Abstract
Background. A measles outbreak in Pohnpei State, Federated States of Micronesia in 2014 affected many persons who had received ≥1 dose of measles-containing vaccine (MCV). A mass vaccination campaign targeted persons aged 6 months to 49 years, regardless of prior vaccination.
Methods. We evaluated vaccine effectiveness (VE) of MCV by comparing secondary attack rates among vaccinated and unvaccinated contacts after household exposure to measles.
Results. Among 318 contacts, VE for precampaign MCV was 23.1% (95% confidence interval [CI], −425 to 87.3) for 1 dose, 63.4% (95% CI, −103 to 90.6) for 2 doses, and 95.9% (95% CI, 45.0 to 100) for 3 doses. Vaccine effectiveness was 78.7% (95% CI, 10.1 to 97.7) for campaign doses received ≥5 days before rash onset in the primary case and 50.4% (95% CI, −52.1 to 87.9) for doses received 4 days before to 3 days after rash onset in the primary case. Vaccine effectiveness for most recent doses received before 2010 ranged from 51% to 57%, but it increased to 84% for second doses received in 2010 or later.
Conclusions. Low VE was a major source of measles susceptibility in this outbreak; potential reasons include historical cold chain inadequacies or waning of immunity. Vaccine effectiveness of campaign doses supports rapid implementation of vaccination campaigns in outbreak settings.
Keywords: Federated States of Micronesia, measles, measles vaccine, MMR, vaccine effectiveness
The Federated States of Micronesia (FSM) is an independent nation comprising 607 islands located just north of the Equator in the Western Pacific Ocean. The Federated States of Micronesia is considered by the World Bank as a lower-middle income country and is linked through a Compact of Free Association with the United States, which provides funding for its immunization program. Pohnpei, 1 of 4 FSM states, has a population of approximately 36 000 persons and includes a main island, where 96% of the population resides, and 8 outer islands. The median age of Pohnpei residents is 21.8 years [1].
As a member country of the World Health Organization (WHO) Western Pacific Region (WPR), FSM supports the regional goal to eliminate endemic measles through strategies including achieving high (>95%) coverage with 2 doses of measles-containing vaccine (MCV) for every birth cohort, implementing supplementary immunization activities (SIAs) when required, conducting high-quality surveillance including laboratory surveillance, maintaining measles outbreak preparedness for rapid response, and ensuring appropriate case management [2]. Measles vaccination was introduced in FSM in 1963 with a single dose of monovalent measles vaccine administered at 9 months of age; it was replaced with a combined measles, mumps, and rubella (MMR) vaccine in 1971. A second dose of MMR was introduced in 1995 and was recommended for administration at school entry. Since 1997, the recommendation has been to administer the first dose at 12 months of age and the second dose at 13 months [3]. Two doses of MMR have been required for school entry since the early 1990s, but enforcement varies. In 2013, Pohnpei State had 1-dose MCV (MCV1) and 2-dose MCV (MCV2) coverage among 2 year olds of 85% and 72%, respectively (FSM national immunization information system, or WebIZ). Pohnpei also conducted an SIA with MMR in 2011, targeting children aged 1–6 years and attaining 96% coverage in that age group [4].
Before 2014, the last reported measles cases in FSM occurred in 1994 when Pohnpei State experienced a limited measles outbreak (26 cases reported) [5]. Before 1994, the last measles outbreak in Pohnpei had occurred in 1968, with 93 cases [6].
In May 2014, Kosrae State reported the first cases of measles in FSM in 2 decades. On June 1, 2014, Pohnpei State reported its first measles case, marking the beginning of an outbreak that spread throughout the main island and resulting in 251 reported cases (overall attack rate = 7 per 1000 persons) and 1 death over the following 3 months. The median age of case patients in the outbreak in Pohnpei was 24 years, with the highest attack rate among infants younger than 1 year (46 per 1000), followed by adults aged 20–29 years (14 per 1000). Overall, 71% of case patients had received at least 1 dose of MCV, and 54% had received at least 2 doses. On June 16, 2014, the Pohnpei State Department of Health Services implemented a nonselective vaccination campaign, targeting persons aged 6 months to 49 years regardless of previous MCV vaccination. By the end of the campaign on September 20, 2014, 29 159 persons had been vaccinated [7].
The high number and proportion of vaccine failures (ie, persons who had received 1 or more doses of MCV and developed measles) in the 2014 FSM outbreak raised questions about the effectiveness of MCV in this setting. We assessed the effectiveness of MCV doses received before the 2014 outbreak vaccination campaign and of doses received during the campaign.
METHODS
Study Design
We conducted a secondary attack rate study to evaluate MCV effectiveness in household contacts within 1 incubation period after exposure to the first measles case in the household.
Definitions
We defined a confirmed measles case according to the US Council of State and Territorial Epidemiologists (CSTE) guidelines as a person with acute febrile rash illness with detection of measles-specific nucleic acid from a clinical specimen using polymerase chain reaction testing (PCR), or a positive serologic test for measles immunoglobulin (Ig)M antibody, or direct epidemiologic linkage to another confirmed case [8]. Laboratory testing was performed at the Centers for Disease Control and Prevention. Serum specimens were tested for measles-specific IgM, and throat swabs were tested for the presence of measles ribonucleic acid by reverse transcription-PCR [9, 10]. Households were selected for the study by convenience sampling of confirmed measles cases reported to the Pohnpei State Department of Health Services, with laboratory-confirmed cases prioritized. We defined a primary case as the first confirmed measles case in the household. For each primary case, we conducted face-to-face interviews with 1 or more household informants. If, during interviews, we identified a measles case with rash onset before that in the originally selected case, the case with the earliest rash onset in the household became the primary case. Household contacts were persons who shared meals with the primary case or spent at least 1 night in the household from 3 days before to 3 days after rash onset in the primary case. Secondary cases were defined as household contacts who met the confirmed measles case definition and had rash onset 7 to 21 days after rash onset in the primary case. Confirmed cases among household contacts with rash onset <7 days after rash onset in the primary case were considered coprimary cases.
Vaccination Status
The first case of measles was reported to the Pohnpei State Department of Health Services on June 1, 2014. We considered vaccinations administered before June 1, 2014 as precampaign doses and vaccinations administered on or after June 1, 2014 as campaign doses. We defined a pre-exposure campaign dose as a dose received ≥5 days before rash onset in the primary case and a postexposure campaign dose as a dose received between 4 days before to 3 days after rash onset in the primary case. Doses received by contacts ≥4 days after rash onset in the primary case were not counted as campaign doses, because they would not be expected to protect against disease as a result of the household exposure. For the primary analysis, we included households with a minimum follow-up of 18 days after rash onset in the primary case; we also performed a sensitivity analysis that included only households with at least 21 days of follow-up.
Most informants could not recall the vaccination status of household members and did not possess vaccination cards. To determine the vaccination status of study participants, we first searched WebIZ, which was implemented in 2007. Recording of vaccinations in WebIZ is reported to be comprehensive for vaccinations administered after WebIZ implementation; however, for some people, vaccinations received before 2007 were not consistently entered in WebIZ; therefore, if WebIZ listed other childhood vaccinations for the participant, then the vaccination record was considered to be complete. If the participant was not listed in WebIZ or no childhood vaccinations were documented in WebIZ, we then searched paper vaccination records and considered the vaccination record to be complete if found. We refer to MMR and measles-rubella vaccine collectively as MCV.
Analysis
We excluded from analysis the following: (1) coprimary cases, (2) household contacts aged <6 months (maternal antibodies may confer protection in these infants [11]), (3) household contacts aged ≥40 years (vaccination records were only rarely available for this age group), and (4) persons with incomplete vaccination records.
We used logistic regression to evaluate the independent effects of 1, 2, or 3 precampaign doses in combination with a pre- or postexposure campaign dose on developing measles. Because of the small sample size, we applied Firth's method of penalized maximum likelihood for parameter estimation [12]. Vaccine effectiveness (VE) is the proportionate reduction in measles attack rates among vaccinated persons compared with unvaccinated persons with similar exposure to measles virus. We present VE as 1 minus the odds ratio from the regression model and 95% confidence intervals (CIs) as 1 minus the Wald 95% CI for the odds ratio (OR). The reference (unvaccinated) group for all VE estimates consisted of persons who never received a dose of MCV either before or during the campaign.
To evaluate the effect of time since vaccination on VE, we used Firth logistic regression to calculate VE and 95% CI of 2 pre-exposure doses by calendar year of the second dose, adjusted for any campaign doses received. We categorized the year of the second precampaign dose as follows: before 2000, 2000 to 2009, and 2010 to May 31, 2014. There were insufficient numbers of persons with 1 or 3 precampaign doses to evaluate VE by year of last dose.
The Wilcoxon rank-sum test was used to test the association between the occurrence of household measles transmission and both the number of household members and the number of household members per room. Fisher's exact test was used to test the association between the number of vaccinations received by the primary case patient and the occurrence of secondary transmission in the household.
We used R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria) for data management and statistical analysis [13]. As part of the outbreak investigation, this study was designated exempt from the Centers for Disease Control and Prevention human subject policy.
RESULTS
The primary analysis included 80 primary case patients and their 580 household contacts. Median household size was 6.5 persons (range, 2–26), and the median number of household members per room was 4 (range, 1–18). Sixty-four (80%) of the primary cases were laboratory confirmed. Of the primary case patients, 62 (78%) had complete vaccination records; among these, 15 (24%) were unvaccinated, 12 (19%) had received 1 dose of MCV, 33 (53%) had received 2 doses, and 2 (3%) had received 3 doses. Of the 580 household contacts, the following were excluded: 118 were aged <6 months or ≥40 years, 15 were coprimary cases, and 129 had incomplete vaccination records. Of the remaining 318 household contacts included in the study, the median age was 14 years (range, 6 months–36 years), and 157 (49%) were female. There were 18 secondary cases among contacts (secondary attack rate, 5.7%). Six of the 18 secondary cases (33%) were laboratory confirmed. The median interval between rash onset in the primary and secondary cases was 10 days (range, 7–20 days).
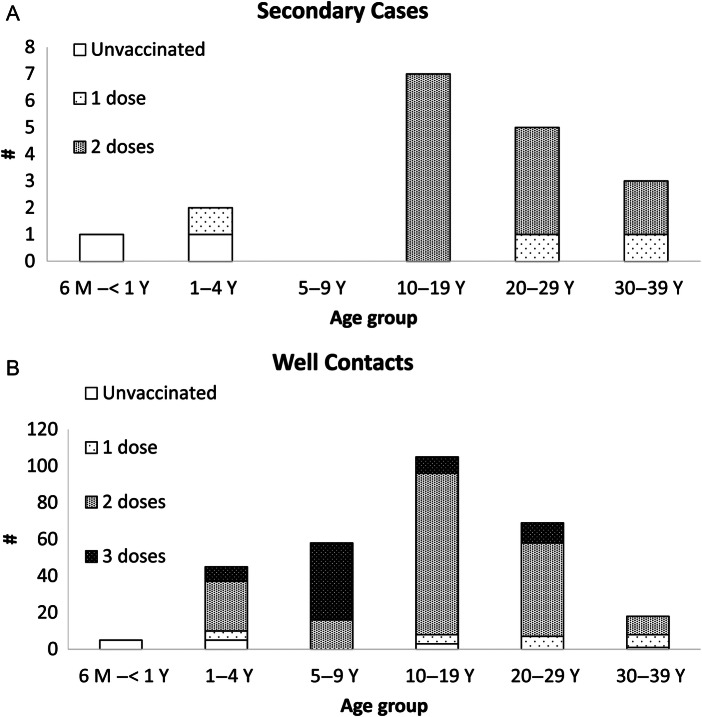
Fifteen (83%) of the 18 secondary cases occurred in adolescents and young adults 10 to 29 years of age. Two (11%) of the 18 secondary case patients had received no precampaign MCV doses, 3 (17%) had received 1 precampaign dose, and 13 (72%) had received 2 precampaign doses (Figure 1, Table 1). Among the 300 well household contacts, 14 (5%) had received no precampaign MCV doses, 24 (8%) had received 1 precampaign dose, 192 (64%) had received 2 doses, and 70 (23%) had received 3 doses. One (6%) secondary case patient and 75 (25%) well household contacts received a pre-exposure campaign dose; 3 (17%) secondary case patients and 77 (26%) well household contacts received a postexposure campaign dose (Table 1). The attack rates for persons who received 0, 1, 2, or 3 precampaign doses (irrespective of any campaign dose) were 13%, 11%, 6%, and 0%, respectively; attack rates for persons who received no campaign dose, received a pre-exposure dose, or received a postexposure campaign dose (irrespective of any precampaign dose) were 9%, 1%, and 4%, respectively.
Figure 1.
Number of precampaign measles-containing vaccine doses in secondary cases (A) and well household contacts (B), by age group—Pohnpei State, Federated States of Micronesia, 2014. All vaccine doses were received at age 6 months or older with at least 28 days between doses if more than 1 dose was received. Abbreviations: M, month; Y, year.
Table 1.
Receipt of Precampaign and Campaign Doses of Measles-Containing Vaccine by Secondary Measles Cases and Well Household Contacts—Pohnpei State, Federated States of Micronesia, 2014
| Campaign Dose | No. of Precampaign Doses Received |
||||
|---|---|---|---|---|---|
| 0 Cases/Totala (Attack Rate) | 1 Cases/Totala (Attack Rate) | 2 Cases/Totala (Attack Rate) | 3 Cases/Totala (Attack Rate) | Total Cases/Totala (Attack Rate) | |
| No campaign dose | 1/7 (14%) | 2/15 (13%) | 11/110 (10%) | 0/30 (0%) | 14/162 (9%) |
| Pre-exposureb | 0/4 (0%) | 1/8 (13%) | 0/44 (0%) | 0/20 (0%) | 1/76 (1%) |
| Postexposurec | 1/5 (20%) | 0/4 (0%) | 2/51 (4%) | 0/20 (0%) | 3/80 (4%) |
| Total | 2/16 (13%) | 3/27 (11%) | 13/205 (6%) | 0/70 (0%) | 18/318 (6%) |
a “Cases” are the secondary household cases. “Total” is the total household contacts (cases + well contacts).
b Received ≥5 days before date of rash onset in the primary case.
c Received between 4 days before to 3 days after date of rash onset in the primary case.
The estimates of VE from the logistic regression model (calculated as 1-OR) of precampaign MCV doses were 23.1% (95% CI, −425 to 87.3) for 1 dose, 63.4% (95% CI, −103 to 90.6) for 2 doses, and 95.9% (95% CI, 45.0 to 100) for 3 doses, independent of receipt of a campaign dose. The estimates of VE of the campaign dose were 78.7% (95% CI, 10.1 to 97.7) for pre-exposure doses and 50.4% (95% CI, −52.1 to 87.9) for postexposure doses independent of receipt of any precampaign doses. Vaccine effectiveness for all combinations of pre- and postcampaign doses are presented in Table 2.
Table 2.
Adjusted Vaccine Effectiveness for Combinations of Precampaign and Campaign Doses of Measles-Containing Vaccine—Pohnpei State, Federated States of Micronesia, 2014
| Campaign Dose | No. of Precampaign Doses |
|||
|---|---|---|---|---|
| 0 % VE (95% CI) | 1 % VE (95% CI) | 2 % VE (95% CI) | 3 % VE (95% CI) | |
| No campaign dose | (Reference) | 23.1 (−425 to 87.3) | 63.4 (−103 to 90.6) | 95.9 (45.0 to 100) |
| Pre-exposureb | 78.7 (10.1 to 97.7) | 83.6 (−93.5 to 98.6) | 92.2 (22.5 to 99.2) | 99.1 (70.9 to 100) |
| Postexposurec | 50.4 (−52.1 to 87.9) | 61.6 (−269.8 to 96) | 81.7 (−33.0 to 97.5) | 97.9 (43.7 to 99.9) |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
a Calculated as (1 minus odds ratio) × 100 from the logistic regression model.
b Received ≥5 days before date of rash onset in the primary case.
c Received between 4 days before to 3 days after date of rash onset in the primary case.
Among persons who had received 2 precampaign doses (13 secondary cases and 192 well contacts), the VE (adjusted for campaign doses received) was 50.6% (95% CI, −206 to 90.0) for second doses given before 2000, 56.6% (95% CI, −157 to 90.3) for second doses given from 2000 to 2009, and 83.9% (95% CI, −34.3 to 98.6) for second doses given from 2010 to May 31, 2014. Table 3 shows the raw data and attack rates by year of the second precampaign dose and receipt of a campaign dose.
Table 3.
Receipt of 2 Precampaign Doses and Campaign Dose of Measles-Containing Vaccine Among Secondary Measles Cases and Well Household Contacts—Pohnpei State, Federated States of Micronesia, 2014
| Campaign Dose | Year of Second Precampaign Dose |
|||
|---|---|---|---|---|
| Before 2000 Cases/Totala (Attack Rate) | 2000 to 2009 Cases/Totala (Attack Rate) | 2010 to 1 June 2014 Cases/Totala (Attack Rate) | Total Cases/Totala (Attack Rate) | |
| No campaign dose | 2/19 (11%) | 8/62 (13%) | 1/29 (3%) | 11/110 (10%) |
| Pre-exposureb | 0/1 (0%) | 0/32 (0%) | 0/11 (0%) | 0/44 (0%) |
| Postexposurec | 0/7 (0%) | 1/25 (4%) | 1/19 (5%) | 2/102 (2%) |
| Total | 2/27 (7%) | 9/119 (8%) | 2/59 (3%) | 13/205 (6%) |
a “Cases” are the secondary household cases. “Total” is the total household contacts (cases + well contacts).
b Received ≥5 days before date of rash onset in the primary case.
c Received between 4 days before to 3 days after date of rash onset in the primary case.
There were no differences between households with measles transmission and households without transmission with regard to the number of household members (median 8 vs 6, respectively, P = .14) or the number of persons per room (median 4.0 vs 3.5, respectively, P = .11). There was no significant association between the number of measles vaccinations the primary case had received and the occurrence of secondary measles transmission within the household (P = .11).
The sensitivity analysis of VE of pre-exposure doses and campaign doses restricted to the 72 (90%) households with at least 21 days of follow up after rash onset in the primary case were not substantially different than the VE in the primary analysis.
DISCUSSION
Pohnpei, a Western Pacific island, with 85% 1-dose measles vaccination coverage among 2 year olds experienced a widespread measles outbreak after 20 years without reported measles. In the context of the WPR measles elimination goal, this outbreak highlights the ongoing need for high coverage, high-quality surveillance, and rapid outbreak response capability, even in countries with long periods without endemic measles transmission. The high rates of infection among vaccinated case patients, in the overall outbreak, and in primary and secondary case patients in this study, with very few cases among unvaccinated persons, all strongly support the conclusion that vaccine failure was a major source of susceptibility in this population. Campaign doses of MCV administered during the pre- and postexposure periods as part of the outbreak response campaign had relatively high VE and support rapid implementation of outbreak response vaccination.
In this household exposure study the overall secondary attack rate was 6%. The attack rate among contacts with no recorded precampaign or campaign MCV doses was 14%, much lower than the 80%–90% attack rates seen in other high-intensity exposure studies [14, 15] and lower than the 52% secondary attack rate seen in a similar household study in the Pacific region [16]. Potential reasons for the low attack rate in the unvaccinated population include possible persistent maternal antibody in the unvaccinated infants, vaccination that was not ascertained or previous undetected measles disease in older ages. We did not collect data on previous measles disease, but the probability of study participants having previous disease is very low given that since 1968, only 26 measles cases, which occurred during a 1994 outbreak, have been reported in Pohnpei. Secondary attack rates trended downward from 11% in contacts who had received 1 precampaign dose to 6% among those with 2 doses and 0% among those with 3 doses. The adjusted VE for 1, 2, and 3 precampaign doses of MCV was 23%, 63%, and 98%, respectively. The CIs surrounding the VE point estimates are extremely wide because the reference rate is based on a single secondary case in a very small number of unvaccinated contacts.
The VE estimates in our study are among the lowest reported for the live-attenuated MCV. Previous estimates in the Pacific region for MCV effectiveness from 1978 to 2003 ranged from 84%–92% for 1 dose and 95%–100% for 2 doses [16–18]. Vaccinations administered during the 2011 SIA targeting 1- to 6-year-olds were clearly effective because there was only 1 case reported in this cohort, most of whom fell into the 5-9 year age group 3 years later during the 2014 outbreak [7]. For MCV doses administered before 2010, VE was much lower compared with doses administered in 2010 or later. This low VE might be due to primary vaccine failure, in which a person does not develop protective immunity after vaccination, or secondary vaccine failure, in which a person develops an adequate initial immune response but immunity wanes over time to a nonprotective level, or a combination of the 2.
Vaccine failure observed during this outbreak might have been the result of suboptimal storage and handling of MCV as has been previously documented in the region [18]. In Pohnpei, beginning in the mid-2000s, upgraded cold chain equipment and improvements in vaccine handling and storage, through multiple training programs in cooperation with WHO, UNICEF, and the Japan International Cooperation Agency, have resulted in an improvement in cold chain management and could account for improved VE for more recently administered doses. For example, new refrigerators were installed that provided more temperature stability, and temperature monitoring was instituted during storage and transport, whereas thermometers were previously unavailable. Vaccine transportation has also improved; previously, vaccines were directly (and incorrectly) placed on ice packs and were transported to outer dispensaries in an ice chest in the back of an open pick-up truck, leaving them somewhat exposed to the outside weather. Now, cold boxes and vaccine carriers are used for transporting vaccines. Furthermore, vaccine delivery times from the United States to FSM have been reduced from as long as 1 month to within 3 days, and vaccine packaging during transport has also improved.
The epidemiology of the outbreak, which primarily affected adults vaccinated years ago, and the lower VE for doses administered before 2010 could also suggest waning of immunity over time, or secondary vaccine failure. However, for doses administered before 2010, we did not observe a trend in which VE decreased with time since last vaccination. A 1993 study in Palau also showed no difference in attack rates of measles among those with the most recent dose at least 15 years before exposure compared with those vaccinated within 5 years of exposure [17]. The high rate of vaccine failure contributed to sustained transmission of measles; however, in the absence of pre-exposure antibody titers, it is difficult to distinguish primary from secondary vaccine failure. Assessment of anti-measles IgG antibody titers, plaque reduction neutralization titers, and IgG antibody avidity in acute-phase serum samples could provide evidence to help in making this distinction. Serologic analyses of serum specimens from the FSM outbreak are ongoing.
Measles-containing vaccine administered during the postexposure period from 4 days before to 3 days after rash onset in the household primary case increased protection against measles by 50%. Previous small studies have shown a VE of 91% to 100% for persons vaccinated within 3 days of exposure [19–21]. These studies typically evaluate VE of a first dose of MCV. Although most persons vaccinated in the postexposure period in our study had already received at least 1 dose, regression analysis allowed us to demonstrate the effectiveness of campaign doses independent of receipt of precampaign doses.
Our results support implementation of a vaccination campaign as soon as possible after introduction of measles into a population with suboptimal levels of measles immunity, as evidenced by the protective effect of both pre-exposure and postexposure campaign doses. During the 1991–1994 measles outbreak in Micronesia, duration of the outbreak on a given island was reduced by almost 6 days for each week reduction in time required to vaccinate >80% of the target population [5]. During the 2014 FSM outbreak, the attack rate in Pohnpei was lower compared with Kosrae, where sustained measles transmission occurred for weeks before it was recognized, therefore delaying implementation of the vaccination campaign [7] there.
This study had several limitations. Although all primary or secondary measles cases met the CSTE case definition of a confirmed case, not all were laboratory confirmed. The paucity of unvaccinated contacts in the study and the fact that only a single secondary case was unvaccinated severely widen the CI on the very low estimate of VE and precluded evaluation of VE by important characteristics such as age, timing of first and subsequent vaccinations, and primary case vaccination status.
Only 7 persons (1 case and 6 well contacts) were never vaccinated either before or during the campaign, 6 of whom (including the 1 case) were under 5 years of age. This reflects the high vaccination rate before and during the campaign and the stringent criteria used to document that a participant was unvaccinated. The disproportionally low number of unvaccinated contacts reduced the precision of (1) the attack rate estimate in the unvaccinated persons and (2) the VE calculations. Only a single secondary unvaccinated case was detected, and adding or subtracting a single case would significantly affect the attack rate. Although we may have excluded some truly unvaccinated participants when no vaccination record existed, among 129 persons who were excluded from this analysis because they had unknown pre-exposure vaccination status, the measles attack rate was 4.7%, suggesting that many of them were actually vaccinated.
CONCLUSIONS
Mathematical models predict that populations must maintain 92%–95% immunity to prevent prolonged or endemic transmission of measles [22]. Before the 2014 outbreak, Pohnpei State had suboptimal MCV coverage, 85% with 1 dose and 72% with 2 doses. Our study shows that the unusually low effectiveness of MCV in Pohnpei, particularly for doses received before 2010, resulted in a high rate of susceptibility to measles and sustained the outbreak. Improvements in cold chain management beginning in the early 2000s may explain higher VE observed for more recent vaccinations. Improved thermostable vaccines that do not require cold chain management would be extremely beneficial in reducing the logistical difficulties and cost associated with the cold chain and the consequences of cold chain lapses. Furthermore, improved protection among persons who received vaccinations postexposure highlights the importance of identification and vaccination of persons exposed to measles to reduce transmission. Similar investigations in populations that experience a measles outbreak preceded by a prolonged measles-free period would be useful to further evaluate duration of immunity. Although FSM and many Pacific Island nations have populations too small to sustain endemic transmission, it is critical to maintain high population immunity with high 2-dose coverage through routine vaccinations and SIAs, maintain vigilance in cold chain management, and strengthen surveillance to prevent future outbreaks from occurring when measles virus is reintroduced.
Acknowledgments
We thank the Federated States of Micronesia (FSM) Department of Health and Social Affairs Secretary Dr. Vita Skilling, Assistant Secretary Marcus Samo, FSM National Laboratory Coordinator Lisa Barrow, and FSM National Surveillance Coordinator Eliashib Edward for support. We appreciate staff from the immunization program of Pohnpei State and FSM National, including Spencer Donre, Mercedes Gilmete, Carter Apaisam, Augustus Elias, and other staff of the Division of Primary Health Care in Pohnpei for their dedication in collecting data for this study, as well as Rebecca NcNall, Nobia Williams, Marcia McGrew, and Sun Bae Sowers for laboratory testing at the Centers for Disease Control and Prevention.
Disclaimers. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Office of Statistics, Budget and Economic Management, Overseas Development Assistance, and Compact Management. 2010 FSM-wide Census of Population and Housing-Pohnpei State. Available at: http://www.sboc.fm/index.php?id1=Vm0xMFlWWXhWWGhTYmxKV1YwZFNUMVpzV21GVk1WbDNXa2M1VmxKdGVGbGFWVnBoVlVaV1ZVMUVhejA9 Accessed 10 April 2015.
- 2.Centers for Disease Control and Prevention (CDC). Progress toward measles elimination--Western Pacific Region, 2009–2012. MMWR Morb Mortal Wkly Rep 2013; 62:443–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health, Education, and Social Affairs. Federated States of Micronesia National Measles Elimination Plan. Federated States of Micronesia 2004.
- 4.World Health Organization. Federated States of Micronesia Country Profile - Measles Elimination, 2014. Available at: http://www.wpro.who.int/immunization/documents/measles_country_profile_sep2014_fsm.pdf Accessed 30 April 2015.
- 5.Guris D, Auerbach SB, Vitek C et al. Measles outbreaks in Micronesia, 1991 to 1994. Pediatr Infect Dis J 1998; 17:33–9. [DOI] [PubMed] [Google Scholar]
- 6.Gould KL, Herrman KL, Witte JJ. The epidemiology of measles in the U.S. Trust Territory of the Pacific Islands. Am J Public Health 1971; 61:1602–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breakwell L, Moturi E, Helgenberger L et al. Measles outbreak associated with vaccine failure in adults–Federated States of Micronesia, February-August 2014. MMWR Morb Mortal Wkly Rep 2015; 64:1088–92. [DOI] [PubMed] [Google Scholar]
- 8.Council of State and Territorial Epidemiologists. Public Health Reporting and National Notification for Measles. CSTE Position Statement 12-ID-07, 2012. Available at: http://c.ymcdn.com/sites/www.cste.org/resource/resmgr/PS/12-ID-07FINAL.pdf. Accessed 13 March 2015.
- 9.Hummel KB, Erdman DD, Heath J, Bellini WJ. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol 1992; 30:2874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hummel KB, Lowe L, Bellini WJ, Rota PA. Development of quantitative gene-specific real-time RT-PCR assays for the detection of measles virus in clinical specimens. J Virol Methods 2006; 132:166–73. [DOI] [PubMed] [Google Scholar]
- 11.Caceres VM, Strebel PM, Sutter RW. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin Infect Dis 2000; 31:110–9. [DOI] [PubMed] [Google Scholar]
- 12.Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002; 21:2409–19. [DOI] [PubMed] [Google Scholar]
- 13.Georg Heinze, Meinhard Ploner, Daniela Dunkler and Harry Southworth (2013). logistf: Firth's bias reduced logistic regression. R package version 1.21. http://CRAN.R-project.org/package=logistf..
- 14.Bhuniya S, Maji D, Mandal D, Mondal N. Measles outbreak among the Dukpa tribe of Buxa hills in West Bengal, India: epidemiology and vaccine efficacy. Indian J Public Health 2013; 57:272–5. [DOI] [PubMed] [Google Scholar]
- 15.De Serres G, Boulianne N, Defay F et al. Higher risk of measles when the first dose of a two-dose schedule is given at 12–14 versus 15 months of age. Clin Infect Dis 2012; 55:394–402. [DOI] [PubMed] [Google Scholar]
- 16.Marin M, Nguyen HQ, Langidrik JR et al. Measles transmission and vaccine effectiveness during a large outbreak on a densely populated island: implications for vaccination policy. Clin Infect Dis 2006; 42:315–9. [DOI] [PubMed] [Google Scholar]
- 17.Guris D, McCready J, Watson JC et al. Measles vaccine effectiveness and duration of vaccine-induced immunity in the absence of boosting from exposure to measles virus. Pediatr Infect Dis J 1996; 15:1082–6. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre RC, Preblud SR, Polloi A, Korean M. Measles and measles vaccine efficacy in a remote island population. Bull World Health Organ 1982; 60:767–75. [PMC free article] [PubMed] [Google Scholar]
- 19.Watson GI. Protection after exposure to measles by attenuated vaccine without gamma-globulin. Br Med J 1963; 1:860–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheppeard V, Forssman B, Ferson MJ et al. The effectiveness of prophylaxis for measles contacts in NSW. N S W Public Health Bull 2009; 20:81–5. [DOI] [PubMed] [Google Scholar]
- 21.Barrabeig I, Rovira A, Rius C et al. Effectiveness of measles vaccination for control of exposed children. Pediatr Infect Dis J 2011; 30:78–80. [DOI] [PubMed] [Google Scholar]
- 22.Fine PE, Mulholland K.. Community immunity. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines . 6th ed Philadelphia, PA: Elsevier Inc., 2013; 20:381. [Google Scholar]