs [11,20,27]. The actions of S1P have been found to be mediated by a family of G protein-coupled receptors including sphingosine-1-phosphate receptors [21]. In vitro studies showed that S1P can increase electric resistance and decrease albumin permeability across endothelial cell monolayers [18,24]. An investigation in the perfused lung with added S1P demonstrated that S1P reversed the vascular permeability increase via engagement of endothelial sphingosine-1-phosphate receptor 1 (S1P1) [37,43]. Recent studies investigated S1P effects on hydraulic conductivity (Lp) and solute permeability (P) of intact post-capillary venules in rat mesentery [2,4,5,11,25,43]. These results showed that via receptor S1P1, S1P can attenuate acute microvascular permeability increases by inflammatory agents such as platelet-activating factor (PAF) [4,25,43] and bradykinin [4,5]. In addition to attenuating increased microvessel permeability by inflammatory mediators, 1 μM S1P decreased basal Lp by 63% for a group of vessels with slightly higher Lp [25] and by 30% for those with normal Lp [4]. 1μM S1P also maintained normal albumin permeability in the absence of BSA in the perfusate [2].
To investigate the underlying structural mechanisms by which S1P maintains microvessel permeability, in an in vitro study using HMVEC (human dermal microvascular endothelial cells) monolayers, as well as in intact microvessels of rat mesentery, Adamson et al. [4] found that S1P pretreatment inhibited rearrangements of VE-cadherin and occludin induced by PAF or bradykinin and preserved peripheral cortactin. In addition to endothelial junction proteins, endothelial surface glycocalyx (ESG) forms a barrier between blood circulation and the surrounding tissues and plays an important role in controlling microvessel permeability, especially microvessel permeability to large molecules. A recent study by Zeng et al [42] using an in vitro cultured cell monolayer (rat fat-pad endothelial cells) has shown that S1P plays a critical role in protecting the ESG via the S1P1 receptor and inhibits the matrix metalloproteinase (MMP) activity-dependent shedding of heparan sulfate (HS), chondroitin sulfate (CS) and the ectodomain of syndecan-1. The aim of the present study was to further test the hypothesis that S1P contributes to the maintenance of normal vascular permeability by protecting the ESG in intact microvessels. To test this hypothesis, we first quantified the ESG in the post-capillary venules of rat mesentery by immunostaining of heparan sulfate, the most abundant glycosaminoglycans (GAGs) of the ESG in the presence and absence of S1P. Secondly, we measured the microvessel solute permeability P to albumin (Stokes radius ~3.6 nm) in the presence and absence of S1P, as well as in the presence of MMP inhibitors. We also measured microvessel permeability P to a small solute, sodium fluorescein (Stokes radius, 0.45 nm) in the presence and absence of S1P. Thirdly, we applied a mathematical model for the inter-endothelial cleft to predict the possible changes in Lp and P by changing the structural components of the microvessel wall, e.g., degrading the surface glycocalyx, increasing the gap between endothelial cells, and increasing the size or the number of junction pores. Fourthly, we compared the measured Lp and P, as well as the ESG with those predicted by the mathematical model and found the most likely structural changes in the presence and absence of S1P to confirm our hypothesis.
MATERIAL AND METHODS
Animal Preparation
All experiments were performed on female Sprague-Dawley rats (250–300g), supplied by Hilltop Laboratory Animals (Scottsdale, PA). All procedures were approved by the Animal Care and Use Committees at the City College of the City University of New York. The methods used to prepare rat mesenteries, perfusate solutions, and micropipettes for microperfusion experiments have been described in detail in [9,14,34].
Rats were first anaesthetized with pentobarbital sodium given subcutaneously into the loose skin over the back of the neck. The initial dosage was 65 mg/kg (0.81-0.98 ml 20mg/ml pentobarbital sodium for the rats used in this study). The depth of anesthesia was monitored for the absence of withdrawal reflex to toe or ear pinch, and absence of blink reflex. After 60min injection of the initial dosage, an additional 3 mg/dose (0.15 ml) was given if the criteria for the anesthesia were not satisfied. After a rat was anesthetized, a midline surgical incision (2–3 cm) was made in the abdominal wall. The rat was then transferred to a tray and kept warm on a heating pad. The mesentery was gently taken out from the abdominal cavity and spread on a glass coverslip, which formed the base of the observation platform as previously described [34]. The gut was gently pinned out against a silicon elastomer barrier to maintain the spread of the mesentery. The upper surface of the mesentery was continuously superfused by a dripper with mammalian Ringer solution at 35–37°C, which was regulated by a controlled water bath and monitored regularly by a thermometer probe. The microvessels chosen for the study were post-capillary venules, with diameters of 35–50 μm. All vessels had brisk blood flow immediately before cannulation and had no marginating white cells. At the end of experiments, the animals were euthanized with excess anesthetic. The thorax was opened to ensure death.
Solutions and Reagents
Mammalian Ringer solution was used for all dissections, perfusion, and superfusion. The solution compositions are (in mM) 132 NaCl, 4.6 KCl, 1.2 MgSO4, 2.0 CaCl2, 5.0 NaHCO3, 5.5 glucose, and 20 HEPES. Its pH was balanced to 7.4 by adjusting the ratio of HEPES acid to base. In addition, the perfusate into the microvessel lumen contained fatty acid-free bovine serum albumin (BSA, A0281, Sigma) at 10 mg/ml (1% BSA-Ringer solution). This fatty acid-free BSA has about 50 nM S1P [42], which is negligible compared to 1 μM. FITC-conjugated mouse anti-human HS (anti-HS, 10e4 epitope) was purchased from United States Biological (Swampscott, MA). It was diluted to 1:50 (20 μg/ml) in 1% BSA-Ringer solution for labeling HS in the microvascular ESG [40]. A blocking solution was made of 5% goat serum (Invitrogen, Eugene, OR) in 1% BSA-Ringer. All of the solutions described above were made at the time when the experiment was performed and were discarded at the end of the day. Sphingosine-1-phosphate (S1P) (73914, Sigma) was first dissolved in 95% methanol and prepared as a stock solution of 125 μM in 0.4% fatty acid-free BSA [42]. Specific MMP-9 and -13 inhibitors, a broad-spectrum hydroxamic acid inhibitor of MMPs, GM6001 and its negative control GM6001 NC were purchased from EMD Millipore.
To determine microvessel permeability to albumin, fatty acid-free bovine serum albumin (A0281, Sigma, 66kDa, ~3.6 nm Stokes radius) was labeled with Alexa fluor 555 according to the manufacture's (Invitrogen) instructions. The details for generating AlexFluor 555-BSA were described in [2]. We also measured the microvessel permeability to sodium fluorescein (Sigma, 367Da, ~0.45 nm Stokes radius).
Immuno-Labeling and Quantification of Microvessel Endothelial Surface Glycocalyx (ESG)
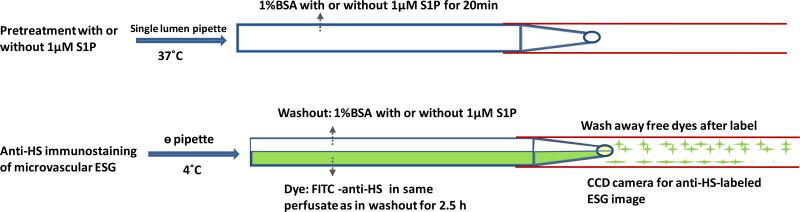
To compare the ESG of the microvessel wall in the presence or absence of S1P, FITC-conjugated heparan sulphate (HS) antibody was used to label HS, one of the most abundant glycosaminoglycans (GAGs) forming the ESG [15,31]. The detailed method was described in [5,9,40]. Briefly, as shown in Fig. 1, a post-capillary venule was first cannulated with a single lumen micropipette with or without 1 μM S1P in the perfusate and perfused for 20 min. The upper surface of the mesentery was continuously superfused by a dripper with mammalian Ringer solution at 37°C, which was regulated by a controlled water bath and monitored by a thermometer probe. Then the cannulation was changed to a glass θ micropipette with two lumens. The vessel was first perfused for 15 min with a blocking solution of 5% goat serum in 1% BSA-Ringer (with or without S1P) through one lumen of the θ pipette. The superfusion temperature was gradually decreased to ~4°C in ~15 min. Then the perfusion was switched to another lumen of the pipette to inject FITC-conjugated anti-HS in 1%BSA-Ringer (20 μg/ml, with or without S1P) into the microvessels for ~2.5 h. After 15 min perfusion of the first perfusate to wash away the free dye, the vessel with fluorescently labeled glycocalyx (focused at the mid-plane of a vessel) was imaged by the same imaging system used in the P measurement. The intensity of the fluorescently labeled glycocalyx in the vessel segment was measured offline by the InCyt Im™ imaging and analyzing system (Intracellular Imaging Inc., Cincinnati, OH, USA). To test the assumption that the fluorescence intensity is linearly related to the amount of the fluorescently labeled glycocalyx, we did in vitro calibration experiments. We used the same instrument settings in the calibration experiments as those used in the in vivo measurement of the fluorescently labeled glycocalyx. The linear range of FITC-anti-HS concentrations was from 0 to 50 μg/ml under our settings. We thus chose 20 μg/ml FITC-anti-HS in our experiments.
Figure 1.
Schematic showing the single vessel cannulation and perfusion for directly injecting FITC-anti-HS into a post-capillary venule via a theta (θ) micropipette. Upper figure shows the pre-treatment with or without 1μM S1P in 1%BSA Ringer for 20 min via a single lumen micropipette under 37°C superfusate. After pretreatment, the single lumen micropipette was changed to a θ micropipette and the superfusate temperature was gradually decreased to 4 °C. The washout (blocking) solution was first perfused into the vessel through one lumen of the θ micropipette. Then the perfusion was switched to another lumen (Dye) to perfuse FITC-anti-HS for immunolabeling the ESG of the microvessel for 2.5h. After labeling, the perfusion was back to the washout solution to wash away the free FITC-anti-HS.
After that, the vessel was fixed by superfusing the tissue with ice-cold 1% paraformaldehyde for 3-5 min. The animal was killed by anesthetic overdosing. The vessel was perfused until no blood circulation was observed in the tissue and the cannulating micropipette was pulled out of the vessel. The tissue (~1cm×1cm) surrounding the vessel was then dissected, rinsed with ice cold PBS, and mounted on a glass coverslip. A secured-seal spacer (Invitrogen, Eugene, OR) was used to surround the tissue and to make a well about 120 μm deep between two coverslips to retain the three-dimensional structure of the vessel.
Intravital and Confocal Microscopy
A Nikon Eclipse TE2000-E inverted fluorescent microscope was used to observe the mesentery. A 10× lens (NA 0.3, Nikon) gave a field of view of approximately 2 mm in diameter. The tissue was observed with either transmitted white light from a light pipe suspended above the preparation or with fluorescent light from an illumination system (the monochromator with a xenon lamp FSM150Xe, Bentham Instrument Ltd., UK). The monochromator can generate the light of wavelength from 200 to 700 nm. The observation of fluorescently labeled glycocalyx and measurements of P to albumin and sodium fluorescein were done by a high-performance digital 12-bit CCD camera (SensiCam QE, Cooke Corp., Romulus, MI, USA) with a Super Fluor 20x objective lens (NA=0.75, Nikon) and recorded by InCyt Im™ imaging and analyzing system (Intracellular Imaging Inc., Cincinnati, OH, USA).
The cross-sectional view of a vessel with the fluorescently labeled glycocalyx was observed using 12-bit laser scanning confocal microscopy (LSCM, Zeiss LSM 510 Confocal Microscope System) with a 40×/NA1.3 objective lens. Excitation/Emission wavelength (nm) = 490/525 for FITC. Images were collected from the top (near lens) to the bottom (z-direction) for each sample, forming a stack of images along the z-direction. The thickness of each image was 0.2-0.3 μm. This confocal system has a refractive index mismatch correction function to automatically correct the elongation in z-axis due to using an oil-immersed high numerical aperture objective lens for our sample in Ringer (water). The image stacks were analyzed with the public domain National Institutes of Health IMAGE J program [30]. Stacks were reconstructed as a three-dimensional view and re-sliced into ~1 μm thick cross-sections along the vessel axis.
Measurement of Microvessel Solute Permeability P
To further investigate the structural and molecular mechanisms by which S1P maintains normal microvessel permeability, we measured apparent microvessel permeability (P) to a large solute, albumin (AlexFluor 555-BSA, ~3.6 nm Stokes radius), and to a small solute, sodium fluorescein (~0.45 nm Stokes radius). Measurement of P was taken on the individual post-capillary venules in the presence and absence of 1 μM S1P, and in the presence of a generic MMP inhibitor, GM6001 (10 μM), or its negative control GM6001NC (10 μM), or specific inhibitors to MMP-9 (400 nM) and MMP-13 (1 μM). Before P measurement, the microvessels were pretreated with these solutions for 20 min. These concentrations were the optimized concentrations used in [11,25,42,43] for the S1P and inhibitor effects on the endothelial permeability in vivo and in vitro. The detailed method using a theta pipette for P measurement has been previously described in [14,22,34]. Briefly, a post-capillary venule was cannulated with a θ pipette. One lumen was filled with 1% BSA-Ringer (washout) and another lumen with the same solution additionally containing fluorescently labeled solutes (dye). When the dye solution was perfused into the vessel lumen, the vessel was exposed to 555nm wavelength light, the images were recorded simultaneously by a high-performance digital 12-bit charge-coupled device (CCD) camera (SensiCam QE, Cooke, Romulus, MI) with a Super Fluor x20 objective lens (NA = 0.75, Nikon). Then the P was determined offline. The total fluorescence intensity (I) in the lumen of a straight vessel and surrounding tissue was determined by image analysis software (Intracellular Imaging, Cincinnati, OH). The measuring window was 200-500 μm long and 100-200 μm wide and was set at least 100 μm from the cannulation site and from the base of the bifurcation to avoid solute contamination from the cannulation site and from the side arms. P was calculated by P=(1/ΔI0)(dI/dt)0(r/2), where ΔI0 was the step increase in fluorescence intensity in the measuring window when the perfused dye just filled up the vessel lumen, (dI/dt)0 was the initial rate of increase in fluorescence intensity after the dye filled the lumen and began to accumulate in the tissue, and r was the vessel radius. The assumption for using the above equation for determining the P was that the fluorescence intensity is linearly related to the fluorescence concentration. Concentrations of 0.75 mg/ml and 0.1 mg/ml were used in the experiment, respectively, for AlexFluor 555-BSA and sodium fluorescein. These concentration were in the linear range under our imaging settings according to our in vitro calibrations [14,22].
Data Analysis
Data are presented as means ± SE, unless indicated otherwise. Statistical analysis was performed by a T-test or two-way ANOVA using Sigma Plot 11.2 from Systat Software Inc. (San Jose, CA). A level of p < 0.05 was considered significant difference in all experiments.
RESULTS
S1P Protects ESG of the Microvessel
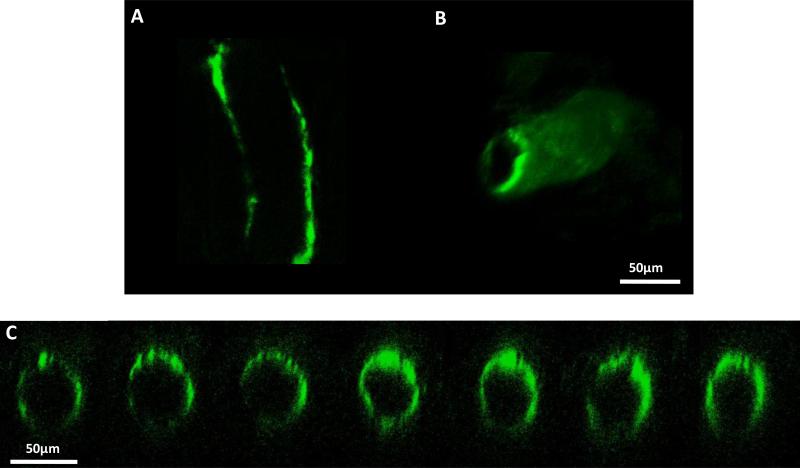
To test the hypothesis that S1P can protect the ESG in intact microvessels as in cultured endothelial cells [42], we perfused the post-capillary venule in the presence or absence of 1 μM S1P in 1%BSA-Ringer for 20 min at 37°C degree. Then we quantified the microvessel ESG by immunolabeling HS, one of the most abundant GAGs in the ESG. Figure 2 shows the confocal images of the FITC-anti-HS labeled ESG in a post-capillary venule in the presence of S1P. Figure 2A shows the mid-plane view and Figure 2B shows the 3D re-construction of the ESG in this vessel. Figure 2C demonstrates the cross-sectional view of 7 slices of thickness 1 μm evenly cut along the segment in the middle of the vessel. In the absence of S1P, the FITC-anti-HS labeled ESG at the microvessel wall was almost invisible.
Figure 2.
Confocal images of the FITC-anti-HS labeled ESG in a post-capillary venule. Image at the mid-plane of the vessel (A), 3D reconstruction (B) and cross-sectional views along the vessel axis (C).
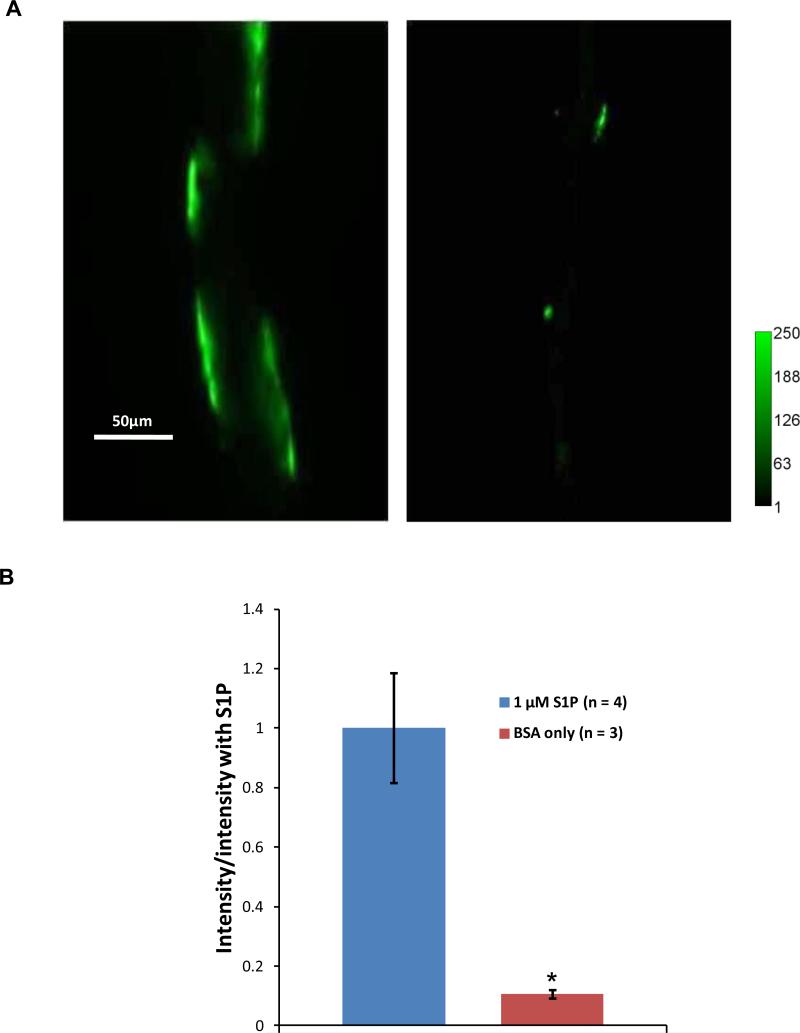
The confocal images in Figure 2 present a visual demonstration of S1P protecting microvessel ESG. Figure 3 shows the mid-plane views of the FITC-anti-HS labeled ESG in the microvessels taken by our highly light-sensitive CCD camera. Since we used a 20x/NA0.75 objective lens to observe the ESG, which has a depth of light collection ~100μm [41], the intensity of the FITC-anti-HS labeled ESG at the top and the bottom of the vessel can both be detected when we focus at the mid-plane of the vessel. The intensity of the FITC-anti-HS was measured in a straight vessel segment or region of interest (ROI) with the vessel diameter as the width and length of 100-200 μm along the vessel walls. There were 3-4 ROIs for each vessel. The intensity per unit area for each vessel is the average over these ROIs. The left image in Fig. 3A shows the FITC-anti-HS labeled ESG in the microvessel in the presence of S1P and the right image is for that in the absence of S1P. Figure 3B compares the intensity of the FITC-anti-HS labeled ESG under these two conditions. In the absence of S1P, the intensity of the FITC-anti-HS labeled ESG was only ~10% of that in the presence of S1P. S1P does protect the ESG in intact microvessels.
Figure 3.
(A) Images of fluorescently labeled heparan sulfate in a post-capillary venule pretreated with 1 μM S1P (left) and a vessel without S1P (right). (B) Comparison of the intensity of the fluorescently labeled heparan sulfate in 4 vessels with S1P and that in 3 vessels without S1P. * p < 0.001.
S1P Maintains Normal Microvessel Permeability
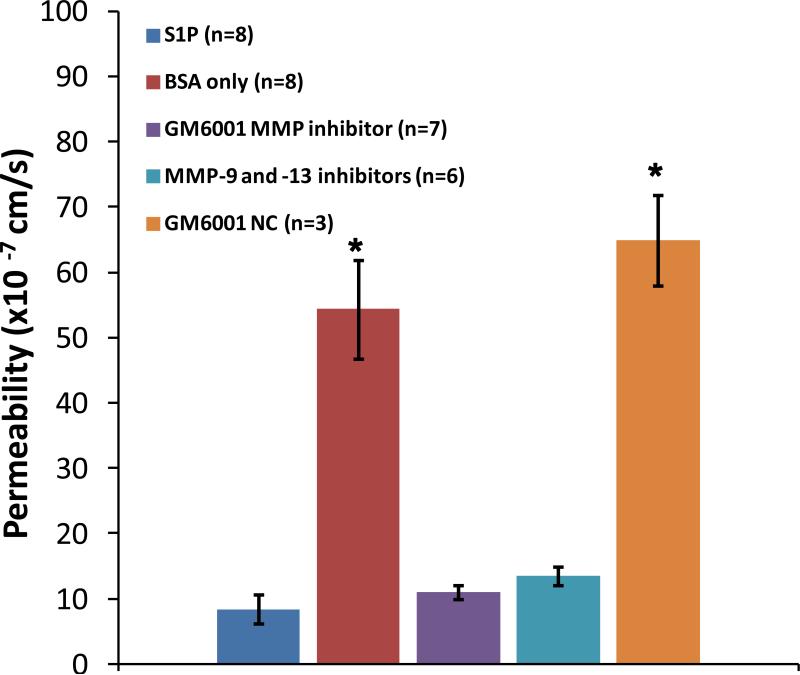
To investigate whether S1P maintains normal microvessel permeability through protecting the ESG, we measured microvessel permeability P to albumin in the presence and absence of S1P. Figure 4 demonstrates that after 20 min pretreatment with 1 μM S1P, P to albumin was 8.4 ± 2.2 ×10−7 cm/s (n=8), while in the absence of S1P, P increased by ~6.5-fold, to 54.4 ± 7.6 ×10−7 cm/s (n=8) (p < 0.001).
Figure 4.
Permeability to BSA under various treatments: S1P, BSA only, generic MMP inhibitor GM 6001, specific inhibitors to MMP-9 and -13, and negative control GM 6001 NC. *p < 0.001 compared to that under S1P treatment. n: number of vessels in each group.
A recent study on an in vitro cultured endothelial cell monolayer has shown that S1P plays a critical role in protecting the ESG by inhibiting the matrix metalloproteinase (MMP) activity-dependent shedding of heparan sulfate (HS), chondroitin sulfate (CS) and the ectodomain of syndecan-1 [42]. We then tested the effects of MMP inhibition on microvessel permeability to albumin. Figure 4 demonstrates that after 20 min pretreatment with a generic MMP inhibitor GM6001, P to albumin was 10.9 ± 1.1 × 10−7cm/s (n = 7), which was not different from that in the presence of S1P (p > 0.3); however its negative control GM6001 NC significantly increased P to albumin to 64.9 ± 6.9 × 10−7cm/s (n = 3) (p < 0.001). After 20 min pretreatment with the specific inhibitors to MMP-9 and -13, P to albumin was 13.5 ± 1.5 × 10−7cm/s, not significantly different from that in the presence of S1P (p > 0.1). We also determined P to a small solute, sodium fluorescein, in the presence of S1P, which was 1.1 ± 0.16 × 10−5cm/s (n = 6), and in the absence of S1P, which was 2.5 ± 0.32 × 10−5cm/s (n = 6). They are significantly different (p < 0.001).
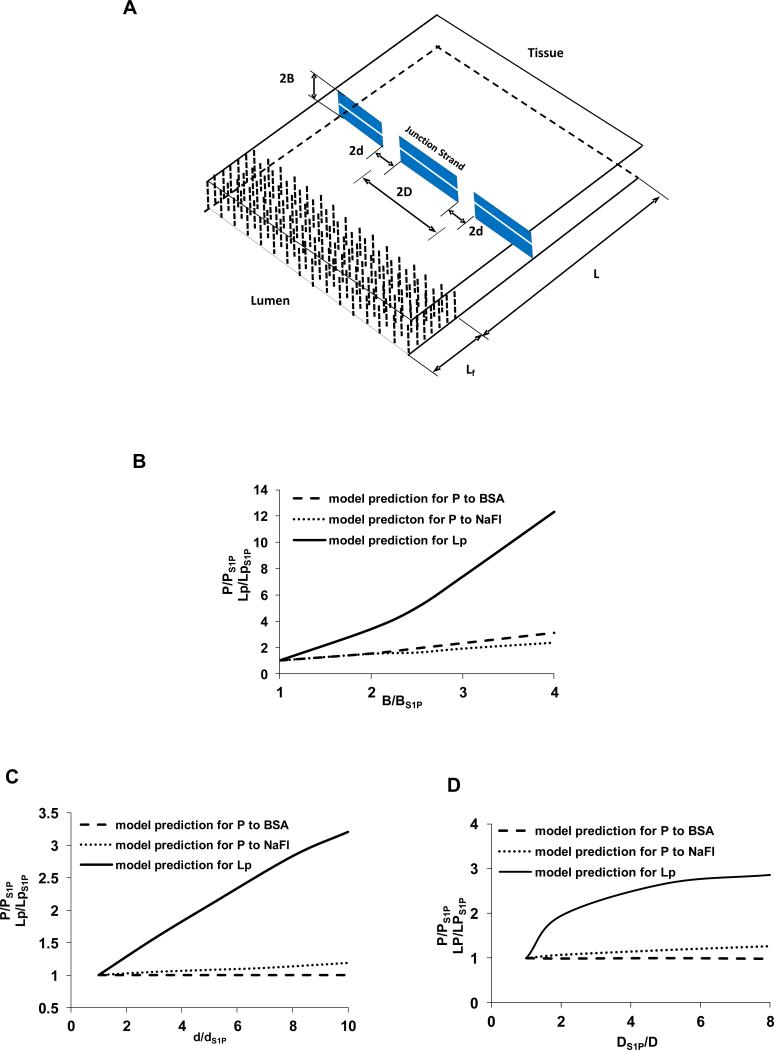
Model Predictions
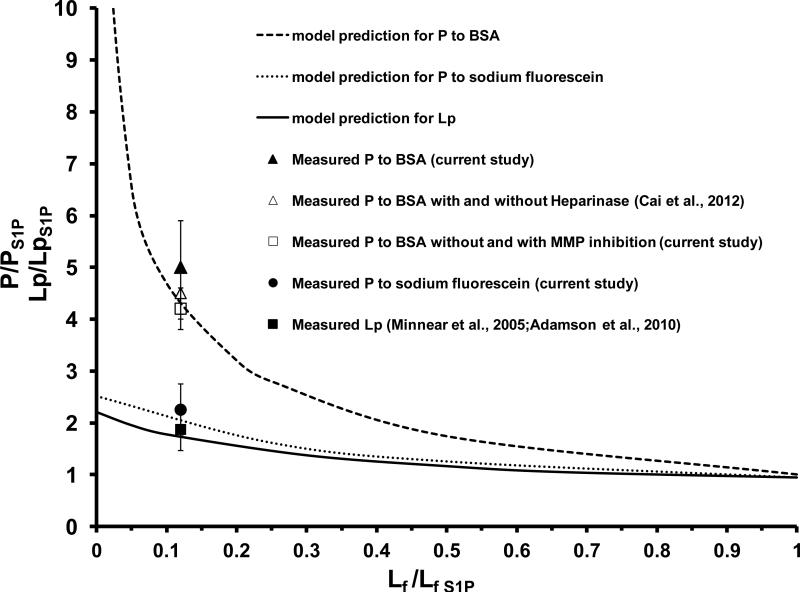
The above experiments of immunostaining for the ESG in the intact microvessel and measurement of microvessel permeability have shown that S1P does protect ESG and maintain normal microvessel permeability. To further quantitatively investigate the underlying structural mechanisms by which S1P maintains microvessel permeability, we adapted the mathematical model for inter-endothelial transport developed by Fu et al [9,16,22]. Figure 5A shows a 3-D model geometry for the inter-endothelial cleft in the wall of a rat mesenteric microvessel (revised from Fu et al. 1994) [16]. There are junction strands with discontinuous large breaks and a continuous small slit within the cleft [1,3], and a surface glycocalyx layer [6,35,40] at the luminal entrance of the cleft. Under normal physiological conditions, electron microscopy studies on rat mesenteric microvessels [3] revealed that on average, the cleft width 2B = 18 nm, break width 2d = 315 nm, spacing between adjacent large breaks 3590 nm, cleft depth L = 411 nm. The thickness Lf of the molecular sieve part of the surface glycocalyx is from 100 to 400 nm with an average 250 nm [17,35]. The glycocalyx fiber radius is 6 nm, and the gap spacing between fibers is 8 nm [6]. There is a small slit of ~1.5 nm width along the junction strand as estimated by Adamson and Michel (1993) [1] and was used to predict the microvessel permeability to small solutes [1,16]. The effective diffusion coefficients for solutes and effective viscosities for water in the fiber matrix (glycocalyx) and in the cleft were calculated by the corresponding models summarized in Sugihara-Seki and Fu (2005)[23]. Our predictions for the permeability increase are based on these baseline parameters which can fit the measured baseline permeability the best. Out of all the model predictions for the increased P to albumin, increased P to sodium fluorescein, as well as the increased hydraulic conductivity Lp measured in [4,25] in the absence of S1P, by changing the structural components of the inter-endothelial cleft, e.g., degrading the surface glycocalyx (decreasing Lf), increasing the gap (2B) between endothelial cells (endothelial cells contract or shrink) (Fig. 5B), increasing the size (2d) (Fig. 5C) or number of junction breaks in the cleft (Fig. 5D), we found that while changes in the gap width of the cleft and the size and frequency of the breaks in the junctional strand could contribute to increased permeability, the only case that accounts for the large differential effect of S1P on the permeability to albumin relative to the smaller solute and water was the degrading of ESG (Fig. 6). Model predictions that reconcile the measured permeability data and ESG quantity with the model predictions are shown in Fig. 6. The solid line is the model prediction for Lp, the dashed line for P to albumin, and the dotted line for P to sodium fluorescein. To account for the measured ratio of Lp [4,25] (solid square), and that of P to sodium fluorescein (SF) (solid circle), as well as that of P to albumin (solid triangle), in the absence and presence of S1P, the predicted Lf/Lf S1P would be close to 0.1, suggesting that ~90% ESG is degraded in the absence of S1P. This result is consistent with the ratio of fluorescent intensities in Fig. 3, assuming a proportional relation between glycocalyx thickness and fluorescent intensity. The ratio of P to albumin in the absence and presence of S1P in Fig. 5B was ~5.1, which was the ratio of respective diffusive permeability data exclusive of the solvent drag (convection) contributions. The solvent drag contributed about ~5% in the presence of S1P, and ~20% in the absence of S1P based on the measured Lp [4,25] and P to albumin (current study) according to the formula in [9]. Comparison of the model predictions with the in vivo measurements quantitatively confirmed that S1P maintains microvessel permeability by preserving ESG of microvessels.
Figure 5.
(A) A three dimensional view of the model geometry for the interendothelial cleft in the wall of a rat mesenteric post-capillary venule (revised from Fu et al., 1994). A junction strand with periodic large breaks of width 2d lies parallel to the luminal front of the cleft. The distance between adjacent large break is 2D. There is a continuous small slit also in the junction strand. An endothelial surface glycocalyx layer (ESG) of thickness Lf is at the cleft entrance; L, the total depth of the cleft; and 2B, the width of the cleft. (B)-(D) model predictions for the effect of changing structural components of the interendothelial cleft on P to BSA, P to sodium fluorescein (NaFl) and hydraulic conductivity Lp. (B): increasing the cleft width 2B. (C): increasing the junction break width 2d. (D): increasing the number of junction breaks (decreasing 2D). PS1P, LpS1P, BS1P, dS1P, DS1P are control values in the presence of S1P.
Figure 6.
Comparison of the model predictions for P to BSA, P to sodium fluorescein and hydraulic conductivity Lp by removing ESG in the absence of S1P and those measured from the experiments. Values are mean ± SE. PS1P, LpS1P, LfS1P are control values in the presence of S1P.
DISCUSSIONS
It is well established that the ESG contains a wide variety of membrane-bound carbohydrate-rich macromolecules including sulfated proteoglycans and glycosaminoglycans (GAGs). Due to its composition and unique location at the interface of circulating blood and the vessel wall, this ESG plays an important role in maintaining vascular permeability, attenuating interactions between circulating blood cells and the endothelial cells forming the vessel wall, as well as sensing the hydrodynamic changes in the blood flow [10,12,13,15,26,29,32,36]. Syndecans-1, -2, -4 and Glypican-1 are major proteoglycans found in ESG, which anchor GAGs to the endothelial cell cytoskeleton, membrane and cytoplasm. GAGs are further categorized as heparan sulfate (HS), chondroitin sulfate (CS), dermatan sulfate, keratin sulfate and hyaluronic acid or hyaluronan (HA) [28,32,33]. HS is the most abundant one, which is ~50-90% of the total GAGs [28,36]. Our newly developed technique of in situ immunolabeling of HS, CS and HA in intact post-capillary venules of rat mesentery [9,40] revealed that compared to HS, the amount of CS and HA can be neglected in these microvessels [39]. By employing this newly developed technique for in situ immunostaining of the ESG in an individual microvessel and the measurement of microvessel solute permeability in the same type of intact microvessels in the presence and absence of S1P, we have confirmed that S1P contributes to the maintenance of normal vascular permeability by protecting the ESG in intact microvessels.
The cellular mechanisms by which S1P acts to enhance endothelial barrier functions both in vivo and in vitro were thought to be via S1P1 ligation[11,18,24,25,38,42,43]. Ligation of S1P1 receptor activates the Rho family small GTPase Rac 1, leading to peripheral localization of cytoskeletal effectors such as cortactin. This localization promotes adherens junction and tight junction formation [4], which promotes cell-cell adhesion and cell-substrate adhesion, resulting in enhancing endothelial barrier function. Via S1P1 receptor, another mechanism of S1P action is to stabilize the endothelial surface glycocalyx (ESG) by reducing matrix metalloproteinase (MMP) activation and attenuating the loss of ESG [42].
Our in situ immunolabeling of the ESG directly showed that S1P can preserve the ESG in intact microvessels (Figs. 2, 3), the same as that observed in cultured endothelial cell monolayers. Our results also demonstrated that S1P can maintain normal microvessel permeability P to a large molecule, albumin (Stokes radius ~3.6 nm) as well as to a small molecule, sodium fluorescein (Stokes radius ~0.45 nm). Inhibitors to MMPs have the same effect as S1P in preserving microvessel permeability to albumin (Fig. 4). To further confirm that MMP inhibition maintains microvessel permeability to albumin by preserving the ESG, we did the immunolabeling of the ESG in the post-capillary venule in the absence of S1P but in the presence of a generic MMP inhibitor. We found that the intensity of the FITC-anti-HS labeled ESG in 3 vessels was 88 ± 11 % of that in the presence of S1P (p = 0.56). MMP inhibition did prevent the loss of ESG. These results are consistent with observations on the same type of intact microvessels in rat mesentery, [11] who reported that S1P stabilizes the basal hydraulic conductivity Lp and P to albumin via receptor S1P1. Our conclusion that the 5 fold increases in albumin permeability in the absence of S1P largely reflect loss of resistance to albumin in the glycocalyx is also consistent with our previous observation that significant loss of the glycocalyx after degradation of the glycocalyx by heparinase III results in a 4.6 fold increase in albumin permeability [9].
To quantitatively investigate the structure-function of endothelial barriers between circulating blood and body tissues, we employed a mathematical model for the paracellular pathway of the microvessel wall (Fig. 5A) to predict the effect of S1P on preserving the endothelial barrier structures. Figure 6 revealed that to account for our ESG observation and the measured permeability data in intact microvessels, the most likely and primary structural change in the interendothelial cleft would be a loss of ESG in the absence of S1P. In Fig. 6, we also plotted the measured P to albumin with and without heparinase III treatment (empty triangle) [9] and that without and with MMP inhibition (empty square). Figure 6 showed that although the model prediction for ~90% loss of ESG fits well with the measured P to albumin with and without enzyme treatment and that without and with MMP inhibition, both of which only affect ESG, it slightly underestimates the measured P to solutes and measured hydraulic conductivity Lp. One explanation for this is that in addition to preserving ESG, S1P can enhance the endothelial adherens and tight junctions in the interendothelial cleft as demonstrated in Adamson et al [4]. In the absence of S1P, the relative increase in Lp and P should be slightly higher than what are predicted by the model which assumed that only ESG is degraded in the absence of S1P.
In Gao and Lipowsky (2010) [17], the diffusion coefficient of FITC in the glycocalyx (Dg) was estimated as ~2.3 ×10−9 cm2/s by using a 1-D unsteady state diffusion equation in the glycocalyx layer and the thickness of the glycocalyx layer of ~0.5μm [17]. This value is three orders of magnitude lower than its free diffusion coefficient (Dfree). In the current study, the measured microvessel permeability to similar sized sodium fluorescein P is 1.1 × 10−5 cm/s in the presence of S1P (or intact glycocalyx). For a 1-D structure filled with the glycocalyx, the P is proportional to Dg by a length scale of the structure (Lg), Dg ~ P*Lg. If assuming that the paracellular pathway across the microvessel wall is a 1-D glycocalyx filled structure and assuming that its thickness is ~1 μm [40], then Dg of sodium fluorescein can be estimated as ~10−9 cm2/s, similar to what was estimated for FITC in [17]. However, for a 3-D structure in the cleft (Fig. 5A) with the junction strand and pores, P and Dg no longer have a simple relation as for a 1-D structure. The detailed relation was described in Fu et al. (1994)[16] for P and structural components of the paracellular pathway across the microvessel wall. In that relation, the Dg was calculated from a fiber matrix model in Sugihara-Seki and Fu (2005)[23]. As for the fiber matrix (glycocalyx) described earlier in the Model Predictions, Dg/Dfree for sodium fluorescein is ~0.2. This value can lead us to the predicted P, which is ~10−5 cm/s, similar to the measured value. Dg/Dfree of 0.2 is 100 times what was estimated in [17], in which a 1-D structure filled with the glycocalyx was assumed for the paracellular pathway across the microvessel wall, indicating that there is a significant resistance to the transport of small solutes from the cleft following the ESG. This can be seen from Fig. 6, which shows that the complete removal of the ESG only increases P to sodium fluorescein by 2.5 fold, but increases P to a larger solute, albumin, by more than 10 fold. If R is the total resistance for the solute transport across the microvessel wall, R = Rg + Rc, Rg is the resistance from the ESG and Rc is that from the cleft (refer to Fig. 5A). When the ESG is intact, P = 1/R = 1/(Rg + Rc), while when the ESG is completely removed, P’ = 1/Rc. For sodium fluorescein P’/P = 2.5, we have Rg = 1.5Rc. The cleft provides a comparable resistance to a small solute like sodium fluorescein. For albumin, P’/P=10, Rg = 9Rc. The ESG provides much larger resistance to a large solute, albumin, than that to a small solute, sodium fluorescein.
The molecular mechanism by which S1P preserves the ESG was investigated in cultured cell monolayers of rat fat-pad endothelial cells [42]. They found that S1P, acting via S1P1 receptors, suppresses the MMP activity and inhibits the shedding of HS, CS and the syndecan-1 ectodomain. Via S1P1 receptors, S1P can also attenuate acute microvascular permeability increases by inflammatory agents such as platelet-activating factor (PAF) [4,25,43] and bradykinin [4,5], as well as maintain normal permeability in intact microvessels [2]. Consistent with their observations, our present study showed that inhibition of MMP activities prevents the increase in microvessel permeability to albumin, the same effect as S1P.
PERSPECTIVES
In summary, we have shown in this study that S1P maintains normal microvessel permeability by protecting the ESG in intact microvessels. Our observations imply possible new therapeutic strategies through S1P to preserve ESG, which were found to be damaged during a variety of vascular diseases and tumor hematogenous metastasis.
Acknowledgments
Supported by NIH SC1CA153325 (BMF), R01HL094889 (JMT and BMF) and HL28607 (FEC)
REFERENCES
- 1.Adamson RH, CC M. Pathways through the intercellular clefts of frog mesenteric capillaries. Journal of Physiology. 1993;466:303–327. [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson RH, Clark JF, Radeva M, Kheirolomoom A, Ferrara KW, Curry FE. Albumin modulates S1P delivery from red blood cells in perfused microvessels: mechanism of the protein effect. Am J Physiol Heart Circ Physiol. 2014;306:H1011–1017. doi: 10.1152/ajpheart.00829.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557:889–907. doi: 10.1113/jphysiol.2003.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamson RH, Sarai RK, Altangerel A, Thirkill TL, Clark JF, Curry FR. Sphingosine-1-phosphate modulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc Res. 2010;88:344–351. doi: 10.1093/cvr/cvq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamson RH, Sarai RK, Clark JF, Altangerel A, Thirkill TL, Curry FE. Attenuation by sphingosine-1-phosphate of rat microvessel acute permeability response to bradykinin is rapidly reversible. Am J Physiol Heart Circ Physiol. 2012;302:H1929–1935. doi: 10.1152/ajpheart.00614.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arkill KP, Knupp C, Michel CC, Neal CR, Qvortrup K, Rostgaard J, Squire JM. Similar endothelial glycocalyx structures in microvessels from a range of mammalian tissues: evidence for a common filtering mechanism? Biophysical Journal. 2011;101:1046–1056. doi: 10.1016/j.bpj.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode CSS, Peest U, Beutel G, Thol F, Levkau B, Li Z, Bittman RHT, Tolle M, van der Giet M, Graler MH. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. Journal of Cellular Biochemistry. 2010;109:1232–1243. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- 8.Bouma HR, Kroese FG, Kok JW, Talaei F, Boerema AS, Herwig A, Draghiciu O, van Buiten A, Epema AH, van Dam A, Strijkstra AM, Henning RH. Low body temperature governs the decline of circulating lymphocytes during hibernation through sphingosine-1-phosphate. Proc Natl Acad Sci U S A. 2011;108:2052–2057. doi: 10.1073/pnas.1008823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai B, Fan J, Zeng M, Zhang L, Fu BM. Adhesion of malignant mammary tumor cells MDA-MB-231 to microvessel wall increases microvascular permeability via degradation of endothelial surface glycocalyx. J Appl Physiol (1985) 2012;113:1141–1153. doi: 10.1152/japplphysiol.00479.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:1541–1547. doi: 10.1161/01.ATV.0000085630.24353.3D. [DOI] [PubMed] [Google Scholar]
- 11.Curry FE, Clark JF, Adamson RH. Erythrocyte-derived sphingosine-1-phosphate stabilizes basal hydraulic conductivity and solute permeability in rat microvessels. Am J Physiol Heart Circ Physiol. 2012;303:H825–834. doi: 10.1152/ajpheart.00181.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curry FR, Adamson RH. Vascular permeability modulation at the cell, microvessel, or whole organ level: towards closing gaps in our knowledge. Cardiovasc Res. 2010;87:218–229. doi: 10.1093/cvr/cvq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curry FR, Noll T. Spotlight on microvascular permeability. Cardiovasc Res. 2010;87:195–197. doi: 10.1093/cvr/cvq188. [DOI] [PubMed] [Google Scholar]
- 14.Fu BM, Shen S. Acute VEGF effect on solute permeability of mammalian microvessels in vivo. Microvasc Res. 2004;68:51–62. doi: 10.1016/j.mvr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Fu BM, Tarbell JM. Mechano-sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med. 2013;5:381–390. doi: 10.1002/wsbm.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu BM, Weinbaum S, Tsay RY, Curry FE. A junction-orifice-fiber entrance layer model for capillary permeability: application to frog mesenteric capillaries. J Biomech Eng. 1994;116:502–513. doi: 10.1115/1.2895802. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Lipowsky HH. Composition of the endothelial glycocalyx and its relation to its thickness and diffusion of small solutes. Microvasc Res. 2010;80:394–401. doi: 10.1016/j.mvr.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 20.Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Lee MJVBJ, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Zeng M, Fu BM. Microvascular hyperpermeability and thrombosis induced by light/dye treatment. Biomech Model Mechanobiol. 2011;10:235–247. doi: 10.1007/s10237-010-0230-x. [DOI] [PubMed] [Google Scholar]
- 23.Sugihara-Sekia M, Fu BM. Blood flow and permeability in microvessels. Fluid Dynamics Research. 2005;37:82–132. [Google Scholar]
- 24.McVerry BJ, Garcia JG. Endothelial cell barrier regulation by sphingosine 1-phosphate. J Cell Biochem. 2004;92:1075–1085. doi: 10.1002/jcb.20088. [DOI] [PubMed] [Google Scholar]
- 25.Minnear FL, Zhu L, He P. Sphingosine 1-phosphate prevents platelet-activating factor-induced increase in hydraulic conductivity in rat mesenteric venules: pertussis toxin sensitive. Am J Physiol Heart Circ Physiol. 2005;289:H840–844. doi: 10.1152/ajpheart.00026.2005. [DOI] [PubMed] [Google Scholar]
- 26.Mulivor AW, Lipowsky HH. Inhibition of glycan shedding and leukocyte-endothelial adhesion in postcapillary venules by suppression of matrixmetalloprotease activity with doxycycline. Microcirculation. 2009;16:657–666. doi: 10.3109/10739680903133714. [DOI] [PubMed] [Google Scholar]
- 27.Ohkawa R, Nakamura K, Okubo S, Hosogaya S, Ozaki Y, Tozuka M, Osima N, Yokota H, Ikeda H, Yatomi Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem. 2008;45:356–363. doi: 10.1258/acb.2007.007189. [DOI] [PubMed] [Google Scholar]
- 28.Oohira A, Wight TN, Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983;258:2014–2021. [PubMed] [Google Scholar]
- 29.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 30.Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997-2012. [Google Scholar]
- 31.Reitsma S, oude Egbrink MG, Vink H, van den Berg BM, Passos VL, Engels W, Slaaf DW, van Zandvoort MA. Endothelial glycocalyx structure in the intact carotid artery: a two-photon laser scanning microscopy study. J Vasc Res. 2011;48:297–306. doi: 10.1159/000322176. [DOI] [PubMed] [Google Scholar]
- 32.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359. doi: 10.1007/s00424-007-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnitzer JE, Carley WW, Palade GE. Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci U S A. 1988;85:6773–6777. doi: 10.1073/pnas.85.18.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen S, Fan J, Cai B, Lv Y, Zeng M, Hao Y, Giancotti FG, Fu BM. Vascular endothelial growth factor enhances cancer cell adhesion to microvascular endothelium in vivo. Experimental physiology. 2010;95:369–379. doi: 10.1113/expphysiol.2009.050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith ML, Long DS, Damiano ER, Ley K. Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys J. 2003;85:637–645. doi: 10.1016/s0006-3495(03)74507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259:339–350. doi: 10.1111/j.1365-2796.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 37.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yen WY, Cai B, Yang JL, Zhang L, Zeng M, Tarbell JM, Fu BMM. Endothelial Surface Glycocalyx Can Regulate Flow-Induced Nitric Oxide Production in Microvessels In Vivo. Plos One. 2015;10 doi: 10.1371/journal.pone.0117133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yen WY, Cai B, Zeng M, Tarbell JM, Fu BM. Quantification of the endothelial surface glycocalyx on rat and mouse blood vessels. Microvasc Res. 2012 doi: 10.1016/j.mvr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan W, Lv YG, Zeng M, Fu BM. Non-invasive measurement of solute permeability in cerebral microvessels of the rat. Microvasc Res. 2009;77:166–173. doi: 10.1016/j.mvr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Zeng Y, Adamson RH, Curry FR, Tarbell JM. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am J Physiol Heart Circ Physiol. 2014;306:H363–372. doi: 10.1152/ajpheart.00687.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang G, Xu S, Qian Y, He P. Sphingosine-1-phosphate prevents permeability increases via activation of endothelial sphingosine-1-phosphate receptor 1 in rat venules. Am J Physiol Heart Circ Physiol. 2010;299:H1494–1504. doi: 10.1152/ajpheart.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]