Abstract
Background. The effect of Xpert MTB/RIF (Xpert) scale-up on patient outcomes in low-income settings with a high tuberculosis (TB) burden has not been established. We sought to characterize the effectiveness of Xpert as implemented across different levels of the healthcare system in Uganda.
Methods. We reviewed laboratory records from 2012 to 2014 at 18 health facilities throughout Uganda. In 8 facilities, Xpert had been implemented onsite since 2012, and in 10 sites Xpert was available as an offsite referral test from another facility. We describe Xpert testing volumes by facility, Xpert and smear microscopy results, and downtime due to malfunction and cartridge stockouts. We compare TB treatment initiation as well as time to treatment between facilities implementing Xpert and those that did not.
Results. The median number of Xpert assays run at implementing facilities was 25/month (interquartile range [IQR], 10–63), amounting to 8% of total capacity. Among 1251 assays run for a new TB diagnosis, 19% were positive. Among 1899 patients with smear-negative presumptive TB, the proportion starting TB treatment was similar between Xpert facilities (11%; 95% confidence interval [CI], 9%–13%) and non-Xpert facilities (9%; 95% CI, 8%–11%; P = .325). In Xpert facilities, a positive Xpert preceded TB treatment initiation in only 12 of 70 (17%) smear-negative patients initiated on treatment.
Conclusions. Xpert was underutilized in Uganda and did not significantly increase the number of patients starting treatment for TB. Greater attention must be paid to appropriate implementation of novel diagnostic tests for TB if these new tools are to impact patient important outcomes.
Keywords: implementation science, tuberculosis, Uganda, Xpert MTB/RIF
Tuberculosis (TB) was first declared a global health emergency by the World Health Organization (WHO) in 1993 [1]. Despite the worldwide adoption of standardized TB control practices, the disease is now the leading cause of infectious mortality worldwide, with an estimated 9.6 million new TB cases and 1.5 million deaths in 2014 [2]. Insufficient case detection is a persistent obstacle to furthering the WHO's stated goal of TB elimination by 2035 [3]. Global case detection has remained stagnant at 66% for the last several years, translating to 3.3 million active TB cases per year who are not notified to public health authorities [2]. One strategy to augment TB case finding is to implement new diagnostic tests with improved sensitivity and turnaround time, particularly in settings that have previously relied on sputum smear microscopy as the primary TB diagnostic. Xpert MTB/RIF ([Xpert]; Cepheid, Inc., Sunnyvale, CA) is a new molecular test that can rapidly detect Mycobacterium tuberculosis (MTB) in sputum, has substantially better sensitivity than sputum smear microscopy, and can identify rifampin resistance [4, 5]. The assay is now recommended by the WHO as the primary diagnostic test for those with human immunodeficiency virus (HIV)-associated TB, as well as those with presumed multidrug resistant (MDR)-TB [6]. The global scale-up of Xpert has been rapid and impressive, with over 18 000 test modules and 10 million assay cartridges procured through 2014 [7]. Published evaluations of the impact of routine Xpert implementation on patient-level outcomes have been performed mostly in clinical trials or in middle-income settings (eg, South Africa), and they have shown a decrease in time to TB treatment, little effect on the overall proportion starting TB treatment, and minimal impact on patient morbidity or mortality [8–10]. In contrast to these middle-income and controlled research settings, most low-income countries remain reliant on smear microscopy for TB diagnosis, often with little empiric treatment. It might therefore be expected that new diagnostics such as Xpert would have substantially greater impact in these areas. However, these settings also have weak referral and specimen transport networks, incomplete linkage to TB treatment, and unreliable infrastructure (eg, electricity), making the implementation of diagnostic tests such as Xpert more challenging. The effect of implementing Xpert on patient outcomes in such settings has not been established, and the identification of contextual factors affecting the success (or failure) of implementation will be valuable in informing and improving comparable efforts in similar settings. Therefore, we sought to characterize the effectiveness of Xpert, as implemented in actual clinical settings across different levels of the healthcare system in Uganda since the scale-up of Xpert in that country began in 2012.
METHODS
Study Setting and Design
In 2012, the Uganda Ministry of Health implemented Xpert in select facilities, recommending Xpert testing for those with presumed TB who are HIV-infected and smear negative, children (0–14 years), healthcare workers, or contacts of MDR-TB cases. The Xpert test was further recommended for previously treated smear-positive TB cases to evaluate for rifampin resistance [11].
We reviewed laboratory and patient records at 18 Ugandan health facilities selected to be representative of both geography and health system level at which Xpert was implemented. We compared 8 facilities where Xpert was implemented onsite for TB diagnosis since 2012 against 10 sites where Xpert was not available onsite. Sites included regional referral hospitals, district-level hospitals, and subdistrict-level health centers. Each facility had a laboratory capable of performing sputum smear microscopy. To ascertain facility-level Xpert testing volumes, we abstracted Xpert and smear results from 12 months of laboratory records, including electronic Xpert machine logs and TB laboratory registers. We interviewed laboratory personnel to ascertain the Xpert testing algorithm used in each facility, as well as the duration and reasons for Xpert machine downtime in the preceding 12 months. To ascertain linkages between Xpert results and treatment initiation, we abstracted all data from TB laboratory and treatment registers for 100 consecutive patients who provided sputum samples for TB evaluation at each facility.
Definitions and Statistical Analysis
We characterized facility-level Xpert testing volumes and patient-level testing results as the median and interquartile range (IQR) of monthly volumes measured throughout the year. We constructed Kaplan-Meier curves to describe the time to treatment initiation, comparing Xpert and non-Xpert facilities, and used the log-rank statistic to test for equality of survival functions. We used Fisher's exact test to compare proportions of patients started on treatment between Xpert and non-Xpert facilities.
To calculate the proportion of Xpert capacity that was being used at each facility, we assumed that a laboratory with a single 4-module Xpert instrument could run 320 assays per month at maximum capacity (4 assays per module per 8-hour workday with 20 workdays per month). We calculated time to TB treatment as the number of days elapsed between provision of the first sputum sample at the laboratory and the recorded start of treatment.
Ethical Considerations
This study was approved by institutional review boards at Makerere University of Health Sciences in Kampala, Uganda, the Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, and the University of California San Francisco in San Francisco, California.
RESULTS
Study Facilities and Xpert Algorithms
The study included 18 health facilities. The mean number of sputa processed for TB diagnosis per month ranged from 8 to 119 per facility (Table 1). Fidelity to the national recommended Xpert testing algorithm varied by facility, with 2 (25%) facilities implementing Xpert using a more restrictive algorithm, 4 (50%) implementing according to guidelines, and 2 (25%) extending the recommendations to include clinician requests (and at 1 site to all HIV-infected individuals regardless of smear result).
Table 1.
Facility Characteristics
| Facility | Onsite Xpert Available? | Facility Level | Urban/Rural | Monthly Sputa for New TB Evaluation (SD) | Xpert Algorithm |
|---|---|---|---|---|---|
| Bwizibwera | Yes | Subdistrict | Rural | 28 (10) | On clinician request |
| Luwero | Yes | Subdistrict | Urban | 37 (12) | National guidelines* |
| Kayunga | Yes | District hospital | Urban | 39 (8) | On clinician request |
| Masaka | Yes | District hospital | Urban | 92 (21) | National guidelines* |
| Soroti | Yes | Regional hospital | Urban | 66 (17) | National guidelines* plus HIV infected individuals regardless of smear status and on clinician request |
| Jinja | Yes | Regional hospital | Urban | 104 (16) | National guidelines* |
| Arua | Yes | Regional hospital | Urban | 107 (39) | National guidelines* plus on clinician request |
| Fort Portal | Yes | Regional hospital | Urban | 119 (22) | National guidelines* |
| Bushenyi | No | Subdistrict | Urban | 8 (3) | N/A |
| Nakasongola | No | Subdistrict | Urban | 20 (6) | N/A |
| Kuluva | No | District hospital | Rural | 11 (6) | N/A |
| Nyenga | No | District hospital | Rural | 25 (8) | N/A |
| Nsambya | No | District hospital | Urban | 48 (22) | N/A |
| Lyantonde | No | District hospital | Urban | 47 (12) | N/A |
| Iganga | No | District hospital | Urban | 67 (12) | N/A |
| Mengo | No | District hospital | Urban | 78 (32) | N/A |
| Tororo | No | District hospital | Urban | 109 (21) | N/A |
| Hoima | No | Regional hospital | Urban | 64 (17) | N/A |
Abbreviations: HIV, human immunodeficiency virus; MDR, multidrug resistant; MTB, Mycobacterium tuberculosis; N/A, not applicable; SD, standard deviation; TB, tuberculosis; Xpert, Xpert MTB/RIF.
*Uganda National guidelines recommend Xpert for the following: those with presumed TB who are HIV-infected and smear negative, children (0–14 years), healthcare workers or contacts of MDR-TB cases, and known TB cases suspected of MDR-TB.
Monthly Facility-Level Xpert Volumes
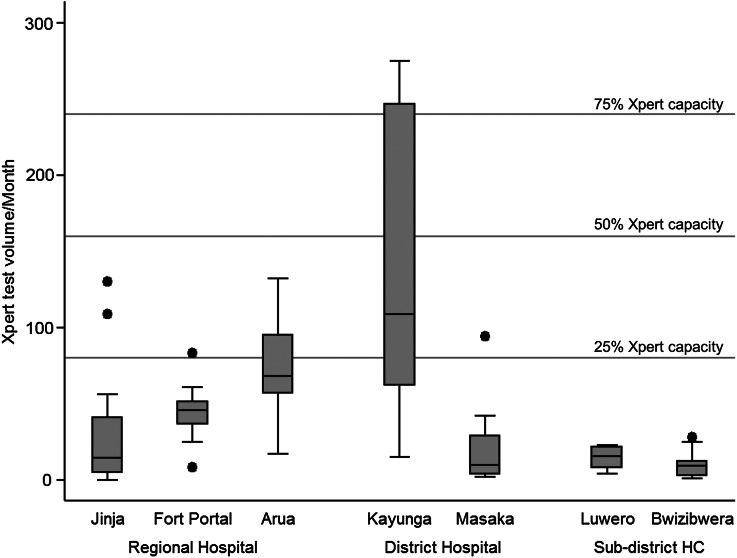
From March 2013 to October 2014, 3561 Xpert assays were performed over 74 months at 7 facilities where Xpert was implemented. The Xpert computer logs were unavailable for abstraction at the eighth Xpert facility. A median of 25 Xpert assays were run per facility per month (IQR, 10–63 assays), amounting to 8% of total Xpert assay testing capacity. The highest median monthly Xpert volume was achieved by Kayunga District Hospital, at 109 assays, amounting to 34% of total Xpert capacity; however, 4 of 7 facilities ran fewer than 20 Xpert assays per month on average (<6% of capacity), equating to less than 1 Xpert assay performed per work day (Figure 1). Among sputum specimens from patients evaluated for a new TB diagnosis at sites with onsite Xpert capability, a median of 21% (IQR, 6%–37%) had a corresponding Xpert test performed.
Figure 1.
Boxplots of monthly Xpert MTB/RIF (Xpert) test volumes by facility. Maximum Xpert capacity is considered 320 tests per month. Abbreviation: HC, health center.
Facility-Level Xpert Results
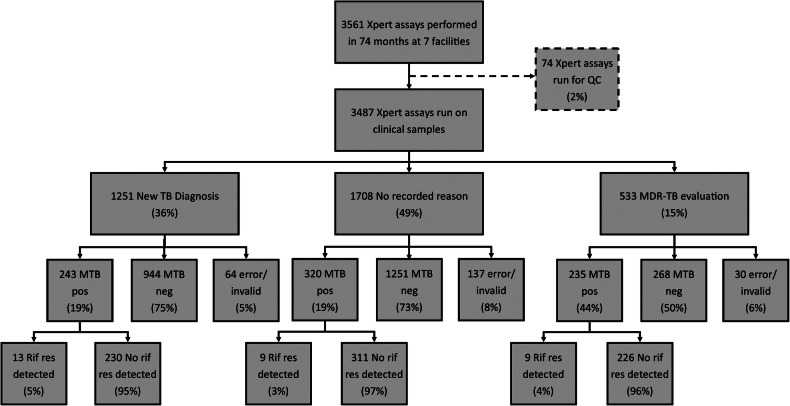
Among the 3487 assays performed on patent samples, 1251 (36%) were for new TB diagnosis, 533 (15%) were for MDR-TB evaluation, and 1708 (49%) had no recorded reason for evaluation (Figure 2). Among samples run for new TB diagnosis, 243 (19%) were positive for MTB, of which 13 (5%) had rifampicin resistance detected. Among those run for MDR-TB evaluation, 235 (44%) were positive for MTB, of which 9 (4%) had rifampin resistance detected. Among samples with no recorded reason for evaluation, 320 (19%) were positive for MTB, of which 9 (3%) had rifampicin resistance detected.
Figure 2.
Xpert MTB/RIF (Xpert) results from 7 Ugandan health facilities implementing Xpert. Xpert computer logs were unavailable for abstraction at the eighth Xpert facility. Percentage may not add to 100% due to rounding. Abbreviations: MDR-TB, multidrug resistant TB; MTB, Mycobacterium tuberculosis; neg, negative; pos, positive; QC, quality control; res, resistance; Rif, rifampicin; TB, tuberculosis.
Patient-Level Results and Time to Treatment
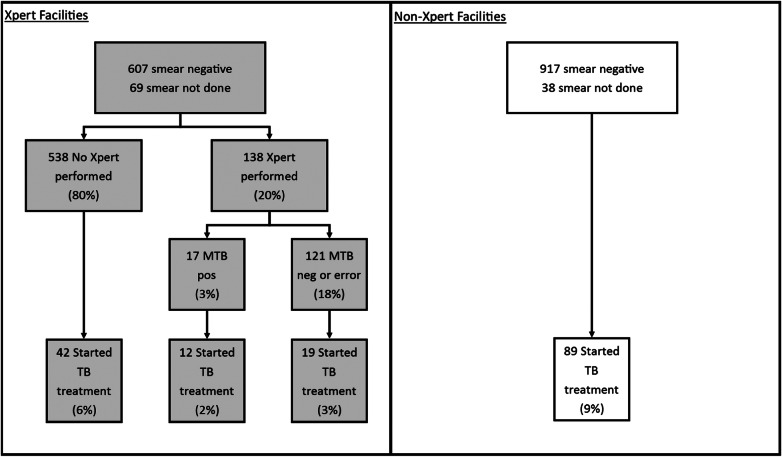
We reviewed laboratory and treatment records from 1899 patients (100 consecutive patients at each of 18 facilities, minus 1 record excluded as incomplete, and 100 consecutive patients from each of 2 separate TB clinics at 1 facility). Of these records, 800 came from 8 facilities implementing Xpert, and 1099 came from 10 non-Xpert facilities. At the Xpert facilities, 607 of 800 patients (76%) were sputum smear negative, and an additional 69 (9%) had no sputum smear result (Figure 3). Among these 676 smear-negative or unknown patients, Xpert assays were performed on 138 (20%), and 17 (12%) were positive for MTB. Twelve (71%) Xpert-positive patients were started on TB treatment, in addition to 16 of 115 (14%) who tested Xpert negative, and 42 of 538 (8%) with no Xpert performed. Thus, a positive Xpert result for MTB preceded only 12 of 70 (17%) patients initiated on treatment with a negative smear or no smear performed.
Figure 3.
The flow of diagnostic testing and tuberculosis (TB) treatment initiation for patients with presumptive TB at 18 Ugandan health facilities who were smear negative or had no smear microscopy done. In total, 11% (73 of 676) patients at Xpert MTB/RIF (Xpert) facilities were started on TB treatment by 60 days after sputum collection, similar to 9% (89 of 955) at facilities without Xpert (P = .325). Abbreviations: MTB, Mycobacterium tuberculosis; neg, negative; pos, positive.
At the 10 non-Xpert facilities, a similar proportion of patients had a negative (or no) initial smear result (87% [95% confidence interval [CI], 85%–89%] vs 85% [95% CI, 82%–87%] at Xpert sites; P = .547), of whom a similar proportion started on TB treatment by 60 days after sputum provision (11% [95% CI, 9%–13%] vs 9% [95% CI, 8%–11%]; P = .325). The median time to treatment for patients with a positive Xpert was 1 day (IQR, 1–2 days), for those with a positive smear was likewise 1 day (IQR, 0–3 days; P = .345 for comparison with Xpert-positives), and for patients started on treatment with no positive Xpert or smear result was 3 days (IQR, 0–7 days; P = .004). Time to TB treatment for patients with negative or no smear results was likewise similar between Xpert and non-Xpert facilities (P = .43) (Figure 4).
Figure 4.
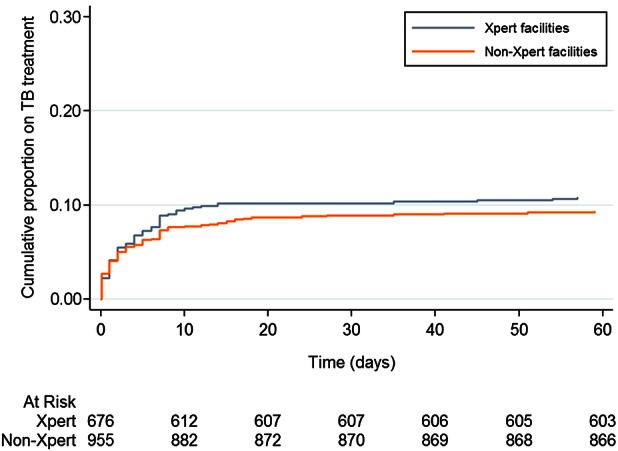
Time to treatment among presumptive tuberculosis (TB) cases with a negative or no smear result at 8 Xpert MTB/RIF (Xpert) and10 non-Xpert health facilities in Uganda.
Assay Errors, Stockouts, and Module Malfunction
Among the 3487 Xpert assays, 429 (12%) had an invalid or error result, of which 231 (54%) were repeated with a valid result, leaving 198 assays (6% overall) without a valid result. The proportion of assays with an error result remained constant over the study period (data not shown). Three facilities (43%) reported periods when Xpert assays could not be run due to cartridge stockouts. These periods ranged from 1 to 20 weeks in duration and totaled 7 months of machine downtime due to cartridge stockouts, or 9% of the entire study period. Module malfunction to the point of requiring repair was reported at 2 of 7 facilities, with the duration of module downtime ranging from 1 to 16 weeks. This represents a 5% reduction in total Xpert module capacity among all facilities during the study period.
DISCUSSION
This evaluation of implementation across 18 clinic sites demonstrates that Xpert is greatly underutilized in Uganda. In particular, testing volume was low, with only 8% of total testing capacity utilized, and only 21% of individuals with TB symptoms and a negative sputum smear receiving Xpert testing. At sites with Xpert available onsite, only 17% of all smear-negative patients started on TB treatment did so as a result of a positive Xpert test. Cartridge stockouts, module malfunction, and consistently high invalid/error rates were common problems. As a result, despite a high level of Xpert positivity (19% of all tests for new diagnosis), facilities with onsite Xpert had no detectable increase in the total proportion of symptomatic patients started on TB treatment, nor any decrease in time to treatment initiation relative to sites in which Xpert was not available.
Low Xpert utilization has been demonstrated in 2 other recent implementation studies. In Mozambique and Swaziland, Xpert was used at less than two-thirds capacity despite a higher burden of TB and concomitant rollout of a specimen transport network, coordination of procurement and supply networks, and additional laboratory training and general process oversight [12, 13]. Mozambique was nonetheless able to achieve a 69% increase in case detection among smear-negative individuals with presumptive TB, although treatment initiation in Xpert-positive individuals lagged behind that of those who were smear positive. Our findings from Uganda—where Xpert is recommended and often available, but not supported to the degree seen in Swaziland and Mozambique—are likely to be more reflective of actual implementation in the majority of low-income settings, underscoring the fact that implementing a novel test without such concomitant support may not markedly improve patient outcomes. In addition to supportive infrastructure, programs should consider testing algorithms that take advantage of existing capacity before rushing to increase capacity and testing volumes.
Our findings are also in keeping with several recent studies examining the patient-level impact of Xpert. Two pragmatic trials, both set in South Africa, compared Xpert against smear microscopy as the primary diagnostic for those with symptoms of TB, with testing available onsite in the TB-NEAT study [10] or at an offsite laboratory in the XTEND trial [8]. No impact of Xpert was observed on patient morbidity or mortality, and both studies found that Xpert led to no increase in TB treatment initiation. Our study suggests that these trial findings may also hold in actual implementation in low-income settings, where culture is not routinely available; an Xpert test result preceded only a small minority of TB treatment initiation in facilities with onsite testing available. The absence of patient impact, even in settings with excellent utilization and increased case detection, highlights the importance of strengthening entire the TB care cascade. Likewise, frequent practice of empiric treatment may obscure or erode the benefit of new diagnostics, because such tests may only serve to replace rather than augment clinical decision making [14].
Our study adds an important perspective—of real-world clinical implementation in a low-income setting—to a growing body of work that describes how this new technology might or might not improve patient-important outcomes. To optimize the impact of Xpert, greater attention must be paid to enhancing the utilization of this test and linking test results to treatment outcomes. Interventions that might fill this implementation gap include those that have been used successfully to promote treatment adherence, such as phone reminders, counselling and education, monetary or other incentives, or assistance by lay health workers or case managers [15–18]. Updating estimates of the cost-effectiveness of Xpert [19, 20] to account for implementation in clinical settings could also help to inform policy.
Findings from this study should be considered in light of several limitations. These data consisted mainly of laboratory records collected under routine programmatic conditions rather than in a stringently controlled research setting. Data on patient impact were therefore limited primarily to TB treatment initiation and time to treatment, and we were unable to link these data to mortality or treatment completion. The proportion of patients started on treatment after a positive Xpert may therefore serve more as an upper bound than as a precise estimate of Xpert's impact on patient important outcomes. In addition, HIV status was not reliably captured in the laboratory records, and thus it did not allow us to consider the specific impact of Xpert in this important subgroup, nor to evaluate how closely facilities adhered to testing algorithms. Nevertheless, these findings from a large sample size across a range of facility levels throughout Uganda are a real-world reflection of how Xpert testing is conducted in the complex context of a developing country health system, and the results allowed us to highlight challenges in implementation such as low patient volumes and cartridge stockouts. As a result, our findings are likely to generalize to similar low-income settings in sub-Saharan Africa.
CONCLUSIONS
In summary, our data show that Xpert is not well implemented in Uganda. As a result, despite approximately 1 in 5 Xpert tests for new TB diagnosis being positive for MTB, we observed no significant differences in treatment initiation, comparing clinics with onsite Xpert capacity to those in which Xpert had not been implemented. The average facility performed approximately 1 Xpert test per day, such that only 1 in 50 people presenting with TB symptoms ultimately received a positive Xpert test result that was followed by treatment initiation. These findings should serve as a warning that implementation of new diagnostic technology that is limited to procurement of machines and laboratory training alone is unlikely to have meaningful impact on patient outcomes at the facility level. Before indiscriminately scaling up Xpert, TB control programs should consider whether funding, infrastructure, and human capacity allow for an appropriately expansive testing algorithm that is capable of improving health outcomes for patients with symptoms of TB.
Acknowledgments
We graciously thank the laboratory and clinical staff at the study health facilities.
Financial support. This work was supported by the National Institute for Allergy and Infectious Diseases at the National Institutes of Health (grant number R21 AI106031).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization. A Review of Current Epidemiological Data and Estimation of Future Tuberculosis Incidence and Mortality. Geneva; World Health Organization; 1993. [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report. Geneva, Switzerland; World Health Organization; 2015. [Google Scholar]
- 3.World Health Organization. Global Strategy and Targets for Tuberculosis Prevention, Care and Control After 2015. Geneva, Switzerland; World Health Organization; 2014. [Google Scholar]
- 4.Boehme CC, Nabeta P, Hillemann D et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steingart KR, Sohn H, Schiller I et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2013; 1:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Policy Statement: Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System. Geneva, Switzerland; World Health Organization; 2011. [PubMed] [Google Scholar]
- 7.World Health Organization. WHO monitoring of Xpert MTB/RIF roll-out. Available at: http://www.who.int/tb/laboratory/mtbrifrollout/en/ Accessed 15 August 2015.
- 8.Churchyard GJ, Stevens WS, Mametja LD et al. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Global Health 2015; 3:e450–7. [DOI] [PubMed] [Google Scholar]
- 9.Cox HS, Mbhele S, Mohess N et al. Impact of Xpert MTB/RIF for TB diagnosis in a primary care clinic with high TB and HIV prevalence in South Africa: a pragmatic randomised trial. PLoS Med 2014; 11:e1001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Theron G, Zijenah L, Chanda D et al. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 2014; 383:424–35. [DOI] [PubMed] [Google Scholar]
- 11.Health UMo. National TB and Leprosy Program guidelines. Kampala, Uganda, 2012. [Google Scholar]
- 12.Cowan J, Michel C, Manhica I et al. Implementing rapid testing for tuberculosis in Mozambique. Bull World Health Organ 2015; 93:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sikhondze W, Dlamini T, Khumalo D et al. Countrywide roll-out of Xpert((R)) MTB/RIF in Swaziland: the first three years of implementation. Public Health Action 2015; 5:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theron G, Peter J, Dowdy D et al. Do high rates of empirical treatment undermine the potential effect of new diagnostic tests for tuberculosis in high-burden settings? Lancet Infect Dis 2014; 14:527–32. [DOI] [PubMed] [Google Scholar]
- 15.Liu Q, Abba K, Alejandria MM et al. Reminder systems to improve patient adherence to tuberculosis clinic appointments for diagnosis and treatment. Cochrane Database Syst Rev 2014; 11:CD006594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutge EE, Wiysonge CS, Knight SE et al. Incentives and enablers to improve adherence in tuberculosis. Cochrane Database Syst Rev 2015; 9:CD007952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.M'Imunya JM, Kredo T, Volmink J. Patient education and counselling for promoting adherence to treatment for tuberculosis. Cochrane Database Syst Rev 2012; 5:CD006591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwankeeree W, Picheansathian W. Strategies to promote adherence to treatment by pulmonary tuberculosis patients: a systematic review. Intern J Evid Based Healthc 2014; 12:3–16. [DOI] [PubMed] [Google Scholar]
- 19.Menzies NA, Cohen T, Lin HH et al. Population health impact and cost-effectiveness of tuberculosis diagnosis with Xpert MTB/RIF: a dynamic simulation and economic evaluation. PLoS Med 2012; 9:e1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassall A, van Kampen S, Sohn H et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med 2011; 8:e1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]