Abstract
In an observational study of 582 patients with laboratory-confirmed influenza virus infections and their household contacts, we found that the initiation of oseltamivir within 24 hours was associated with shorter duration of self-reported illness symptoms (56% reduction in duration; 95% confidence interval, 41%–67%). However, we did not find any association of oseltamivir treatment with duration of viral shedding by polymerase chain reaction or with the risk of household transmission.
Keywords: influenza, oseltamivir, acute respiratory illness
Oseltamivir is a neuraminidase inhibitor commonly prescribed as both prophylaxis for and treatment of influenza virus infections [1, 2]. Recent articles have discussed the strengths and weaknesses of evidence on the benefits and harms of oseltamivir from randomized trials and observational studies [2–4]. The randomized trials demonstrated that oseltamivir has moderate efficacy in reducing the duration of fever and respiratory symptoms among children and adults with uncomplicated medically attended influenza virus infections [2, 5]. There is less observational evidence for its effectiveness in reducing symptoms when prescribed to patients in community settings, and few studies have investigated its effect on viral shedding and on prevention of secondary infections within households [3, 6–8]. In the present study, we aimed to examine the effect of oseltamivir on the reduction of self-reported symptoms, virus shedding, and household transmission in a community setting.
METHODS
We analyzed data from a community-based study of household transmission of influenza virus in Hong Kong (2008–2013), including data from 2008 that were published elsewhere [8]. In the present study, household index case patients were recruited from outpatient clinics if they met the following inclusion criteria: ≥2 symptoms of acute respiratory illness with onset within 48 hours of recruitment, no reported acute respiratory illness in the 14 days before recruitment, and living with ≥2 other individuals in the same household, none of whom reported acute illnesses in the prior 14 days. We excluded data from our pilot phase using viral culture as a confirmatory test and retained only index patients confirmed by real-time reverse-transcriptase (RT) polymerase chain reaction (PCR).
Further details of case confirmation, recruitment, consent, and biological sample extraction procedures are available elsewhere [8, 9]. In brief, trained study nurses collected nasal and throat swab specimens from index patients at baseline (day 0) and at 2–3 subsequent visits at approximately 3-day intervals (a total of 7–10 days). Specimens were also collected from household contacts regardless of illness during each home visit. At the initial home visit, we provided structured diaries for both index patient and household contacts to record their daily symptoms and personal digital tympanic thermometers to monitor their daily body temperatures. We obtained information on medications prescribed to index patients at baseline from respective clinic sites and further ascertained this information with index patients during home visits. The Institutional Review Board at the University of Hong Kong approved the study protocol; all participants ≥18 years old provided written informed consent, while written parental consent was obtained for participants <18 years old.
The respiratory symptom score (range, 0–4) was defined as the presence of self-reported sore throat, cough, coryza, and phlegm extracted from participants' diary entries. The total symptom score (range, 0–7) was calculated from respiratory symptom score plus any presence of fever (≥37.8°C), monitored by participants using personal digital tympanic thermometers, headache, and myalgia. Time to symptom alleviation was defined as the time from symptom onset to the first day scoring a 0 on the symptom scores (total and respiratory) and body temperature <37.8°C (fever symptom). Household transmission was assessed based on the number of household contacts (nonindex) of index patients who had developed a new PCR-confirmed influenza virus infection at each household visit.
We conducted descriptive analyses, used Kaplan–Meier estimates to estimate time to cessation of self-reported symptoms, the Turnbull nonparametric estimator for time to cessation of virus shedding, and Weibull accelerated failure time regression models to examine factors affecting time to cessation of self-reported symptoms and virus shedding. The Turnbull estimator accounted for interval censoring, because the presence or absence of virus shedding was measured only every 3 days on average, and the Weibull analysis of time to cessation of virus shedding accounted for interval censoring in the likelihood function [10].
Finally, we used an individual-based hazard model [11, 12] to estimate the effect of factors affecting influenza transmission. In this model, the risk of infection for a household contact depended on their age and vaccination status as well as the number and characteristics of infected persons in the household. The model allowed for the possibility of tertiary as well as secondary infections, in households with >1 infected household contact. It incorporated the level of influenza activity in the community so that infections from outside the household could be accounted for. We used the model to estimate the association between oseltamivir treatment and infectivity by including the oseltamivir treatment status of index patients.
To account for the potential for immortal time bias [13] we stratified contacts into 3 groups, depending on the delay from onset to treatment of the index patient in their household, and compared the risk of infection of contacts between those with index patients who were or were not treated with oseltamivir in each of the 3 strata. We fitted our model in a Bayesian framework and used a Markov chain Monte Carlo algorithm to estimate posterior distributions of the unknown parameters. The adequacy of the model was tested with the use of a simulation-based χ2 test comparing observed and expected distributions of the number of cases by household size [11]. The posterior deviance information criterion was used to assess model fit.
RESULTS
Between 2008 and 2013, a total of 4301 index patients were recruited, 876 (20%) had a positive rapid test result, and 697 were followed up with home visits. Among the 697 patients, we excluded 107 patients without RT-PCR confirmed influenza at baseline and another 8 patients who were prescribed an antiviral other than oseltamivir. This left 582 index patients for analysis, of whom 121 were children aged <5 years and 250 (43%) were aged 6–12 years (Appendix Table A1). Patients treated with oseltamivir were generally similar to those not treated (Appendix Table A1), except for a significantly higher probability to report muscle pain at baseline, and a lower probability of being prescribed antipyretics or antihistamines. The mean number of symptoms reported at baseline was 4.8 in the oseltamivir group versus 4.7 in the nontreated group (P = .21; t test). The mean number of household contacts per index patient was 3.04.
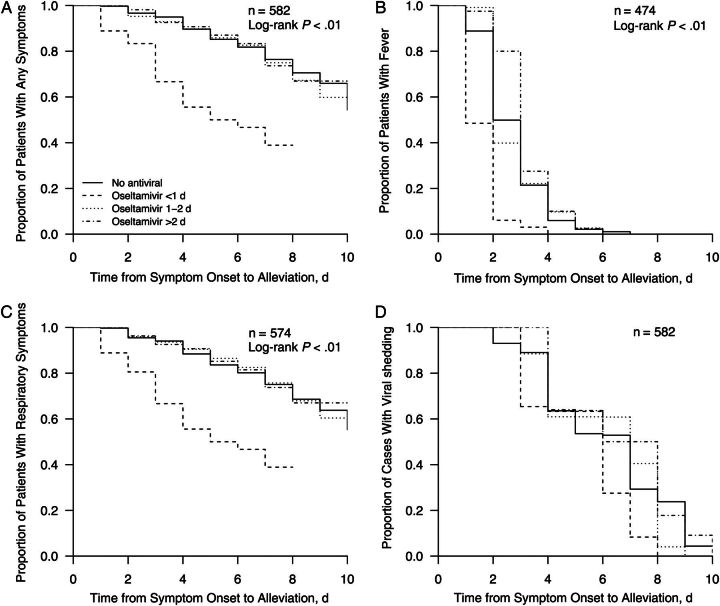
The median duration of illness (all self-reported symptoms) was significantly shorter for patients treated with oseltamivir within 24 hours of onset than for the other patients (Appendix Figure A1). Initiation of oseltamivir treatment within 24 hours of symptom onset was associated with a 56% reduction in time to cessation of all influenza symptoms (acceleration factor [AF], 0.44; 95% confidence interval [CI], .33–.59), a 47% reduction in time to alleviation of fever (AF, 0.52; 95% CI, .45–.63), and a 56% reduction in time to cessation of respiratory symptoms (AF, 0.44; 95% CI, .33–.59) compared with results in index patients without any antiviral treatment, after adjustment for potential confounders. There was no significant association of oseltamivir treatment with the duration of virus shedding by PCR (Table 1).
Table 1.
Relative Duration of Influenza Symptoms and Viral Shedding During Oseltamivir Treatment in Hong Kong (2008–2013)a
| Oseltamivir Therapy | All Symptoms |
Feverb |
Respiratory Symptomsc |
Viral Sheddingd |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients, No. | AF (95% CI) | P Value | Patients, No. | AF (95% CI) | P Value | Patients, No. | AF (95% CI) | P Value | Patients, No. | AF (95% CI) | P Value | |
| Started <1 d after illness onset | 36 | 0.44 (.33–.59) | <.01 | 33 | 0.53 (.45–.63) | <.01 | 36 | 0.44 (.33–.59) | <.01 | 37 | 0.83 (.67–1.04) | .11 |
| Started 1–2 d after illness onset | 129 | 0.83 (.66–1.03) | .09 | 114 | 1.06 (.96–1.17) | .23 | 128 | 0.88 (.70–1.11) | .28 | 130 | 1.06 (.92–1.22) | .42 |
| Started >2 d after illness onset | 54 | 0.84 (.61–1.16) | .29 | 40 | 1.08 (.92–1.27) | .32 | 54 | 0.88 (.63–1.23) | .45 | 54 | 0.96 (.78–1.17) | .66 |
| No antiviral | 360 | 1.00 | 287 | 1.00 | 356 | 1.00 | 361 | 1.00 | ||||
Abbreviations: AF, acceleration factor; CI, confidence interval.
a All models were adjusted for index patient characteristics, including age; sex; influenza vaccination in the past year; number of symptoms at baseline; influenza type; presence of any chronic conditions; use of antibiotics, antipyretics, or antihistamines; and study year.
b Not all index patients had fever or respiratory symptoms at baseline.
c Respiratory symptoms included sore throat, cough, coryza, and phlegm.
d Estimated time to cessation of viral shedding by oseltamivir exposure groups, allowing for interval censoring.
Regarding household transmission, index patients with at least 1 household contact who had viral shedding detected by RT-PCR at initial home visits (ie, co–index patients) were excluded from our analyses (n = 115). In the remaining households analyzed (n = 467), 139 of 1420 household contacts (9.8%) had laboratory-confirmed influenza virus infections. There was no significant association between oseltamivir treatment and infectivity of index patients, regardless of the delay between symptom onset and treatment (Table 2).
Table 2.
Factors Associated With Transmission of Influenza Virus Infection From Index Patients to Household Contactsa
| Stratum | Index Patients, No. | OR (95% CrI) | P Value |
|---|---|---|---|
| Oseltamivir therapy vs no treatment | |||
| Started at <1 d | 69 | 0.53 (.18–1.24) | .17 |
| Started at 1–2 d | 237 | 0.65 (.37–1.14) | .10 |
| Started at >2 d | 161 | 1.15 (.59–2.18) | .67 |
Abbreviations: CrI, credible interval; OR, odds ratio.
a Household contacts were stratified into 3 groups, based on the time from illness onset to medical consultation for the index patient in their household. The ORs presented here reflect the risk of infection of a household contact, comparing household contacts of index patients treated and of those not treated with oseltamivir in each stratum. The analysis was also adjusted for age and vaccination status of the household contacts and accounted for tertiary infections and infections from outside the household (see “Methods” section).
DISCUSSION
Congruent with our previous report [8] based on a smaller sample size, in the present study we found additional evidence to support the effectiveness of early initiation of oseltamivir (<24 hours after symptom onset) in shortening the duration of self-reported symptoms among patients in a community setting (Table 1). Recent evidence from a randomized controlled trial involving mostly children from an urban setting in Bangladesh suggests that oseltamivir may be efficacious in shortening the duration of self-reported influenza symptoms and viral shedding even when started >48 hours after onset [14]. However, we did not find observational evidence to support the effectiveness of oseltamivir in shortening the duration of viral shedding by PCR or preventing transmission within the household (Table 2).
The association between oseltamivir treatment and alleviation of influenza symptoms in the absence of a significant reduction in viral shedding has several plausible explanations. We measured virus shedding by PCR, and this was therefore a quantification of the amount of viral element in the nose and throat swab rather than infectious virions; direct assessment of virus replication (eg, plaque assays [median tissue culture infective dose]), would have been preferable, but requires substantial resources and has other limitations, including the difficulty in culturing some viruses and the consequences of freeze-thaw cycles for viability. In addition, many of the symptoms of influenza virus infections may be caused by the immune response to infection, and inflammatory cytokines in particular, rather than the damage to cells caused directly to the virus, and therefore a reduction in the duration of viral shedding after onset may have a limited effect on symptoms [15]. Finally, our sample size was relatively small, and a larger study would have had greater statistical power to identify small to medium effects of oseltamivir on virus shedding and household transmission.
Results from our study should be interpreted in light of several limitations. First, the observational design is subject to unmeasured confounders such as physician training and socioeconomic status. However, we are optimistic that these biases could have been minimized after controlling for other covariates including vaccination and medication prescriptions other than antivirals [3]. Second, we excluded households with concurrent influenza infection among household contacts other than the index patient at study enrollment, but these could indeed be secondary infections. Finally, self-reported influenza symptoms were measured only once a day; the use of several measurements throughout the day may have higher resolution in capturing self-reported symptom duration.
Overall, our data show that oseltamivir has moderate effect on fever and self-reported respiratory symptom alleviation among index patients when prescribed in a community setting within 24 hours of the onset of influenza symptoms. Although existing guidelines do not recommend the prescription of oseltamivir for outpatients without risk factors for progression to more severe disease [1], our results demonstrate benefits for outpatient use in terms of faster self-reported symptom alleviation.
Notes
Acknowledgments. We thank all the physicians, nurses, and staff members at the participating centers for facilitating recruitment; the dedicated team of healthcare workers who conducted the home visits; and Philip Au, Chan Kit Man, Calvin Cheng, Rita Fung, Erin Li, Ho Yuk Ling, Lam Yiu Pong, Lincoln Lau, Anita Li, Tom Lui, Tong Hok Leung, Loretta Mak, and Teresa So for research support.
Financial support. This project was supported by the National Institute of Allergy and Infectious Diseases (NIAID) (contract HHSN266200700005C; ADB No. N01-AI-70005 [NIAID Centers for Excellence in Influenza Research and Surveillance]); the Government of the Hong Kong Special Administrative Region (commissioned grant from the Health and Medical Research Fund); the Harvard Center for Communicable Disease Dynamics, National Institute of General Medical Sciences (grant U54 GM088558); the Research Grants Council of the Hong Kong Special Administrative Region (project T11-705/14N); and L'Oreal Hong Kong (research scholarship to T. K. T.).
Potential conflicts of interest. D. K. M. I. has received research funding from Hoffmann–La Roche. J. S. M. P. receives research funding from Crucell and serves as an ad hoc consultant for GlaxoSmithKline and Sanofi Pasteur. G. M. L. has received speaker honoraria from HSBC and CLSA. B. J. C. has received research funding from MedImmune and Sanofi Pasteur and consults for Crucell. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
Figure A1.
Time to alleviation of all symptoms (n = 582) (A), fever (n = 474) (B), respiratory symptoms (n = 574) (C), and viral shedding (D) by oseltamivir prescription among index patients with polymerase chain reaction-confirmed influenza virus infection. Note that not all patients had fever or respiratory symptoms at baseline.
Table A1.
Characteristics at Baseline by Oseltamivir Prescriptiona
| Characteristic | Patients, No. (%) |
P Value | |
|---|---|---|---|
| Oseltamivir (n = 223) | No Antiviral (n = 359) | ||
| Male sex | 117 (52.5) | 168 (46.8) | .21 |
| Age group, y | .15 | ||
| ≤5 | 84 (37.7) | 166 (46.2) | |
| 6–12 | 24 (10.8) | 38 (10.6) | |
| 13–17 | 66 (29.6) | 81 (22.6) | |
| ≥18 | 84 (37.7) | 166 (46.2) | |
| Vaccinated in past 1 y | 35 (15.7) | 46 (12.8) | .39 |
| Influenza A | 185 (83) | 258 (71.9) | .34 |
| Influenza B | 38 (17) | 101 (28.1) | .52 |
| Any chronic medical condition | 30 (13.5) | 28 (7.8) | .67 |
| Fever ≥37.8°C | 191 (85.7) | 286 (79.7) | .09 |
| Headache | 109 (48.9) | 185 (51.5) | .59 |
| Sore throat | 139 (62.3) | 220 (61.3) | .87 |
| Cough | 197 (88.3) | 305 (85) | .30 |
| Muscle pain | 104 (46.6) | 137 (38.2) | .05 |
| Coryza | 205 (91.9) | 318 (88.6) | .25 |
| Phlegm | 134 (60.1) | 233 (64.9) | .28 |
| Delay between illness onset and enrollment, h | .51 | ||
| ≤24 | 151 (67.7) | 237 (66) | |
| 25–48 | 72 (32.3) | 120 (33.4) | |
| >48 | 0 (0) | 2 (0.6) | |
| Prescribed antibiotics | 52 (23.3) | 64 (17.8) | .13 |
| Prescribed antipyretics | 130 (58.3) | 259 (72.1) | <.01 |
| Prescribed antihistamines | 124 (55.6) | 250 (69.6) | <.01 |
a P values were estimated using χ2 tests.
References
- 1.World Health Organization. WHO guidelines for pharmacological management of pandemic influenza A(H1N1) 2009 and other influenza viruses. Geneva, Switzerland: World Health Organization, 2010. [PubMed] [Google Scholar]
- 2.Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev 2014; 4:CD008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freemantle N, Shallcross LJ, Kyte D, Rader T, Calvert MJ. Oseltamivir: the real world data. BMJ 2014; 348:g2371. [DOI] [PubMed] [Google Scholar]
- 4.Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ 2014; 348:g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hernan MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis 2011; 53:277–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayden FG, Belshe R, Villanueva C, et al. Management of influenza in households: a prospective, randomized comparison of oseltamivir treatment with or without postexposure prophylaxis. J Infect Dis 2004; 189:440–9. [DOI] [PubMed] [Google Scholar]
- 7.Welliver R, Monto AS, Carewicz O, et al. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA 2001; 285:748–54. [DOI] [PubMed] [Google Scholar]
- 8.Ng S, Cowling BJ, Fang VJ, et al. Effects of oseltamivir treatment on duration of clinical illness and viral shedding and household transmission of influenza virus. Clin Infect Dis 2009; 50:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a randomized trial. Ann Intern Med 2009; 151:437–46. [DOI] [PubMed] [Google Scholar]
- 10.Lindsey JC, Ryan LM. Tutorial in biostatistics: methods for interval-censored data. Stat Med 1998; 17:219–38. [DOI] [PubMed] [Google Scholar]
- 11.Cauchemez S, Donnelly CA, Reed C, et al. Transmission of novel influenza A (H1N1) virus in households in the USA. N Engl J Med 2009; 361:2619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang TK, Cauchemez S, Perera RA, et al. Association between antibody titers and protection against influenza virus infection within households. J Infect Dis 2014; 210:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010; 340:b5087. [DOI] [PubMed] [Google Scholar]
- 14.Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis 2014; 14:109–18. [DOI] [PubMed] [Google Scholar]
- 15.Braciale TJ, Sun J, Kim TS. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol 2012; 12:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]