Abstract
mTOR is aberrantly activated in hepatocellular carcinoma (HCC) and plays pivotal roles in tumorigenesis and chemoresistance. Rapamycin has been reported to exert antitumor activity in HCC and sensitizes HCC cells to cytotoxic agents. However, due to feedback activation of AKT after mTOR complex 1 (mTORC1) inhibition, simultaneous targeting of mTORC1/2 may be more effective. In this study, we examined the interaction between the dual mTORC1/2 inhibitor OSI-027 and doxorubicin in vitro and in vivo. OSI-027 was found to reduce phosphorylation of both mTORC1 and mTORC2 substrates, including 4E-BP1, p70S6K, and AKT (Ser473), and inhibit HCC cell proliferation. Similar to OSI-027 treatment, knockdown of mTORC2 induced G0–G1 phase cell-cycle arrest. In contrast, rapamycin or knockdown of mTORC1 increased phosphorylation of AKT (Ser473), yet had little antiproliferative effect. Notably, OSI-027 synergized with doxorubicin for the antiproliferative efficacy in a manner dependent of MDR1 expression in HCC cells. The synergistic antitumor effect of OSI-027 and doxorubicin was also observed in a HCC xenograft mouse model. Moreover, AKT was required for OSI-027–induced cell-cycle arrest and downregulation of MDR1. Our findings provide a rationale for dual mTORC1/mTORC2 inhibitors, such as OSI-027, as monotherapy or in combination with cytotoxic agents to treat HCC.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer and second leading cause of cancer-related deaths worldwide (1). Surgery is often unsuitable in advanced disease, although surgical resection or liver transplantations are suitable therapeutic approaches for early-stage HCC (2). Doxorubicin is widely used to treat HCC (3, 4), despite the fact that monotherapies such as doxorubicin have shown limited efficacy in clinical trials (2, 4, 5). Thus, research into novel effective chemotherapeutic strategies continues; combination therapy based on traditional chemotherapeutic agents and small-molecule inhibitors that selectively target cancer cells represents a potentially promising approach.
The mammalian target of rapamycin (mTOR) is a critical mediator of numerous cellular signals in oncogenesis (6). The mTOR complex is comprised of two distinct components: mTORC1 and mTORC2. Rapamycin-sensitive mTORC1 directly targets ribosomal protein S6 kinase (p70S6K) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) to promote cap-dependent protein translation (7, 8). Rapamycin-insensitive mTORC2 phosphorylates the hydrophobic motif (Ser473) of the prosurvival kinase AKT, which subsequently facilitates autophosphorylation of AKT on Thr308 to maximize AKT activity (9). Hyperactivation of AKT promotes cell growth, proliferation, and survival and inhibits apoptosis (10, 11). At molecular level, Ser2448 and Ser2481 are two of the most studied phosphorylation sites on mTOR. Recent studies have demonstrated that mTORC1 contains mTOR phosphorylated predominantly on S2448, whereas mTORC2 contains mTOR on S2481. Moreover, phosphorylation of mTOR on 2448 is a biomarker of mTORC1 activity, whereas phosphorylation of the protein on Ser2481 represents mTORC2 activity (12).
MTOR is aberrantly activated in human HCC (13) and plays a pivotal role in HCC tumorigenesis (14). Targeting mTOR using rapamycin can sensitize tumor cells to cisplatin (15), doxorubicin (16), and other targeted therapeutic agents such as histone deacetylase inhibitors (17). However, mTORC1 inhibition activates mTORC2 signaling via disengaging the p70S6K-define (IRS) negative feedback loop, thus limiting the antitumor efficacy of this strategy (18). Therefore, efforts are now underway to identify a mechanism of targeting mTORC2 in cancer.
OSI-027, a novel ATP-competitive inhibitor of mTOR, inhibits both components of the mTOR complex and has demonstrated potent anticancer effects in colorectal cancer, breast cancer, and lymphoma (19–21). However, OSI-027 has been reported to enhance the cytotoxicity of cisplatin and EGFR inhibitor (EGFRi) in breast cancer and head/neck squamous cell carcinoma, respectively (22, 23). Given that rapamycin and rapalog (RAD001) exert additive antitumor effects when administered with doxorubicin in preclinical models of HCC (16, 24), we investigated the antitumor effect of OSI-027 alone and in combination with doxorubicin. In the present study, we demonstrate that inhibition of mTORC2, but not mTORC1, abrogated hyperactivation of AKT and consequently promoted cell-cycle arrest in a panel of HCC lines. Furthermore, inhibition of mTORC2 potently sensitized HCC cancer cells to doxorubicin both in vitro and in vivo. This study provides a rationale for the use of dual mTORC1/mTORC2 inhibitors as a monotherapy or in combination with traditional chemotherapeutic agents for the treatment of HCC.
Materials and Methods
Cell lines and cell culture
Human HCC cell lines and HL-7702 cells were obtained from Shanghai Institute for Biological Sciences, China on February 10, 2012. Huh-7 and SK-Hep1 cells were cultured in high glucose DMEM (Gibco) supplemented with 10% FBS (Gibco) and 1% penicillin/streptomycin (Sigma-Aldrich); Hep3B cells, MEM (Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin; SNU387, SNU-449, and HL-7702 cells, RPMI-1640 (Gibco) supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were maintained at 37°C in a humidified incubator with 5% CO2 and were used within 3 months of resuscitation. We have had all the cell lines authenticated by a professional biotechnology company in 2014.
Reagents
OSI-027 and GSK690693 were purchased from Selleck; rapamycin, doxorubicin, MG-132, and cycloheximide (CHX) from Sigma-Aldrich. Stock solutions were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich), stored at −20°C, and diluted in fresh medium for each experiment. The final concentration of DMSO did not exceed 0.5% in any experiment.
Cell viability and proliferation assays
To evaluate relative cell viability, HCC cells (5,000 per well) were seeded into 96-well microplates, incubated overnight, the culture medium was replaced with complete media containing diverse concentrations of rapamycin (0.1, 0.4, 1.6, 6.4, 25.6, 102.4, and 409.6 µmol/L) or OSI-027 (0.3125, 0.625, 1.25, 2.5, 5.0, 10, and 20 µmol/L) for 48 hours, then the cell counting kit-8 assay (CCK-8; KeyGEN) was conducted following the manufacturer's instructions. The dosages of OSI-027 to treat HL-7702 cells were 0.25, 0.50, 1.0, 2.0, 4.0, 8.0, and 16 µmol/L. Cell viability was expressed relative to untreated control cells.
To evaluate cell proliferation, SNU-387 cells were treated with 10 µmol/L rapamycin or 2.0, 5.0, and 10 µmol/L OSI-027, respectively, while other four cell lines were incubated with 2.0 µmol/L rapamycin or indicated concentrations of OSI-027 (0.5, 1.0, 2.0 µmol/L). Then cells were assayed using the Click-iT 5-ethynyl-20-deoxyuridine (EdU) Imaging Kit (Invitrogen) following the manufacturer's instructions and counterstained with Hoechst 33342. The percentage of proliferating cells in five random fields of view per slide was determined under an inverted fluorescence microscope (Olympus) and expressed relative to untreated control cells.
Apoptosis and cell-cycle analysis
Cells were treated with OSI-027 (5.0, 10, and 20 µmol/L for SNU-387 cells, 1.0, 2.0, and 4.0 µmol/L for other four HCC cell lines) for 48 hours, stained using the Annexin V-PE/7AAD apoptosis kit (BD Biosciences) according to the manufacturer's protocol, and analyzed using a BD FACS Caliber flow cytometer using BD Cell Quest software.
For cell-cycle analysis, cells were treated with DMSO, rapamycin (20 µmol/L for SNU-387 and 4 µmol/L for other four HCC cell lines) or OSI-027 as described for apoptosis assay for 48 hours, stained with propidium iodide (PI; Dawen) and analyzed by flow cytometry. Quantitative cell-cycle analysis was conducted using ModFit software (Verity Software House). Cell proliferation was expressed as the percentage of S + G2–M phase cells (25).
Drug combination analysis
Multiple drug-effect analysis was used to evaluate the effects of combined drug treatment, as described by Chou and Talalay (26). The results of the CCK-8 assay were calculated using the following equation: combination index (CI) = (D)1/(Dx)1 + (D)2/(Dx)2, where (Dx)1 and (Dx)2 are the concentrations of drug 1 and drug 2 alone that achieve effect fa; (D)1 and (D)2 are the concentrations of drug 1 and drug 2 in combination that give the same effect fa. A CI < 1 indicates synergism; CI = 1 and CI > 1 indicate an additive effect and antagonism, respectively.
RNAi
Human siRNAs specific for Raptor and Rictor were synthesized by GenePharma Co., Ltd. Detailed information of Raptor and Rictor siRNAs is described in the Supplementary Materials and Methods. MDR1, mTOR, and AKT-specific siRNAs were purchased from Cell Signaling Technology. Cells were transfected with siRNAs using Roche X-tremeGENE 9 (Roche Applied Science) following the manufacturer's instructions. Transfection medium was replaced with complete medium 6 hours later, and efficiency of siRNA knockdown was confirmed by immunoblotting.
Quantitative real-time PCR
Cells were treated with DMSO, OSI-027 (double the IC50 concentrations), doxorubicin (IC50 concentrations), or OSI-027 plus doxorubicin for 24 hours, and total RNA was extracted using TRIzol Reagent (Invitrogen) and reverse transcribed using the Prime Script reagent RT Kit (Takara Biotechnology). Primers for MDR1 were designed and purchased from Takara. PCR was performed on an ABI Prism 7900HT Real-Time System (Applied Biosystems Inc). MDR1 mRNA expression was normalized to β-actin and determined using the comparative 2−ΔΔCt method (27). Detailed sequences of primers for MDR1 and β-actin are given in the Supplementary Materials and Methods.
Immunoblotting
Immunoblotting was performed using standard protocols. Briefly, 20 µg protein lysates were fractionated on 8% to 12% Tris-glycine polyacrylamide gels, transferred to polyvinylidene difluoride membranes, blocked, incubated overnight with primary antibodies (all 1:1,000 dilution) followed by the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (1:2,000 dilution), and developed using enhanced chemiluminescence. All antibodies were obtained from Cell Signaling Technology, with the exception of anti-Cyclin D1 (Epitomics) and the HRP-conjugated secondary antibodies (Beyotime Institute of Biotechnology).
Xenograft study
Male athymic nude mice (3–4 week old; 18–20 g) were purchased from Shanghai Experimental Animal Centre and housed in a specific pathogen-free facility. Animal care was in compliance with the guidelines of the Animal Ethics Committee of Zhejiang University. Mice were inoculated subcutaneously in the left flank with 106 Huh-7 cells in 100 µL saline solution.
Two weeks after tumor inoculation, twenty tumor-bearing mice were divided into four groups (n = 5 each): control group; OSI-027 group [orally administered 30 mg/kg OSI-027 in 20% Trappsol [(CTD Holdings Inc)] every other day; doxorubicin group, injected via the caudal vein with 2 mg/kg doxorubicin in saline solution every other day; and combination group, administered 30 mg/kg OSI-027 and 2 mg/kg doxorubicin every other day. Tumor volume and body weight were measured every 2 days. Tumor volume was calculated using (L × W2)/2, where L is the diameter of the longest dimension and W is the orthogonal diameter. Relative tumor volume (RTV) was calculated using Vt/V0, where Vt is the tumor volume of all the groups at indicated time t and V0 is the original tumor volume of the animals. After 2 weeks, the mice were euthanized by cervical dislocation. Tumor growth inhibition (TGI%) was calculated using {1 − [(Tt/T0)/(Ct/C0)]/1 − [C0/Ct]} × 100, where Tt is the tumor volume of the treated group at indicated time t, T0 is the original tumor volume of the treated animal, Ct is median tumor volume of untreated mice at time t, and C0 is the median original tumor volume of the control group.
Results
OSI-027 inhibits the growth of HCC cells in a concentration-dependent manner in vitro
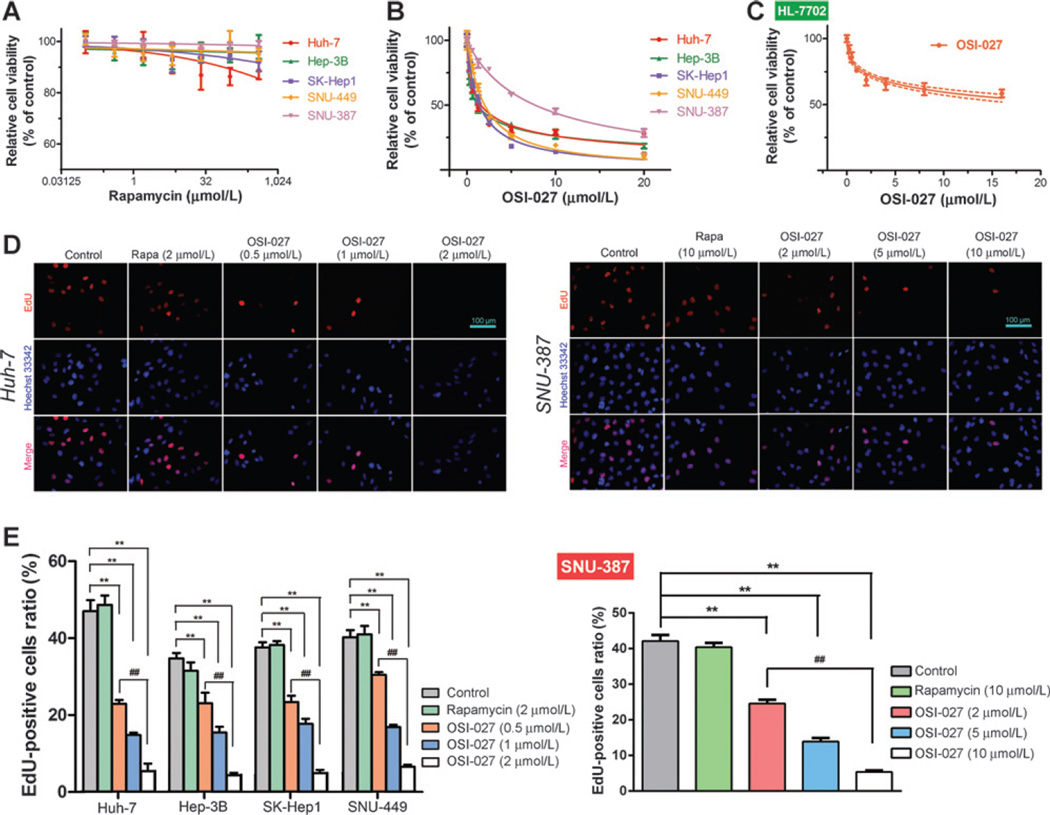
In agreement with a previous study (28), the CCK-8 assay demonstrated rapamycin hardly had any cytotoxicity (IC50 > 100 mmol/L) in all HCC cell lines tested (Fig. 1A). In contrast, the cytotoxicity of OSI-027 varied in HCC cell lines, with IC50 values ranging from 1 to 8 µmol/L (Fig. 1B; Table 1). Moreover, OSI-027 had a lower cytotoxicity in HL-7702 normal human liver cells (IC50 > 25 µmol/L) than HCC cells (Fig. 1C).
Figure 1.
Effect of rapamycin and OSI-027 on HCC cell proliferation. A and B, mean ± SD relative viability of a panel of HCC cells after treatment with various concentrations of rapamycin (A) or OSI-027 (B) for 48 hours. C, mean ± SD relative viability of HL-7702 cells treated with various concentrations of OSI-027 for 48 hours. D and E, EdU staining (D) and relative EdU-positive cell ratios (E) for HCC cells treated with indicated concentration of rapamycin (Rapa) or various concentrations of OSI-027 for 48 hours; ** and ## P < 0.01, one-way ANOVA.
Table 1.
IC50 values and statistical analyses of OSI-027 and doxorubicin (DOX) treatments in HCC cell lines and HL-7702
| IC50 (µmol/L)a | ||||
|---|---|---|---|---|
| HCC cell lines | OSI-027 | DOX | OSI-027+DOX | Combination index |
| Huh-7 | 1.613 (1.445–1.782) | 0.6490 (0.5959–0.7022) | OSI 0.3857 (0.3685–0.4028) DOX 0.3857 (0.3685–0.4028) |
0.8333 |
| Hep-3B | 1.051 (0.8065–1.295) | 1.157 (1.107–1.206) | OSI 0.3551 (0.2977–0.4125) DOX 0.3551 (0.2977–0.4125) |
0.6448 |
| SK-Hep1 | 1.303 (1.175–1.432) | 0.6720 (0.6133–0.7306) | OSI 0.7423 (0.6870–0.7977) DOX 0.1485 (0.1374–0.1595) |
0.7907 |
| SNU-449 | 2.063 (1.975–2.150) | 1.964 (1.831–2.098) | OSI 0.05758 (0.04519–0.06997) DOX 1.152 (1.052–1.252) |
0.6145 |
| SNU-387 | 8.728 (7.486–9.969) | 1.063 (0.9927–1.134) | OSI 0.9397 (0.8569–1.023) DOX 0.4696 (0.4363–0.5029) |
0.5494 |
| HL-7702 | 26.07 (16.85–35.30) | / | / | / |
IC50 values show OSI-027 and doxorubicin concentration [µmol/L, mean (95% confidence intervals)].
As the CCK-8 assay cannot distinguish between cell-cycle arrest and cytotoxicity in continuously cycling cell lines, we used the EdU thymidine analog incorporation assay (29) to estimate proliferation in HCC cells treated with rapamycin or OSI-027 for 48 hours. Consistent with the CCK-8 assay, rapamycin did not significantly affect HCC cell proliferation, whereas OSI-027 significantly inhibited HCC cell proliferation in a dose-dependent manner (Fig. 1D and E and Supplementary Fig. S1A–S1C).
OSI-027, but not rapamycin, inhibits both the mTORC1 and mTORC2 signaling cascades in HCC cells
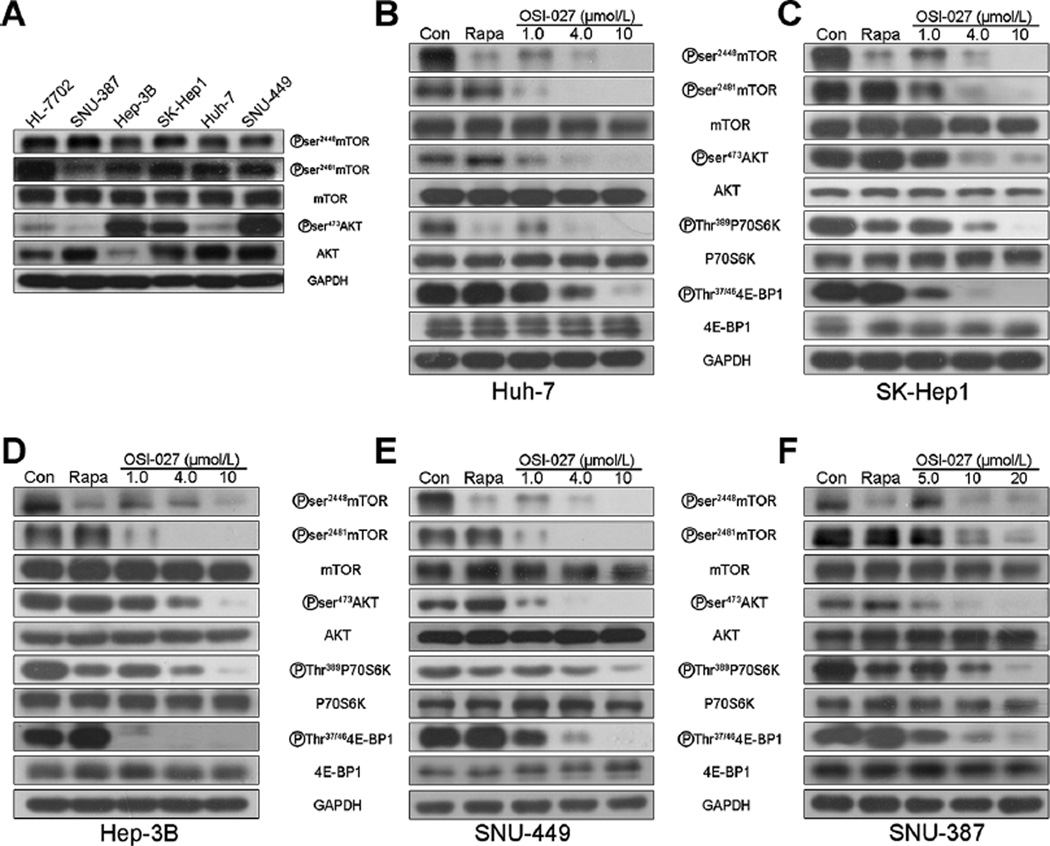
We investigated the mechanism underlying the increased cytotoxicity of OSI-027 compared with rapamycin (Fig. 1). Immunoblotting demonstrated ubiquitous basal activation of mTOR-dependent signaling in HCC cells, as indicated by high levels of phosphorylated mTOR substrates (Fig. 2A). Exposure of HCC cells to OSI-027 for 8 hours completely inhibited phosphorylation of mTOR on Ser2481, the marker of mTORC2 complex activity, and its substrate AKT (Ser473) in a dose-dependent fashion. In contrast, rapamycin inhibitedmTORC1activity (phosphorylation of Ser2448) without affecting mTOR2 activity (Ser2481), and also markedly increased AKT phosphorylation (Ser473; Fig. 2B–F; lanes 1, 2, 4). OSI-027 inhibited the phosphorylation of all mTORC1 substrates examined, including 4E-BP1 (Thr37/46) and p70S6K (Thr389). In contrast, rapamycin inhibited phosphorylation of p70S6K, but not 4E-BP1 (Fig. 2B–F; lanes 6, 8). Therefore, OSI-027 efficiently inhibits both the mTORC1 and mTORC2 signaling cascades in HCC cells, whereas ramamycin partially inhibits mTORC1 but does not inhibit mTORC2, even at relatively high doses in the micromolar range. These results are consistent with previous studies on rapamycin (30, 31) and may explain the poor efficacy of rapamycin in vitro.
Figure 2.
OSI-027 blocks mTORC1/C2 signaling more efficiently than rapamycin. A, immunoblot analysis of basal mTORC1 and mTORC2 signaling in representative HCC cells and HL-7702 cells. GAPDH was used as a protein loading control. B–F, immunoblot analysis of mTORC1 and mTORC2 signaling in (B) Huh-7, (C) SK-Hep1, (D) Hep-3B, and (E) SNU-449 cells treated with 4 µmol/L rapamycin (Rapa) or various concentrations of OSI-027 for 8 hours, and (F) SNU-387 cells treated with 10 µmol/L rapamycin or various concentrations of OSI-027 for 8 hours.
OSI-027, but not rapamycin, induces cell-cycle arrest in HCC cells
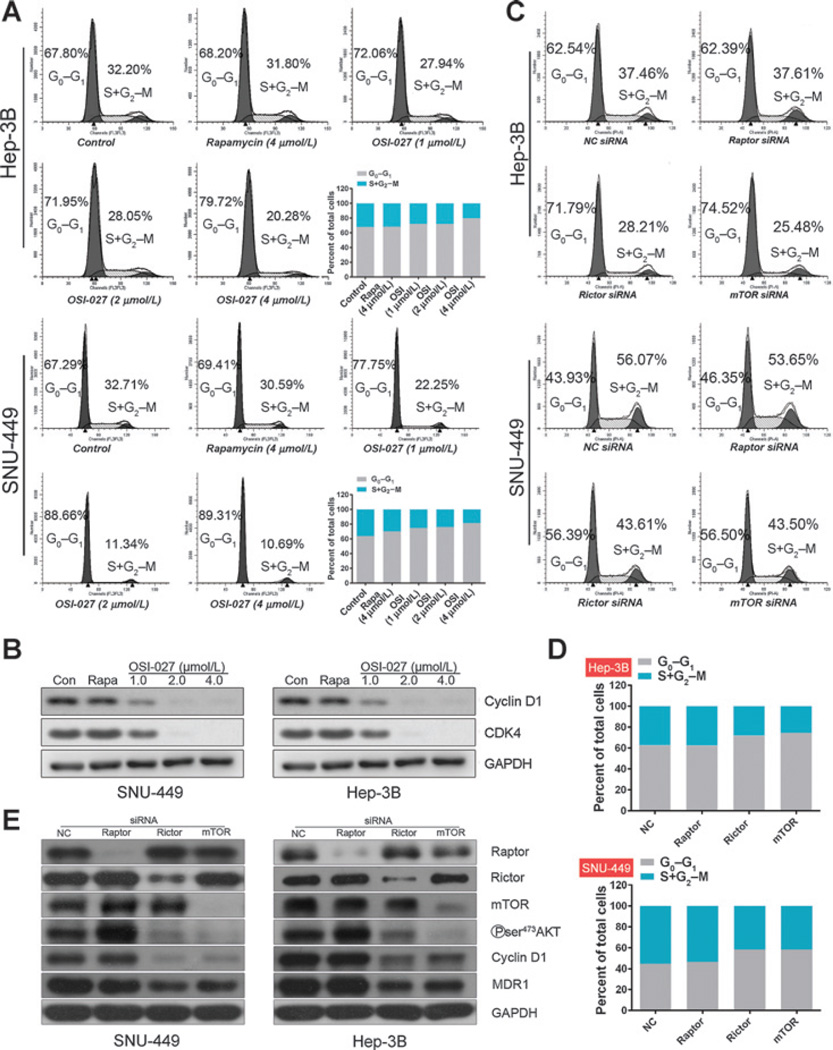
Considering the significance of mTOR in the regulation of cell metabolism, cell-cycle progression and survival (32–35), we investigated the antiproliferative effect of OSI-027 further. Annexin V staining and flow cytometry demonstrated treatment with OSI-027 for 48 hours (Supplementary Fig. S2A and S2B) did not induce apoptosis, as previously reported (19, 21, 36). Nevertheless, OSI-027 increased the proportion of SNU-449 cells in the G0–G1 phase (from 67.29% to 89.31%) in a dose-dependent manner (Fig. 3A). In contrast, rapamycin did not significantly affect the G0–G1 ratio compared with DMSO control-treated cells (Fig. 3A). Similar results were obtained in the other four HCC cell lines, except for SNU-387 cells in which rapamycin induced a low level of cell-cycle arrest (Fig. 3A and Supplementary Fig. S3A–S3C). In agreement with the cell-cycle distribution analysis, OSI-027 significantly reduced the expression of Cyclin D1 and CDK4 in all five HCC cell lines, whereas rapamycin did not significantly affect the expression of these cell cycle-associated proteins (Fig. 3B and Supplementary Fig. S 3D).
Figure 3.
Effect of rapamycin and OSI-027 on HCC cell-cycle progression (A and B), and knockdown of Rictor or mTOR, but not Raptor, results in cell-cycle arrest (C–E). A, flow-cytometric analysis of cell-cycle distribution in Hep-3B and SNU-449 cells treated with 4 µmol/L rapamycin (Rapa) or the indicated concentrations of OSI-027 (OSI) for 48 hours. B, Western blot analysis of Cyclin D1 and CDK4 in HCC cells treated with rapamycin or OSI-027. GAPDH was used as a protein loading control. C–E, HCC cells were transfected with 50 nmol/L of siRNA against Raptor, Rictor, mTOR, or negative control (NC) siRNA as indicated, and incubated for 48 hours. Flow-cytometric analysis (C) and quantification of cell-cycle distribution (D) for Hep-3B and SNU-449 cells. E, immunoblot analysis of Raptor, Rictor, mTOR, p-AKT (S473), Cyclin D1, MDR1, and GAPDH (loading control) in Hep-3B cells and SNU-499 cells. These experiments were repeated three times with similar results.
Targeting of mTORC2, rather than mTORC1, promotes G0–G1-phase arrest in HCC cell lines
To further identify whether mTORC1 or mTORC2 participates in the induction of cell-cycle arrest by OSI-027, the effects of Raptor, Rictor, and mTOR on cell-cycle progression were examined. Raptor is short for regulatory-associated protein of mTOR and regarded as one of the components ofmTORC1. Raptor serves as a binding platform where substrates are presented to mTOR for subsequent activation of mTORC1 (37). As for rapamycin-insensitive companion of mTOR (Rictor), it contributes to the structural foundation of mTORC2. And in the absence of Rictor, mTORC2 becomes inactive (38). Knockdown of Rictor or mTOR clearly increased the number of G0–G1 phase cells in all HCC cell lines tested, whereas knockdown of Raptor had no significant effect (Fig. 3C and D and Supplementary Fig. S4A–S4C). Furthermore, knockdown of Rictor or mTOR, but not Raptor, simultaneously reduced Cyclin D1 expression and AKT phosphorylation (Ser473) in Hep-3B and SNU-449 cells (Fig. 3E; lanes 4, 5). Knockdown of Raptor even slightly increased AKT phosphorylation (Ser473; Fig. 3E; lane 4), consistent with activation of AKT by rapamycin (Fig. 2B–F). These data suggest that targeting of mTORC2, but not mTORC1, promotes G0–G1-phase arrest and phosphorylation of the hydrophobic motif of AKT may be required to maintain Cyclin D1 expression and promote cell-cycle progression in HCC cells.
OSI-027 synergistically enhances the efficacy of doxorubicin via reversing doxorubicin-induced upregulation of MDR1
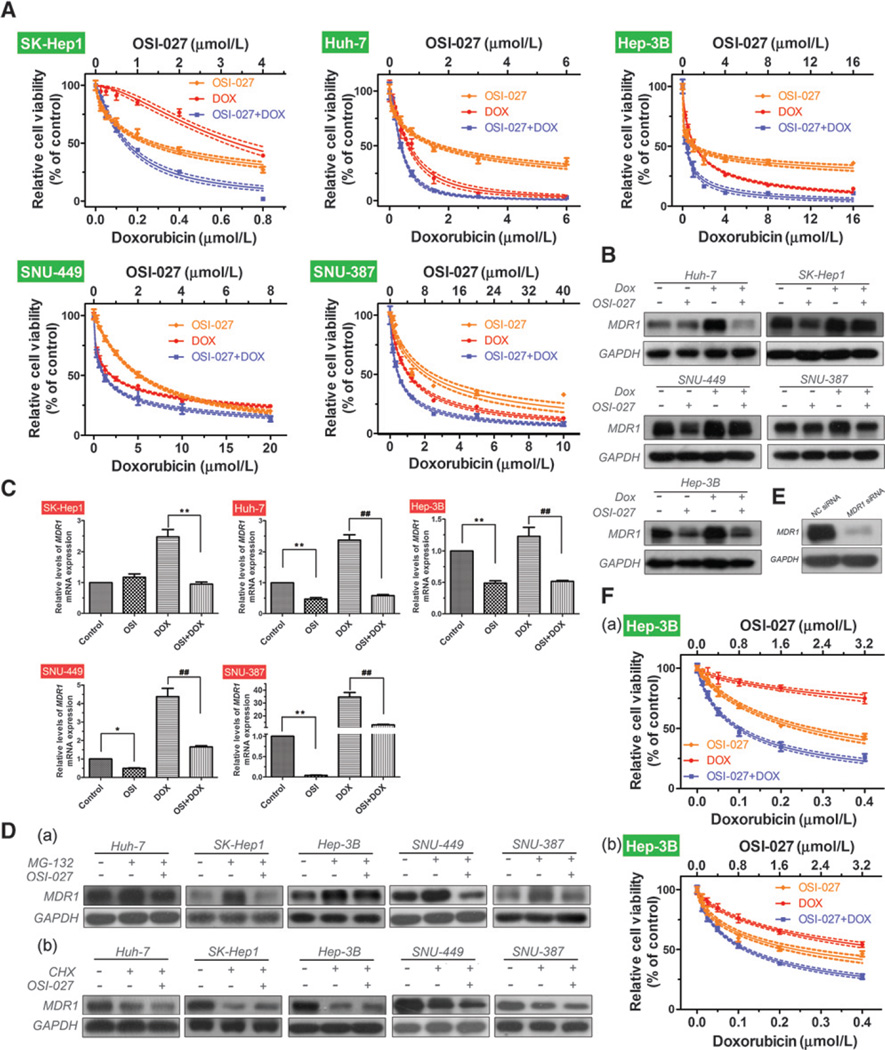
Multidrug resistance protein 1 (MDR1) plays a role in the doxorubicin resistance of HCC cell lines (39). Given that marked downregulation of MDR1 was observed in Rictor-silenced HCC cells (Fig. 3E; lane 6), we examined whether the combination of OSI-027 and doxorubicin led to an additive or synergistic effect. Drug combination index (CI) analysis is a generalized method for analyzing the effects of multiple drugs and identifying addition, synergism, and antagonism. We treated HCC cells with decreasing half-log concentrations of OSI-027, doxorubicin, or a combination of both. The CCK-8 assay was performed and the data were analyzed using method of Chou and Talalay, as described in Materials and Methods. The dose-response curves demonstrated the combined treatment resulted in an enhanced, synergistic effect (CI values ranging from 0.5494 to 0.8333; Fig. 4A; Table 1). Immunoblotting demonstrated downregulation of MDR1 when the cells were treated with approximately double the IC50 concentrations of OSI-027 for 24 h (Fig. 4B). Moreover, upregulation of MDR1 induced by doxorubicin could be reversed by the dual mTORC1/mTORC2 inhibitor OSI-027 (Fig. 4B), especially in Huh-7 and Hep-3B cells. Real-time qRT-PCR confirmed the Western blotting results; the relative expression of MDR1 mRNA reduced by approximately half in OSI-027–treated cells (except in SK-Hep1 cells; Fig. 4C). Furthermore, OSI-027 potently reversed the high levels of MDR1 mRNA expression induced by doxorubicin (Fig. 4C).
Figure 4.
OSI-027 synergizes the efficacy of doxorubicin via reversing the upregulation of MDR1 induced by doxorubicin. A, dose-response curves for HCC cells treated with OSI-027, doxorubicin, or a combination of both for 48 hours. Immunoblotting analysis (B) and RT-PCR analysis ofMDR1 expression (C) in HCC cells treated withOSI-027 (OSI, double the IC50 concentrations for each cell line), doxorubicin (IC50 concentrations), or a combination of both for 24 hours. GAPDH was used as a protein loading control. *, P < 0.05; ** and ##, P < 0.01, one-way ANOVA D, immunoblotting analysis of MDR1 in HCC cells treated with (a) 1 µmol/L MG-132 or (b) 100 µg/mL cycloheximide (CHX) in the presence or absence of OSI-027 (double the IC50 concentrations) for 24 hours. E, immunoblotting analysis of the efficiency of MDR1 siRNA-mediated knockdown compared to control cells (NC). F, dose-effect curves forOSI-027, doxorubicin, and the combination of both drugs in (a) control cells and (b)MDR1-knockdown Hep-3B cells at 48 hours.
Next, we investigated how OSI-027 affects MDR1 expression. As the ubiquitin-proteasome pathway regulates degradation of MDR1 in mammalian cells (40), we treated the cells with a proteasome inhibitor (MG132) alone or combination with OSI-027. MG132 increased the levels of MDR1 in HCC cells, and this effect could be reversed by OSI-027 (Fig. 4D, a). However, no further reduction MDR1 expression was obtained when the cells were treated with a combination of cycloheximide and OSI-027 compared with cycloheximide alone (Fig. 4D, b). Therefore, OSI-027 downregulates MDR1 by inhibiting its synthesis rather than promoting its degradation.
Hep-3B cells were transfected with negative control (NC) siRNA or MDR1 siRNA and subjected to the CCK-8 assay to confirm whether MDR1 is involved in the synergistic effect of OSI-027 and doxorubicin. Knockdown of MDR1 sensitized Hep-3B cells to doxorubicin (IC50 values reduced from 2.016 µmol/L to 0.4886 µmol/L), without affecting the sensitivity of the cells to OSI-027 (Table 2 and Supplementary Fig. S5A). Correspondingly, the dose-response graphs indicated that knockdown of MDR1 attenuated the synergistic effect of OSI-027 and doxorubicin (Fig. 4F); this observation was supported by an increase in the CI values (Table 2). Similar results were observed in SNU-449 cells (Supplementary Fig. S5B–S5D).
Table 2.
IC50 values and statistical analyses of OSI-027 and doxorubicin treatments in MDR1 knockdown HCC cell lines
| HCC cell lines (Treatment) |
IC50 (µmol/L)a | |||
|---|---|---|---|---|
| OSI-027 | DOX | OSI-027+DOX | Combination index | |
| Hep-3B (NC siRNA) |
2.041 (1.849–2.233) | 2.016 (0.9769–3.056) | OSI 0.7953 (0.7377–0.8530) DOX 0.09942 (0.09221–0.1066) |
0.4390 |
| Hep-3B (MDR1 siRNA) |
1.915 (1.641–2.189) | 0.4886 (0.4203–0.5569) | OSI 0.9442 (0.8816–1.007) DOX 0.1180 (0.1102–0.1259) |
0.8164 |
| SNU-449 (NC siRNA) |
2.751 (1.702–3.799) | 3.520 (3.090–3.949) | OSI 0.4973 (0.4534–0.5413) DOX 1.989 (1.813–2.165) |
0.4694 |
| SNU-449 (MDR1 siRNA) |
2.511 (1.603–3.419) | 1.845 (1.702–1.988) | OSI 0.1829 (0.1668–0.1990) DOX 0.7317 (0.6673–0.7961) |
0.7458 |
IC50 values show OSI-027 and doxorubicin concentration [µmol/L, mean (95% confidence intervals)].
Taken together, OSI-027 reverses the high levels of MDR1 expression induced by doxorubicin via inhibiting the synthesis of MDR1, and consequently synergizes the efficacy of doxorubicin in vitro.
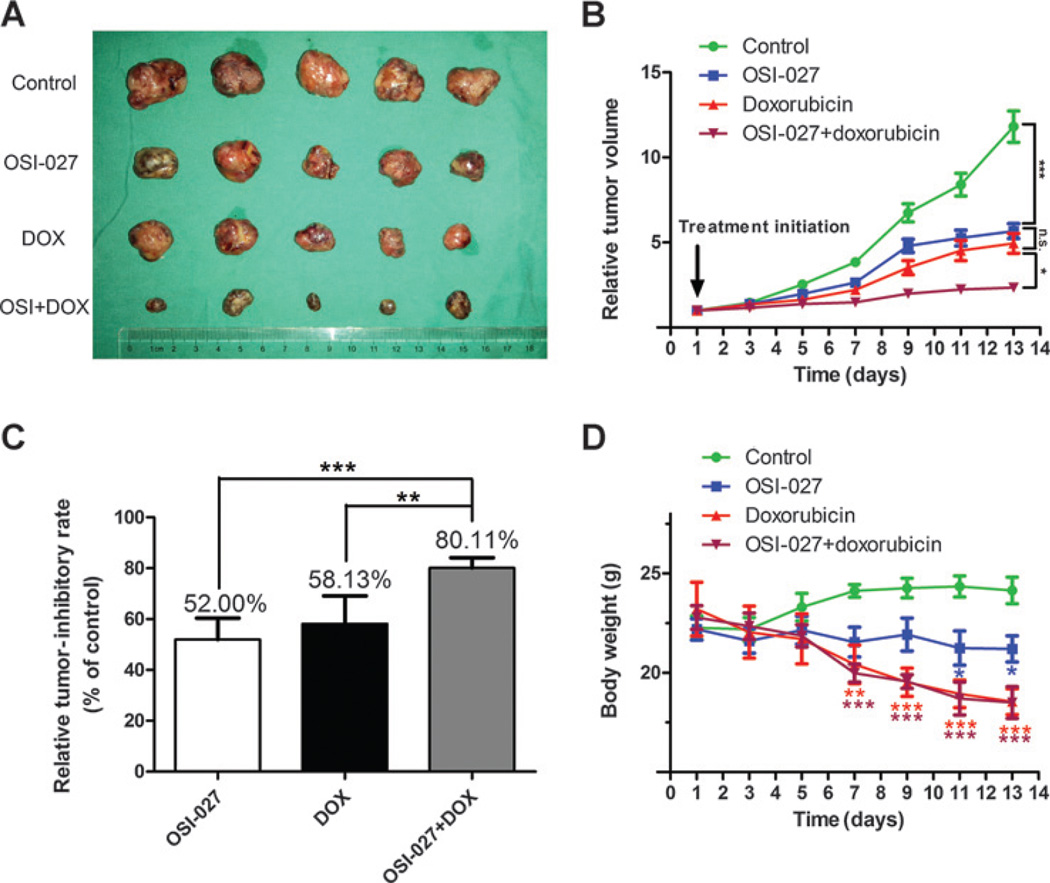
The combination of OSI-027 and doxorubicin significantly enhances tumor growth inhibition in HCC in vivo
OSI-027 inhibits tumor growth more effectively than rapamycin in a number of in vivo cancer models, including colorectal cancer, breast cancer and lymphoma (19–21). Other studies have reported a potent reduction in the tumor burden in mice bearing HCC xenografts treated with a combination of doxorubicin plus rapamycin or rapalog (16, 24). Given the potent synergistic effect of mTORC1/mTORC2 inhibition in combination with doxorubicin in vitro, we assessed the effects of this combination in vivo. Nude mice were inoculated with a representative HCC cell line, Huh-7, and randomized to one of four treatment groups (control, OSI-027 alone, doxorubicin alone, or OSI-027 plus doxorubicin). Tumor growth was significantly delayed in the group receiving both OSI-027 plus doxorubicin compared with either treatment alone or the vehicle control (Fig. 5A and B), indicative of a synergistic effect. In comparison with monotherapy, the combination of OSI-027 plus doxorubicin increased the relative tumor-inhibitory rate from approximately 55% to 80.11% (Fig. 5C). Interestingly, OSI-027 alone exhibited a similar antitumor efficacy to doxorubicin, the typical chemotherapeutic drug for HCC, with no significant difference between the tumor volumes or tumor-inhibitory rate of these groups (Fig. 5B and C).
Figure 5.
Effect of OSI-027 in combination with doxorubicin on HCC xenograft tumor growth in vivo. A xenograft model of HCC was established as described in Materials and Methods. A, size of excised tumors after 2 weeks treatment with OSI-027 in the presence or absence of doxorubicin. B, tumor growth curves. Combined treatment with OSI-027 and doxorubicin (DOX) significantly delayed tumor growth compared with the single agents alone. C, relative tumor inhibitory rate for each treatment; **, P < 0.01; *, P < 0.05, one-way ANOVA. D, change in body weight. *, P < 0.05; **, P < 0.01; ***, P < 0.001 versus control group, one-way ANOVA; n.s., not significant.
OSI-027 was well tolerated using this schedule, with no animals requiring euthanasia for severe weight loss or signs of other toxicities, though the mice receiving OSI-027 did experience modest weight loss. Moreover, the combination of OSI-027 plus doxorubicin did not result in additive toxicity compared with either treatment alone (Fig. 5D).
Cell-cycle arrest induced by targeting mTORC2 and the reversal of MDR1 expression induced by OSI-027 are both AKT dependent
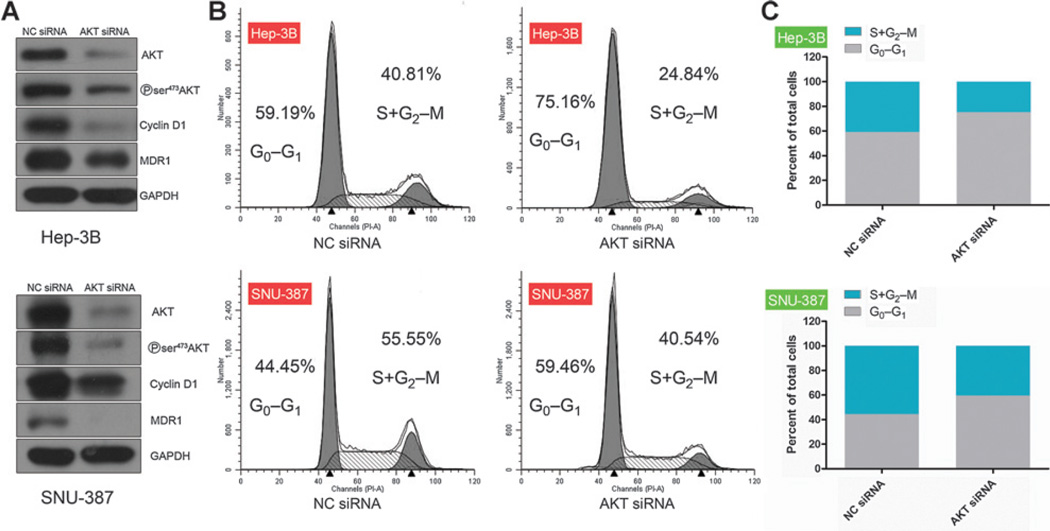
Given that the downregulation of Cyclin D1/CDK4 and MDR1 coincided with decreased AKT phosphorylation (Ser473), we examined the role of AKT in these processes. As expected, knockdown of AKT using a siRNA resulted a robust reduction in AKT phosphorylation (Ser473) and simultaneously downregulated Cyclin D1 (Fig. 6A). Flow cytometry revealed induction of G0–G1-phase arrest after knockdown of AKT in the four HCC cell lines tested compared with NC siRNA-transfected cells (Fig. 6B and C and Supplementary Fig. S6A–S6C); this effect was almost of the same magnitude as the G0–G1-phase arrest induced by knockdown of Rictor/mTOR(Fig. 3A). Knockdown of AKT also markedly downregulated MDR1 (Fig. 6A). Therefore, the cell-cycle arrest, downregulation of Cyclin D1, and reversal of MDR1 expression induced by inhibition of mTORC2 using OSI-027 are AKT dependent.
Figure 6.
Knockdown of AKT promotes G0–G1 arrest and downregulates MDR1. HCC cells were transfected with AKT or control (NC) siRNA. A, immunoblot analysis of p-AKT, MDR1, and cell-cycle–relatedmarkers forHep-3B and SNU-387 cells; GAPDH was used as an internal loading control. B and C, flow-cytometric analysis (B) and quantification (C) of cell-cycle distribution.
Discussion
As a crucial component of the PI3K/AKT pathway that plays a critical role in oncogenesis, mTOR is aberrantly activated in most types of cancer, including human HCC. Increased expression of total p70S6K correlated with activation of mTOR in almost half of patients with HCC (33 out of 73; ref. 13). Consistent with the early study, we found that mTOR and p-mTOR were both overexpressed in either tumor or peritumor tissue compared with normal liver tissue in 60% of the patients detected, indicating the activation of mTOR in HCC (Supplementary Fig. S7). In addition, chromosomal gains in Rictor and positive p-RPS6 staining correlated with tumor recurrence in a larger cohort of patients with HCC (14). In this study, we observed that, though OSI-027 also targets mTORC1, OSI-027 inhibited proliferation and promoted cell-cycle arrest via inhibition of mTORC2, suggesting that mTORC2 plays a critical role in the maintenance of cell viability. We also demonstrated that by inhibiting both mTORC1 and mTORC2, OSI-027 may outperform rapamycin as an anticancer agent. Moreover, OSI-027 exerted a synergistic antitumor effect when combined with doxorubicin. Taken together, this study offers a rationale for clinical testing of mTORC1/mTORC2 inhibitors as monotherapy or concurrently with doxorubicin.
Rapalogs have displayed significant antitumor activity in renal cell carcinoma (41) and have been approved by the U.S. Food and Drug Administration for clinical use. We previously demonstrated that inhibition of mTOR using rapamycin delayed HCC xenograft tumor growth in vivo (42). However, we here found that rapamycin hardly exerted an anticancer effect in HCC in vitro (Fig. 1A and D). These seemingly contradictory findings can be explained by the different microenvironments of in vitro experiments and rodent xenograft models. Inhibition of mTOR using rapamycin significantly reduces HCC and renal cell carcinoma xenograft tumor growth via exerting an antiangiogenic effect (28, 39), and we previously reported that differentiation of monocytes into antitumor M1 macrophages may also participate in the antiangiogenic effect of rapamycin in HCC in vivo (42). In contrast, the present study demonstrated that all of the HCC cell lines tested were sensitive to the antiproliferative effects of mTORC1/mTORC2 inhibition using OSI-027. However, SNU-387 cells exhibited relatively low mTORC2 activity and decreased AKT phosphorylation on S473 (Fig. 2A), which may explain the lower sensitivity of this cell line to OSI-027.
We observed that OSI-027 did not induce apoptosis in HCC cells. OSI-027 induced apoptosis in only three of 22 various cell lines in one study and one of three bladder cancer cell lines in a separate study (19, 36), indicating that apoptosis is not a universal mode of action for this agent. However, inhibition of mTORC1/mTORC2 strongly induced apoptosis when combined with cytotoxic agents such as cisplatin or histone deacetylase inhibitors (17, 22), although we did not examine these combinations in HCC. However, these observations support the ability of mTOR inhibitors to promote apoptosis when administered with other drug combinations.
Targeted inhibition of mTORC2, but not mTORC1, potently prevented cells from transitioning from the G0–G1 phase to S phase (Fig. 3C–E). Further experiments demonstrated that AKT played a critical role in this complex process (Fig. 6). Numerous studies have demonstrated that mTORC2 regulates cell-cycle progression (43, 44); however, the precise mechanisms were poorly characterized. Previous studies reported that targeting mTORC2 suppresses Cyclin D1 translation via suppressing recruitment of Cyclin D1 mRNA to polysomes in acute myeloid leukemia and BCR-ABL–expressing chronic myeloid leukemia cells (45, 46). This mechanism is plausible, as the translational repressor 4E-BP1 that regulates cap-dependent mRNA translation is also deactivated by inhibition of mTORC2 (7, 8). However, decreased phosphorylation of AKT (S473) induced by inhibition of mTORC2 resulted in G0–G1 phase arrest and coincided with downregulation of Cyclin D1. Further investigation is urgently needed to elucidate the precise relationship between AKT and 4E-BP1 in cell-cycle progression.
The ability of hepatoma cells to become resistant to cytotoxic agents remains a significant hurdle to chemotherapy. Intrinsic or acquired multidrug resistances (MDR) are major causes of chemoresistance, especially to doxorubicin. The most extensively studied factor associated with MDR is increased expression of P-glycoprotein (also known as ABCB1 or MDR1; ref. 39), which is encoded byMDR1. Arceci and colleagues reported that rapamycin can reverse the MDR phenotype in human T-cell lymphoblastic leukemia cell lines (47), and increased activation of the PI3K/mTOR pathway was recently associated with overexpression of MDR1 in HCC (48). However, the effect of mTORC2 inhibition on MDR1 expression remained unknown. The present study demonstrates that OSI-027 reserves the overexpression of MDR1 induced by doxorubicin in HCC cells. Furthermore, inhibition of mTORC2 may mainly be responsible for this effect (Fig. 3E). We did not examine whether rapamycin exerts a similar effect on MDR1; however, knockdown of Raptor indicated that inhibition of mTORC1 alone had limited effect on MDR1 expression (Fig. 3E). These results indicate that PI3K/mTOR signaling regulates MDR1 via a number of mechanisms in different human cancer cells.
We then investigated how OSI-027 reduced the expression of MDR1. OSI-027 also downregulated MDR1 mRNA expression (Fig. 4C), thus suppression of cap-dependent mRNA translation of the MDR1 gene secondary to decreased phosphorylation of 4E-BP1 cannot explain this observation. PI3K-AKT signaling regulates transcription of the MDR1 gene. Silencing AKT demonstrated that OSI-027–induced downregulation of MDR1 was AKT dependent. AKT inhibitor was also introduced to further support this finding (Supplementary Fig. S8A and S8B). We also confirmed that AKT plays an essential role in transcription of MDR1 (Supplementary Fig. S9). In agreement with our results, Kuo and colleagues reported that AKT activation induced MDR1 mRNA expression in human HepG2 cells and human embryonic kidney (HEK)-293 cells via NF-κB signaling (49). Therefore, mTORC2 inhibition may suppress MDR1 transcription by reducing AKT activity (Supplementary Fig. S10); further studies are needed to characterize this mechanism. In the present study, knockdown of MDR1 significantly attenuated, but did not completely abrogate, the synergistic effect of OSI-027 and doxorubicin. First, siRNAs induce gene knockdown rather than complete gene knockout. Piguet and colleagues reported that temsirolimus sensitized HCC cells to DNA damage induced by doxorubicin via downregulating p21 (16), suggesting additional pathways may also sensitize HCC cells to doxorubicin.
Although current ATP-competitive mTOR inhibitors potently inhibit mTORC2 activity, they also perturb mTORC1-dependent negative feedback loops, which may limit the therapeutic potential of these agents. Moreover, knockdown of Rictor had less deleterious effects than knockdown of Raptor in mice skeletal muscle (50). To date, the relative significance of mTORC2 versus mTORC1 inhibition in cancer cell proliferation and chemoresistance remain unclear, and may vary in different cells. Therefore, mTORC2-specific inhibitors will prove useful for further preclinical research and may represent promising chemotherapeutic agents.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by the National High Technology Research and Development Program 863 of China (SS2014AA020534; to W. Chen), the National Key Basic Research Program of China (2014CB542101, to T.-B. Liang; 2012CB966600, to X.H. Feng), the National Natural Science Foundation of China (81472212, to T.-B. Liang; 81302071, to W. Chen; 81171884, to X.L. Bai; 81273260, to F. Xue; and 31090360, to X.H. Feng), the National Natural Science Funds for Distinguished Young Scholar of China (30925033, to T.-B. Liang), Project 111 (to X.H. Feng), Project 985 (to X.H. Feng), and the Innovation and High-Level Talent Training Program of Department of Health of Zhejiang (to T.-B. Liang).
Footnotes
Supplementary data for this article are available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: B.W. Chen, W. Chen, C. Liang, X. Zhi, T.-B. Liang
Development of methodology: B.W. Chen, H. Liang, H. Liu, L.-Q. Hu, X.-Z. Yu, F. Xue
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): B.W. Chen, W. Chen, H. Liang, X. Zhi, L.-Q. Hu, T. Wei, L. Zheng, X.-L. Bai
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): B.W. Chen, H. Liang, C. Liang, L.-Q. Hu, X.-Z. Yu, T. Wei, F. Xue, X.-L. Bai
Writing, review, and/or revision of the manuscript: B.W. Chen, W. Chen, H. Liang, B. Zhao, X-H. Feng, X.-L. Bai, T.-B. Liang
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): H. Liu, X.-Z. Yu, T. Ma, B. Zhao
Study supervision: H. Liu, T. Ma, T.-B. Liang
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Lin S, Hoffmann K, Schemmer P. Treatment of hepatocellular carcinoma: a systematic review. Liver Cancer. 2012;1:144–158. doi: 10.1159/000343828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SH, Lee Y, Han SH, Kwon SY, Kwon OS, Kim SS, et al. Systemic chemotherapy with doxorubicin, cisplatin and capecitabine for metastatic hepatocellular carcinoma. BMC Cancer. 2006;6:3. doi: 10.1186/1471-2407-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon a-2b/doxorubicin/fluorouracil(PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. doi: 10.1093/jnci/dji315. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz JD, Schwartz M, Mandeli J, Sung M. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol. 2002;3:593–603. doi: 10.1016/s1470-2045(02)00873-2. [DOI] [PubMed] [Google Scholar]
- 6.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 7.Tamburini J, Green AS, Bardet V, Chapuis N, Park S, Willems L, et al. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood. 2009;114:1618–1627. doi: 10.1182/blood-2008-10-184515. [DOI] [PubMed] [Google Scholar]
- 8.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, et al. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 10.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, et al. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 12.Copp J, Manning G, Hunter T. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 2009;69:1821–1827. doi: 10.1158/0008-5472.CAN-08-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421–8425. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 14.Villanueva A, Chiang DY, Newell P, Peix J, Thung S, Alsinet C, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–1983. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tam KH, Yang ZF, Lau CK, Lam CT, Pang RW, Poon RT. Inhibition of mTOR enhances chemosensitivity in hepatocellular carcinoma. Cancer Lett. 2009;273:201–209. doi: 10.1016/j.canlet.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 16.Piguet AC, Semela D, Keogh A, Wilkens L, Stroka D, Stoupis C, et al. Inhibition of mTOR in combination with doxorubicin in an experimental model of hepatocellular carcinoma. J Hepatol. 2008;49:78–87. doi: 10.1016/j.jhep.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Shao H, Gao C, Tang H, Zhang H, Roberts LR, Hylander BL, et al. Dual targeting of mTORC1/C2 complexes enhances histone deacetylase inhibitor-mediated anti-tumor efficacy in primary HCC cancer in vitro and in vivo. J Hepatol. 2012;56:176–183. doi: 10.1016/j.jhep.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Harkavy B, Shen N, Grohar P, Helman L. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 19.Bhagwat SV, Gokhale PC, Crew AP, Cooke A, Yao Y, Mantis C, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Mol Cancer Ther. 2011;10:1394–1406. doi: 10.1158/1535-7163.MCT-10-1099. [DOI] [PubMed] [Google Scholar]
- 20.Falcon BL, Barr S, Gokhale PC, Chou J, Fogarty J, Depeille P, et al. Reduced VEGF production, angiogenesis, and vascular regrowth contribute to the antitumor properties of dual mTORC1/mTORC2 inhibitors. Cancer Res. 2011;71:1573–1583. doi: 10.1158/0008-5472.CAN-10-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta M, Hendrickson AE, Yun SS, Han JJ, Schneider PA, Koh BD, et al. Dual mTORc1/mTORc2 inhibition diminishes AKT activation and induces PUMA-dependent apoptosis in lymphoid malignancies. Blood. 2012;119:476–487. doi: 10.1182/blood-2011-04-346601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Lin J, Wang X, Yao G, Wang L, Zheng H, et al. Targeting of mTORC2 prevents cell migration and promotes apoptosis in breast cancer. Breast Cancer Res Treat. 2012;134:1057–1066. doi: 10.1007/s10549-012-2036-2. [DOI] [PubMed] [Google Scholar]
- 23.Cassell A, Freilino ML, Lee J, Barr S, Wang L, Panahandeh MC, et al. Targeting TORC1/2 enhances sensitivity to EGFR inhibitors in head and neck cancer preclinical models. Neoplasia. 2012;14:1005–1014. doi: 10.1593/neo.121212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirstein MM, Boukouris AE, Pothiraju D, Buitrago-Molina LE, Marhenke S, Schütt J, et al. Activity of the mTOR inhibitor RAD001, the dual mTOR and PI3-kinase inhibitor BEZ235 and the PI3-kinase inhibitor BKM120 in hepatocellular carcinoma. Liver Int. 2013;33:780–793. doi: 10.1111/liv.12126. [DOI] [PubMed] [Google Scholar]
- 25.Camplejohn RS. Flow cytometric measurement of cell proliferation. Methods Mol Med. 2001;57:133–143. doi: 10.1385/1-59259-136-1:133. [DOI] [PubMed] [Google Scholar]
- 26.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Semela D, Piguet AC, Kolev M, Schmitter K, Hlushchuk R, Djonov V, et al. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46:840–848. doi: 10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:0371–0383. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci U S A. 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 33.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–2287. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker MN, Wu KJ, Marlow LA, Kreinest PA, Vonroemeling CA, Copland JA, et al. The combination of an mTORc1/TORc2 inhibitor with lapatinib is synergistic in bladder cancer in vitro. Urol Oncol. 2014;32:317–326. doi: 10.1016/j.urolonc.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 38.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Park JG, Lee SK, Hong IG, Kim HS, Lim KH, Choe KJ, et al. MDR1 gene expression: its effect on drug resistance to doxorubicin in human hepatocellular carcinoma celllines. J Natl Cancer Inst. 1994;86:700–705. doi: 10.1093/jnci/86.9.700. [DOI] [PubMed] [Google Scholar]
- 40.Katayama K, Noguchi K, Sugimoto Y. FBXO15 regulates P-glycoprotein/ABCB1 expression through the ubiquitin proteasome pathway in cancer cells. Cancer Sci. 2013;104:694–702. doi: 10.1111/cas.12145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oudard S, Medioni J, Aylllon J, Barrascourt E, Elaidi RT, Balcaceres J, et al. Everolimus (RAD001): an mTOR inhibitor for the treatment of metastatic renal cell carcinoma. Expert Rev Anticancer Ther. 2009;9:705–717. doi: 10.1586/era.09.27. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Ma T, Shen XN, Xia XF, Xu GD, Bai XL, et al. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res. 2012;72:1363–1372. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
- 43.Masri J, Bernath A, Martin J, Jo OD, Vartanian R, Funk A, et al. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 2007;67:11712–11720. doi: 10.1158/0008-5472.CAN-07-2223. [DOI] [PubMed] [Google Scholar]
- 44.Smrz D, Kim MS, Zhang S, Mock BA, Smrzová S, DuBois W, et al. mTORC1 and mTORC2 differentially regulate homeostasis of neoplastic and nonneoplastic human mast cells. Blood. 2011;118:6803–6813. doi: 10.1182/blood-2011-06-359984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carayol N, Vakana E, Sassano A, Kaur S, Goussetis DJ, Glaser H, et al. Critical roles for mTORC2- and rapamycin-insensitive mTORC1-complexes in growth and survival of BCR-ABL-expressing leukemic cells. Proc Natl Acad Sci U S A. 2010;107:12469–12474. doi: 10.1073/pnas.1005114107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altman JK, Sassano A, Kaur S, Glaser H, Kroczynska B, Redig AJ. Dual mTORC2/mTORC1 targeting results in potent suppressive effects on acute myeloid leukemia (AML) progenitors. Clin Cancer Res. 2011;17:4378–4388. doi: 10.1158/1078-0432.CCR-10-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arceci RJ, Stieglitz K, Bierer BE. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood. 1992;80:1528–1536. [PubMed] [Google Scholar]
- 48.Wang SF, Chou YC, Mazumder N, Kao FJ, Nagy LD, Guengerich FP, et al. 7-Ketocholesterol induces P-glycoprotein through PI3K/mTOR signaling in hepatoma cells. Biochem Pharmacol. 2013;86:548–560. doi: 10.1016/j.bcp.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo MT, Liu Z, Wei Y, Lin-Lee YC, Tatebe S, Mills GB, et al. Induction of human MDR1 gene expression by 2-acetylaminofluorene is mediated by effectors of the phosphoinositide 3-kinase pathway that activate NF-kB signaling. Oncogene. 2002;21:1945–1954. doi: 10.1038/sj.onc.1205117. [DOI] [PubMed] [Google Scholar]
- 50.Bentzinger CF, Romanino K, Cloëtta D, Lin S, Mascarenhas JB, Oliveri F, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. doi: 10.1016/j.cmet.2008.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.