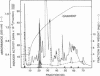
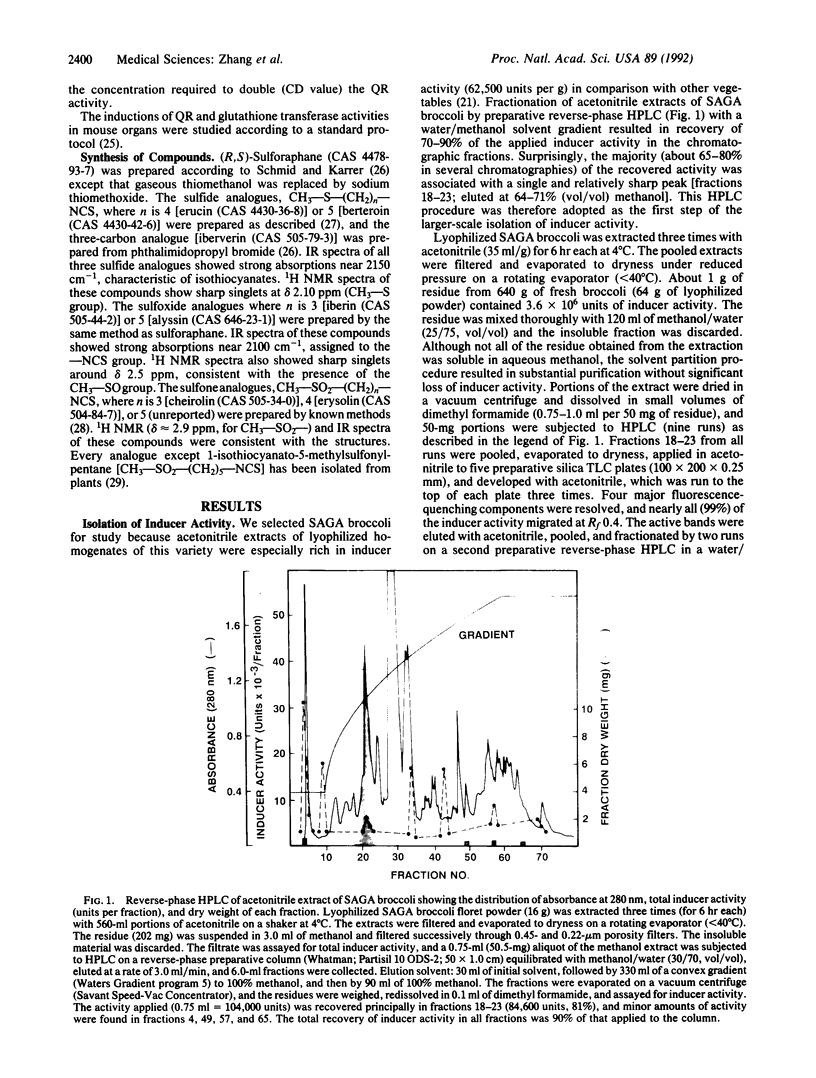
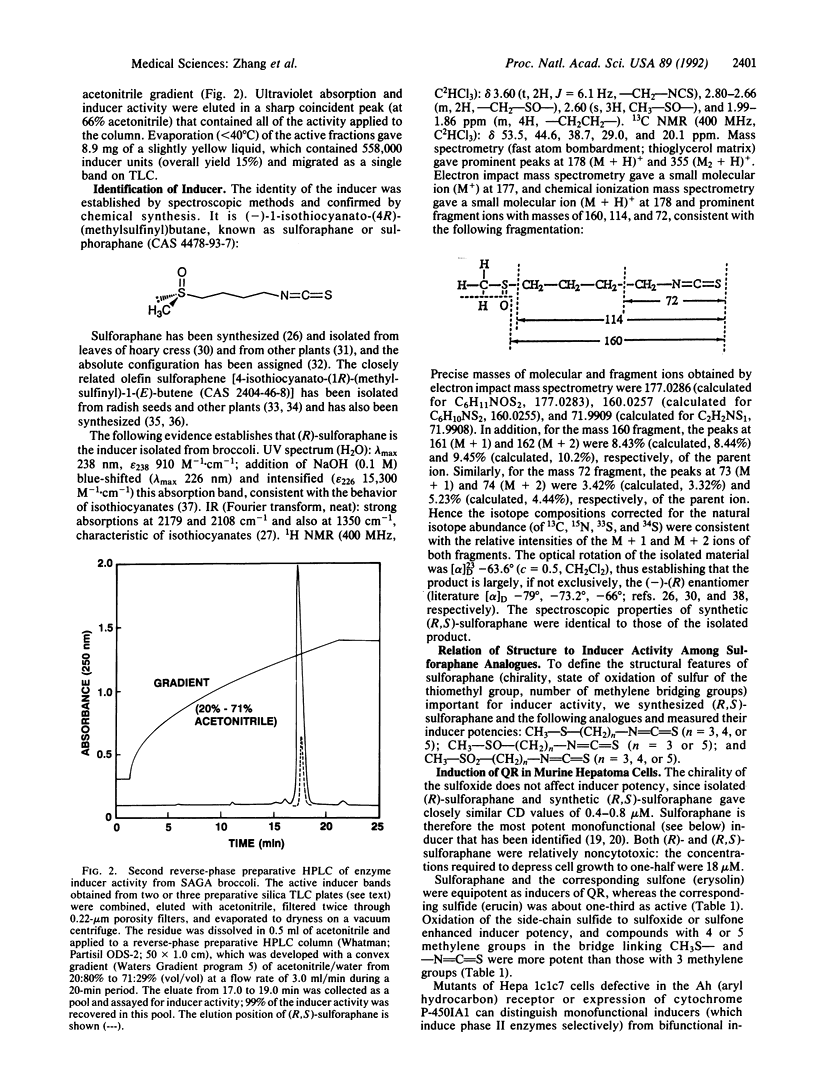
Abstract
Consumption of vegetables, especially crucifers, reduces the risk of developing cancer. Although the mechanisms of this protection are unclear, feeding of vegetables induces enzymes of xenobiotic metabolism and thereby accelerates the metabolic disposal of xenobiotics. Induction of phase II detoxication enzymes, such as quinone reductase [NAD(P)H:(quinone-acceptor) oxidoreductase, EC 1.6.99.2] and glutathione S-transferases (EC 2.5.1.18) in rodent tissues affords protection against carcinogens and other toxic electrophiles. To determine whether enzyme induction is responsible for the protective properties of vegetables in humans requires isolation of enzyme inducers from these sources. By monitoring quinone reductase induction in cultured murine hepatoma cells as the biological assay, we have isolated and identified (-)-1-isothiocyanato-(4R)-(methylsulfinyl)butane [CH3-SO-(CH2)4-NCS, sulforaphane] as a major and very potent phase II enzyme inducer in SAGA broccoli (Brassica oleracea italica). Sulforaphane is a monofunctional inducer, like other anticarcinogenic isothiocyanates, and induces phase II enzymes selectively without the induction of aryl hydrocarbon receptor-dependent cytochromes P-450 (phase I enzymes). To elucidate the structural features responsible for the high inducer potency of sulforaphane, we synthesized racemic sulforaphane and analogues differing in the oxidation state of sulfur and the number of methylene groups: CH3-SOm-(CH2)n-NCS, where m = 0, 1, or 2 and n = 3, 4, or 5, and measured their inducer potencies in murine hepatoma cells. Sulforaphane is the most potent inducer, and the presence of oxygen on sulfur enhances potency. Sulforaphane and its sulfide and sulfone analogues induced both quinone reductase and glutathione transferase activities in several mouse tissues. The induction of detoxication enzymes by sulforaphane may be a significant component of the anticarcinogenic action of broccoli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansher S. S., Dolan P., Bueding E. Biochemical effects of dithiolthiones. Food Chem Toxicol. 1986 May;24(5):405–415. doi: 10.1016/0278-6915(86)90205-x. [DOI] [PubMed] [Google Scholar]
- Aspry K. E., Bjeldanes L. F. Effects of dietary broccoli and butylated hydroxyanisole on liver-mediated metabolism of benzo[a]pyrene. Food Chem Toxicol. 1983 Apr;21(2):133–142. doi: 10.1016/0278-6915(83)90227-2. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Barretto P. B. Effects of disulfiram, diethyldithiocarbamate, bisethylxanthogen, and benzyl isothiocyanate on glutathione transferase activities in mouse organs. Cancer Res. 1985 Sep;45(9):4219–4223. [PubMed] [Google Scholar]
- Benson A. M., Barretto P. B., Stanley J. S. Induction of DT-diaphorase by anticarcinogenic sulfur compounds in mice. J Natl Cancer Inst. 1986 Mar;76(3):467–473. [PubMed] [Google Scholar]
- Boyd J. N., Babish J. G., Stoewsand G. S. Modification of beet and cabbage diets of aflatoxin B1-induced rat plasma alpha-foetoprotein elevation, hepatic tumorigenesis, and mutagenicity of urine. Food Chem Toxicol. 1982 Feb;20(1):47–52. doi: 10.1016/s0278-6915(82)80008-2. [DOI] [PubMed] [Google Scholar]
- Bradfield C. A., Chang Y., Bjeldanes L. F. Effects of commonly consumed vegetables on hepatic xenobiotic-metabolizing enzymes in the mouse. Food Chem Toxicol. 1985 Oct;23(10):899–904. doi: 10.1016/0278-6915(85)90105-x. [DOI] [PubMed] [Google Scholar]
- Colditz G. A., Branch L. G., Lipnick R. J., Willett W. C., Rosner B., Posner B. M., Hennekens C. H. Increased green and yellow vegetable intake and lowered cancer deaths in an elderly population. Am J Clin Nutr. 1985 Jan;41(1):32–36. doi: 10.1093/ajcn/41.1.32. [DOI] [PubMed] [Google Scholar]
- Conney A. H., Pantuck E. J., Hsiao K. C., Kuntzman R., Alvares A. P., Kappas A. Regulation of drug metabolism in man by environmental chemicals and diet. Fed Proc. 1977 Apr;36(5):1647–1652. [PubMed] [Google Scholar]
- De Long M. J., Prochaska H. J., Talalay P. Induction of NAD(P)H:quinone reductase in murine hepatoma cells by phenolic antioxidants, azo dyes, and other chemoprotectors: a model system for the study of anticarcinogens. Proc Natl Acad Sci U S A. 1986 Feb;83(3):787–791. doi: 10.1073/pnas.83.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Long M. J., Prochaska H. J., Talalay P. Tissue-specific induction patterns of cancer-protective enzymes in mice by tert-butyl-4-hydroxyanisole and related substituted phenols. Cancer Res. 1985 Feb;45(2):546–551. [PubMed] [Google Scholar]
- De Long M. J., Santamaria A. B., Talalay P. Role of cytochrome P1-450 in the induction of NAD(P)H:quinone reductase in a murine hepatoma cell line and its mutants. Carcinogenesis. 1987 Oct;8(10):1549–1553. doi: 10.1093/carcin/8.10.1549. [DOI] [PubMed] [Google Scholar]
- Dornberger K., Böckel V., Heyer J., Schönfeld C. H., Tonew M., Tonew E. Untersuchungen über die Isothiocyanate Erysolin und Sulforaphan aus Cardaria draba L. Pharmazie. 1975 Dec;30(12):792–796. [PubMed] [Google Scholar]
- Favreau L. V., Pickett C. B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991 Mar 5;266(7):4556–4561. [PubMed] [Google Scholar]
- Graham S. Results of case-control studies of diet and cancer in Buffalo, New York. Cancer Res. 1983 May;43(5 Suppl):2409s–2413s. [PubMed] [Google Scholar]
- Hankinson O., Andersen R. D., Birren B. W., Sander F., Negishi M., Nebert D. W. Mutations affecting the regulation of transcription of the cytochrome P1-450 gene in the mouse Hepa-1 cell line. J Biol Chem. 1985 Feb 10;260(3):1790–1795. [PubMed] [Google Scholar]
- Ito N., Hiasa Y., Konishi Y., Marugami M. The development of carcinoma in liver of rats treated with m-toluylenediamine and the synergistic and antagonistic effects with other chemicals. Cancer Res. 1969 May;29(5):1137–1145. [PubMed] [Google Scholar]
- Jakoby W. B., Ziegler D. M. The enzymes of detoxication. J Biol Chem. 1990 Dec 5;265(34):20715–20718. [PubMed] [Google Scholar]
- Lacassagne A., Hurst L., Xuong M. D. Inhibition, par deux naphtylisothiocyanates, de l'hépatocancérogenèse produite, chez le rat, par le p-diméthylamino-azobenzène (DAB) C R Seances Soc Biol Fil. 1970 Sep 25;164(2):230–233. [PubMed] [Google Scholar]
- Leonard T. B., Popp J. A., Graichen M. E., Dent J. G. alpha-Naphthylisothiocyanate induced alterations in hepatic drug metabolizing enzymes and liver morphology: implications concerning anticarcinogenesis. Carcinogenesis. 1981;2(6):473–482. doi: 10.1093/carcin/2.6.473. [DOI] [PubMed] [Google Scholar]
- Leonard T. B., Popp J. A., Graichen M. E., Dent J. G. beta-Naphthylisothiocyanate-induced alterations in hepatic drug metabolism and liver morphology. Toxicol Appl Pharmacol. 1981 Sep 30;60(3):527–534. doi: 10.1016/0041-008x(81)90339-2. [DOI] [PubMed] [Google Scholar]
- Makiura S., Kamamoto Y., Sugihara S., Hirao K., Hiasa Y. Effect of 1-naphthyl isothiocyanate and 3-methylcholanthrene on hepatocarcinogenesis in rats treated with diethylnitrosoamine. Gan. 1973 Feb;64(1):101–104. [PubMed] [Google Scholar]
- Miller A. G., Israel D., Whitlock J. P., Jr Biochemical and genetic analysis of variant mouse hepatoma cells defective in the induction of benzo(a)pyrene-metabolizing enzyme activity. J Biol Chem. 1983 Mar 25;258(6):3523–3527. [PubMed] [Google Scholar]
- Morse M. A., Amin S. G., Hecht S. S., Chung F. L. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res. 1989 Jun 1;49(11):2894–2897. [PubMed] [Google Scholar]
- Morse M. A., Eklind K. I., Amin S. G., Hecht S. S., Chung F. L. Effects of alkyl chain length on the inhibition of NNK-induced lung neoplasia in A/J mice by arylalkyl isothiocyanates. Carcinogenesis. 1989 Sep;10(9):1757–1759. doi: 10.1093/carcin/10.9.1757. [DOI] [PubMed] [Google Scholar]
- Pantuck E. J., Pantuck C. B., Garland W. A., Min B. H., Wattenberg L. W., Anderson K. E., Kappas A., Conney A. H. Stimulatory effect of brussels sprouts and cabbage on human drug metabolism. Clin Pharmacol Ther. 1979 Jan;25(1):88–95. doi: 10.1002/cpt197925188. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., De Long M. J., Talalay P. On the mechanisms of induction of cancer-protective enzymes: a unifying proposal. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8232–8236. doi: 10.1073/pnas.82.23.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska H. J., Santamaria A. B. Direct measurement of NAD(P)H:quinone reductase from cells cultured in microtiter wells: a screening assay for anticarcinogenic enzyme inducers. Anal Biochem. 1988 Mar;169(2):328–336. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Santamaria A. B., Talalay P. Rapid detection of inducers of enzymes that protect against carcinogens. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2394–2398. doi: 10.1073/pnas.89.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988 Sep 1;48(17):4776–4782. [PubMed] [Google Scholar]
- Salbe A. D., Bjeldanes L. F. Dietary influences on rat hepatic and intestinal DT-diaphorase activity. Food Chem Toxicol. 1986 Aug;24(8):851–856. doi: 10.1016/0278-6915(86)90076-1. [DOI] [PubMed] [Google Scholar]
- Salbe A. D., Bjeldanes L. F. The effects of dietary brussels sprouts and Schizandra chinensis on the xenobiotic-metabolizing enzymes of the rat small intestine. Food Chem Toxicol. 1985 Jan;23(1):57–65. doi: 10.1016/0278-6915(85)90221-2. [DOI] [PubMed] [Google Scholar]
- Sidransky H., Ito N., Verney E. Influence of alpha-naphthyl-isothiocyanate on liver tumorigenesis in rats ingesting ethionine and N-2-fluorenylacetamide. J Natl Cancer Inst. 1966 Nov;37(5):677–686. [PubMed] [Google Scholar]
- Sparnins V. L., Chuan J., Wattenberg L. W. Enhancement of glutathione S-transferase activity of the esophagus by phenols, lactones, and benzyl isothiocyanate. Cancer Res. 1982 Apr;42(4):1205–1207. [PubMed] [Google Scholar]
- Sparnins V. L., Venegas P. L., Wattenberg L. W. Glutathione S-transferase activity: enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents. J Natl Cancer Inst. 1982 Mar;68(3):493–496. [PubMed] [Google Scholar]
- Sparnins V. L., Venegas P. L., Wattenberg L. W. Glutathione S-transferase activity: enhancement by compounds inhibiting chemical carcinogenesis and by dietary constituents. J Natl Cancer Inst. 1982 Mar;68(3):493–496. [PubMed] [Google Scholar]
- Sparnins V. L., Wattenberg L. W. Enhancement of glutathione S-transferase activity of the mouse forestomach by inhibitors of Benzo[a]pyrene-induced neoplasia of the forestomach. J Natl Cancer Inst. 1981 Apr;66(4):769–771. [PubMed] [Google Scholar]
- Talalay P., De Long M. J., Prochaska H. J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talalay P. Mechanisms of induction of enzymes that protect against chemical carcinogenesis. Adv Enzyme Regul. 1989;28:237–250. doi: 10.1016/0065-2571(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of carcinogen-induced neoplasia by sodium cyanate, tert-butyl isocyanate, and benzyl isothiocyanate administered subsequent to carcinogen exposure. Cancer Res. 1981 Aug;41(8):2991–2994. [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of carcinogenic effects of polycyclic hydrocarbons by benzyl isothiocyanate and related compounds. J Natl Cancer Inst. 1977 Feb;58(2):395–398. doi: 10.1093/jnci/58.2.395. [DOI] [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of neoplasia by minor dietary constituents. Cancer Res. 1983 May;43(5 Suppl):2448s–2453s. [PubMed] [Google Scholar]
- Whitty J. P., Bjeldanes L. F. The effects of dietary cabbage on xenobiotic-metabolizing enzymes and the binding of aflatoxin B1 to hepatic DNA in rats. Food Chem Toxicol. 1987 Aug;25(8):581–587. doi: 10.1016/0278-6915(87)90018-4. [DOI] [PubMed] [Google Scholar]
- Williams R. T. Comparative patterns of drug metabolism. Fed Proc. 1967 Jul-Aug;26(4):1029–1039. [PubMed] [Google Scholar]