Acute kidney injury is common in severe malaria and associated with short- and long-term mortality developing in 50% of cases after admission. Cystatin C and BUN are associated with the severity of AKI, are elevated at admission and predict mortality.
Keywords: acute kidney injury, children, creatinine, inhaled nitric oxide, severe malaria
Abstract
Background. Acute kidney injury (AKI) is a well recognized complication of severe malaria in adults, but the incidence and clinical importance of AKI in pediatric severe malaria (SM) is not well documented.
Methods. One hundred eighty children aged 1 to 10 years with SM were enrolled between 2011 and 2013 in Uganda. Kidney function was monitored daily for 4 days using serum creatinine (Cr). Acute kidney injury was defined using the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. Blood urea nitrogen (BUN) and Cr were assessed using i-STAT, and cystatin C (CysC) was measured by enzyme-linked immunosorbent assay.
Results. Eighty-one (45.5%) children had KDIGO-defined AKI in the study: 42 (51.9%) stage 1, 18 (22.2%) stage 2, and 21 (25.9%) stage 3. Acute kidney injury evolved or developed in 50% of children after admission of hospital. There was an increased risk of AKI in children randomized to inhaled nitric oxide (iNO), with 47 (54.0%) of children in the iNO arm developing AKI compared with 34 (37.4%) in the placebo arm (relative risk, 1.36; 95% confidence interval [CI], 1.03–1.80). Duration of hospitalization increased across stages of AKI (P = .002). Acute kidney injury was associated with neurodisability at discharge in the children receiving placebo (25% in children with AKI vs 1.9% in children with no AKI, P = .002). Mortality increased across stages of AKI (P = .006) in the placebo arm, reaching 37.5% in stage 3 AKI. Acute kidney injury was not associated with neurodisability or mortality at discharge in children receiving iNO (P > .05 for both). Levels of kidney biomarkers were predictive of mortality with areas under the curves (AUCs) of 0.80 (95% CI, .65–.95; P = .006) and 0.72 (95% CI, .57–.87; P < .001), respectively. Admission levels of CysC and BUN were elevated in children who died by 6 months (P < .0001 and P = .009, respectively).
Conclusions. Acute kidney injury is an underrecognized complication in young children with SM and is associated with increased mortality.
Acute kidney injury (AKI) is a common complication of severe malaria (SM) in adults, affecting up to 40% of patients [1, 2]. In the absence of appropriate renal replacement therapy (RRT), the case fatality rate approaches 75% [1, 2]. Clinically and pathologically, AKI in adults with SM resembles acute tubular necrosis with oliguria in 60%–70% of cases [3]. Prompt initiation of RRT has been associated with reduction in malaria-associated AKI in adults [2]. It is recognized that children presenting to hospital with SM and AKI are at increased risk of death [4–7, 8 ]; however, estimates of the incidence of AKI in children with SM are limited with prior studies using measurements of urine output, which may be insensitive to mild to moderate AKI, or single estimates of creatinine (Cr) or blood urea nitrogen (BUN), which may not capture the extent of AKI over time in children with SM and may miss small changes in kidney function that are now known to be associated with less favorable outcomes [9]. Better estimates of the incidence of AKI and the morbidity and mortality associated with AKI in African children with SM are needed.
There is increasing evidence that even small changes in kidney function are associated with increased morbidity, mortality, and risk of developing chronic kidney disease (CKD) [10]. The Kidney Disease: Improving Global Outcomes (KDIGO) guidelines recommend broadening the definition of AKI to encompass acute changes in renal function, including patients with functional impairments relative to physiological demands [10]. In clinical settings, kidney function is assessed using the glomerular filtration rate (GFR) and/or urine output [10]. Glomerular filtration rate is estimated using filtration marker Cr.
Data from a meta-analysis suggest that filtration marker cystatin C (CysC) may be an earlier marker of AKI than Cr [11]. Cystatin C is a 13-kDa nonglycosylated cysteine protease inhibitor produced by nucleated cells, filtered by the renal glomeruli, and reabsorbed and catabolized by proximal tubular cells [12]. Cystatin C is less subject to the effects of age, sex, and race than Cr [13, 14], making it an attractive biomarker of kidney functional status. Elevated CysC has been described in pediatric [15] and adult populations with Plasmodium falciparum infection [12]. Cystatin C has also been identified as a prognostic marker in critically ill children [16].
In this study, we evaluated the incidence and outcome of AKI in Ugandan children with SM enrolled in a clinical trial evaluating inhaled nitric oxide (iNO) as an adjunctive therapy to intravenous artesunate [17]. We also investigated the relationship between kidney biomarkers, AKI, and mortality.
METHODS
Study Design
This study represents a secondary analysis of a randomized, double-blind, placebo-controlled trial evaluating iNO as an adjunctive therapy for SM (2011–2013) [17]. All children were treated with intravenous artesunate. One hundred eighty children aged 1 to 10 years with SM were enrolled at Jinja Regional Referral Hospital where advanced care, including mechanical ventilation and RRT, is not available. Children were eligible if they had a rapid diagnostic test positive for P falciparum histidine-rich protein 2 and parasite lactate dehydrogenase [17] and at least 1 of the following criteria for SM: repeated seizures, prostration, impaired consciousness, or respiratory distress. Exclusion criteria included the following: known chronic illness and severe malnutrition.
Eligible patients were randomly assigned to treatment with iNO (80 ppm) or placebo delivered continuously for up to 72 hours. Renal function was monitored daily for 4 days using Cr (i-STAT CHEM8+ or Crea; Abbott Laboratories, Saint-Laurent, Québec). Creatinine measured by i-STAT is traceable to isotype dilution mass spectrometry and is free of interference from hemoglobin, bilirubin, and glucose [18]. Acute kidney injury with worsening renal function after study gas initiation was a criterion for study gas withdrawal [17].
After discharge from hospital, children were observed at day 14, and children aged <5 years underwent neurocognitive evaluation at 6 months.
Assessment of Kidney Function
Acute kidney injury was defined based on KDIGO guidelines using Cr alone, because data on urine output were not available [19]. Glomerular filtration rate was estimated using the Schwartz equation: Cr clearance (mL/min per 1.73 m2) = [length (cm) × k]/Cr (mg/dL), where k = 0.413 (constant for children aged 1 to 13 years) [20]. Acute kidney injury was defined as follows: a rise in Cr by ≥0.3 mg/dL (26 umol/L) within 48 hours, or a ≥1.5-fold increase in Cr from the reference value. In children meeting the criteria for AKI, it was staged as follows: stage 1, >26 µmol/L within 48 hours or 1.5–1.9× nadir Cr; stage 2, ≥2 to 2.9× nadir Cr; stage 3, ≥3× nadir Cr or ≥4 mg/dL (354 µmol/L) over the course of hospitalization (72 hours) or an estimated GFR (eGFR) <35 mL/min per 1.73 m2 [20]. The baseline Cr was estimated assuming a normal GFR of 120 mL/min per 1.73 m2 [21]. Length data were missing from 15 children so the length was imputed assuming the median length for sex and age using World Health Organization (WHO) growth charts. Renal replacement therapy was not used in the diagnosis or staging of AKI because it was not available.
Assessment of Cystatin C
K2EDTA plasma was collected daily and stored at −80°C until testing. Plasma CysC was measured using an R&D Systems DuoSet ELISA (Burlington, Canada) with the investigator blinded to treatment arm and outcome. Protein concentrations were extrapolated from 4 parameter logistic curves on each plate using Gen5 software (BioTek).
Statistical Analysis
Data were analyzed using IBM SPSS version 20, MedCalc version 13.1.2, and GraphPad Prism version 6. Continuous data are presented as medians (interquartile range [IQR]) and analyzed nonparametrically. Categorical data were analyzed using Pearson χ2 or Fisher's exact tests, as appropriate. To test for trend across stages of AKI, Jonckheere-Terpstra Test for Ordered Alternatives was used for continuous data and χ2 test for linear trend was used for ordinal data. Spearman correlation was used to evaluate associations between renal biomarkers. The relationship between biomarker levels and mortality was explored using Mann-Whitney U test and nonparametric receiver operating characteristic (ROC) curves. Biomarker cut-points were generated using the Youden Index. The areas under independent ROC curves (AUCs) were compared using the method of Delong et al [22].
Ethics, Consent, and Permissions
Ethical approval for this study was granted by the Uganda National Council for Science and Technology, the Uganda National Drug Authority, and Makerere University Research Ethics Committee in Uganda, and the Toronto Academic Health Science Network in Canada. All parents or guardians provided written informed consent for participation in the study.
RESULTS
Incidence of Acute Kidney Injury in Severe Malaria
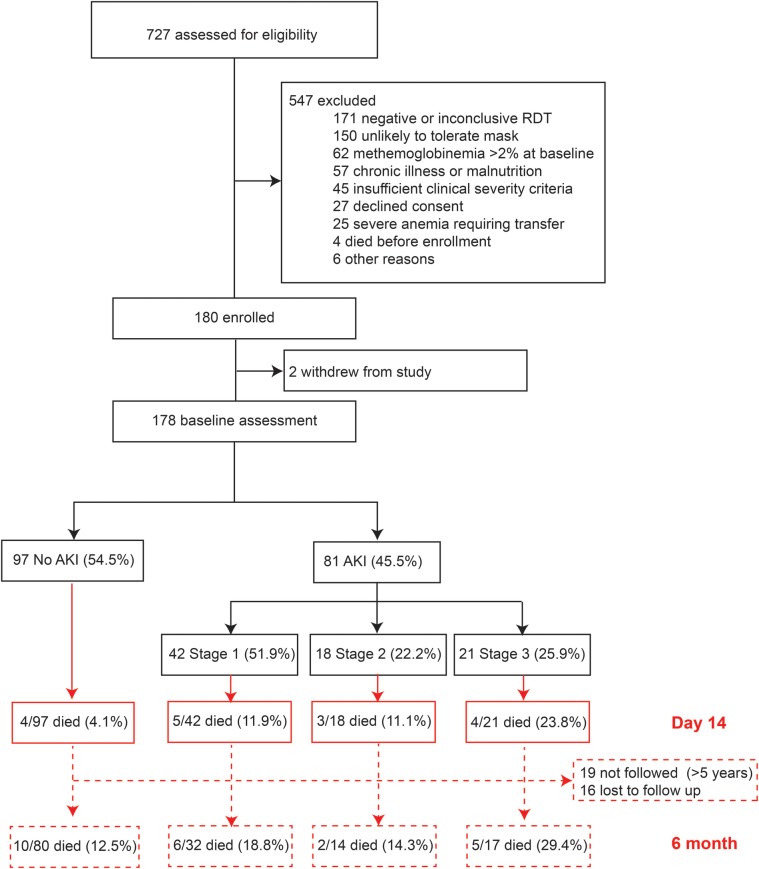
Data were available for 178 children (study flowchart, Figure 1). The median age of children was 2 years (IQR, 1–3), and 56.7% of the children were male. The demographic and clinical characteristics of children in the cohort are shown in Table 1. Overall, 81 children (45.5%) had KDIGO-defined AKI: 42 (51.9%) were stage 1, 18 (22.2%) were stage 2, and 21 (25.9%) were stage 3. Children over 5 years of age were not an increased risk of AKI (relative risk [RR], .86; 95% confidence interval [CI], .57–1.29) compared to younger children. There were trends of lower height-for-age z-scores, systolic blood pressure, base excess, and bicarbonate, and higher pulse rates, lactate, sodium, potassium, and chloride across stages of AKI (P < .05 for all; Table 1).
Figure 1.
Flow chart of study population. A flow chart showing the children enrolled in the study, the incidence of Kidney Disease: Improving Global Outcomes-defined acute kidney injury (AKI) by stage, and mortality across stages of AKI at day 14 and 6-month follow up.
Table 1.
Characteristics of Study Population According to Kidney Function
| Characteristics | Cohort (n = 178) | No AKI (n = 97) | Acute Kidney Injury (n = 81) |
Test-for-Trend | ||
|---|---|---|---|---|---|---|
| Stage 1 (n = 42) | Stage 2 (n = 18) | Stage 3 (n = 21) | ||||
| Demographics | ||||||
| Age | 2 (1–3) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 1.3 (1.0, 2.0) | 1.5 (1.0, 2.8) | 0.120 |
| Age >5 y | 16 (9.0) | 10 (10.3) | 3 (7.1) | 1 (5.6) | 2 (9.5) | 0.673 |
| Males | 101 (56.7) | 53 (54.6) | 19 (47.6) | 18 (88.9) | 12 (57.1) | 0.245 |
| Weight-for-age z-score | −1 (−2, 0) | −1.0 (−2.0, 0.0) | −1.0 (−2.0, 0.0) | −1.0 (−1.3, 0.0) | 0.0 (−1.0, 0.0) | 0.355 |
| Height-for-age z-score | −2 (−3, 0) | −1.0 (−2.3, 0.3) | −2.0 (−3.0, −1.0) | −1 (−3.0, 0.8) | −2.0 (−4.0, 0.0) | 0.022 |
| Fever, days | 3 (2–4) | 3 (3, 5) | 3 (2, 3) | 3 (2, 3) | 3 (2, 4) | 0.014 |
| Clinical | ||||||
| temperature, °C | 37.9 (37.0–38.8) | 37.9 (37.0, 38.9) | 38.2 (37.2, 39.0) | 37.8 (36.7, 38.82) | 37.7 (37.1, 38.8) | 0.938 |
| Respiratory rate, bpm | 48 (38–62) | 46 (36, 58) | 48 (37, 67) | 50 (40, 70) | 53 (43, 62) | 0.126 |
| Pulse rate, bpm | 162 (144–179) | 159 (143, 175) | 162 (140, 175) | 178 (158, 187) | 179 (143, 187) | 0.011 |
| Systolic BP, mmHg | 110 (100–120) | 110 (100, 120) | 110 (100, 127) | 100 (98 120) | 110 (90, 120) | 0.085 |
| Blantyre Coma Score | 2 (2–3) | 2 (2, 3) | 2 (2, 3) | 2 (1, 2.3) | 2 (1, 3) | 0.033 |
| Hemoglobinuriaa | 32 (18.0) | 15 (15.5) | 9 (21.4) | 3 (16.7) | 5 (23.8) | 0.382 |
| Respiratory distress | 100 (56.2) | 50 (51.5) | 23 (54.8) | 14 (77.8) | 13 (61.9) | 0.117 |
| Severe anemiab | 113 (63.5) | 62 (63.9) | 25 (59.5) | 9 (50.0) | 17 (81.0) | 0.501 |
| Shock | 16 (9.0) | 6 (6.2) | 5 (11.9) | 3 (11.1) | 3 (14.3) | 0.179 |
| Laboratory | ||||||
| Parasitemia, parasites/µL | 26 000 (2431–87 120) | 24 440 (2480, 72 110) | 29 400 (4420, 128 120) | 19 240 (750, 64 920) | 22 940 (1320, 81 920) | 0.745 |
| Hb, g/dL | 4.8 (3.2–6.5) | 4.8 (3.3, 6.5) | 4.8 (3.0, 6.5) | 5.5 (2.5, 6.7) | 4. (4.0, 6.7) | 0.978 |
| WBC ×109/L | 11.8 (7.6–19.4) | 10.8 (6.8, 18.0) | 12.4 (8.2, 20.9) | 15.1 (11.7, 22.7) | 17.3 (6.2, 23.2) | 0.061 |
| Platelet ×109/L | 71 (38–126) | 66 (42, 124) | 80 (36, 138) | 74 (34, 180) | 59 (35, 114) | 0.924 |
| Glucose, mmol/L | 6.7 (5.5–8.1) | 6.6 (5.5, 7.9) | 6.7 (5.5, 8.1) | 6.8 (5.8, 7.6) | 7.4 (4.9, 10.3) | 0.356 |
| Lactate, mmol/L | 3.6 (2.1–6.4) | 3.3 (2.1, 6.0) | 3.3 (2.0, 5.7) | 4.0 (2.8, 7.5) | 5.4 (3.2, 12.9) | 0.025 |
| BEecf, mmol/L | −8 (−12, −4) | −7 (−11, −3) | −8 (−12, −5) | −10 (−16, −3) | −12 (−18, −4) | 0.006 |
| HCO3−, mmol/L | 17.3 (13.0–20.1) | 18.1 (14.7, 20.4) | 17.2 (12.3, 19.2) | 15.1 (11.3, 19.9) | 13.2 (8.3, 19.6) | 0.007 |
| Na+, mmol/L | 137 (134–140) | 137 (134, 139) | 139 (135, 142) | 139 (134, 144) | 139 (136, 142) | 0.014 |
| K+, mmol/L | 4.1 (3.7–4.5) | 4.0 (3.6, 4.4) | 4.2 (3.7, 4.7) | 4.2 (3.8, 4.7) | 4.3 (3.8, 4.5) | 0.036 |
| Cl−, mmol/L | 108 (104–112) | 107 (104, 110) | 110 (104, 115) | 110 (104, 115) | 111 (107, 116) | 0.012 |
| Treatment | ||||||
| Nitric oxide | 87 (48.9) | 39 (42.4) | 21 (51.2) | 14 (58.3) | 13 (61.9) | 0.051 |
| Transfusion, no. | 1.0 (0.8–2.0) | 1 (0, 2) | 1 (0.8, 2) | 1 (0.8, 2) | 1 (1, 2) | 0.216 |
| Volume fluid first 48 h, mL | 1000 (500, 1500) | 1000 (500, 1500) | 1020 (760, 1525) | 1010 (526, 1428) | 1410 (510, 1571) | 0.090 |
| Outcomes | ||||||
| Neurodisability at Dischargec | 13 (8.0) | 3 (3.2) | 6 (16.2) | 2 (12.5) | 2 (12.5) | 0.056 |
| Duration hospitalization,c h | 67 (59–85) | 62 (59, 82) | 78 (62, 103) | 80 (76, 84) | 103 (63, 140) | 0.002 |
| Death, 14 d | 16 (9.0) | 4 (4.1) | 5 (11.9) | 3 (11.1) | 4 (23.8) | 0.004 |
| Death, 6 mo | 23 (16.1) | 10 (12.5) | 6 (18.8) | 2 (14.3) | 5 (29.4) | 0.119 |
| Renal Function | ||||||
| Estimated baseline Cr, mg/dL | 0.28 (0.25, 0.31) | 0.29 (0.26, 0.32) | 0.26 (0.24, 0.30) | 0.27 (0.23, 0.30) | 0.27 (0.25, 0.30) | 1.000 |
| Admission Cr, mg/dL | 0.36 (0.27, 0.47) | 0.29 (0.21, 0.35) | 0.40 (0.36, 0.46) | 0.55 (0.39, 0.66) | 0.93 (0.64, 1.68) | <0.001 |
| Nadir Cr | 0.25 (0.20, 0.33) | 0.21 (0.21, 0.25) | 0.28 (0.21, 0.36) | 0.29 (0.20, 0.39) | 0.51 (0.37, 0.76) | <0.001 |
| Peak Cr | 0.41 (0.33, 0.55) | 0.33 (0.27, 0.39) | 0.45 (0.41, 0.54) | 0.59 (0.55, 0.68) | 1.47 (0.87, 2.19) | <0.001 |
| Day Cr nadir | 3 (2, 4) | 3 (1, 4) | 3 (2, 4) | 3 (2, 4) | 3 (1, 4) | 0.286 |
| Day Cr peak | 1.5 (1, 2) | 2 (1, 2) | 2 (1, 2) | 1 (1, 2) | 2 (1, 3) | 0.783 |
Abbreviations: AKI, acute kidney injury; BP, blood pressure; BEecf, base excess; bpm, beats per minute; Cr, creatinine; Hb, hemoglobin; WBC, white blood cell.
a Based on parental report.
b Hemoglobin <5 g/dL.
c In survivors.
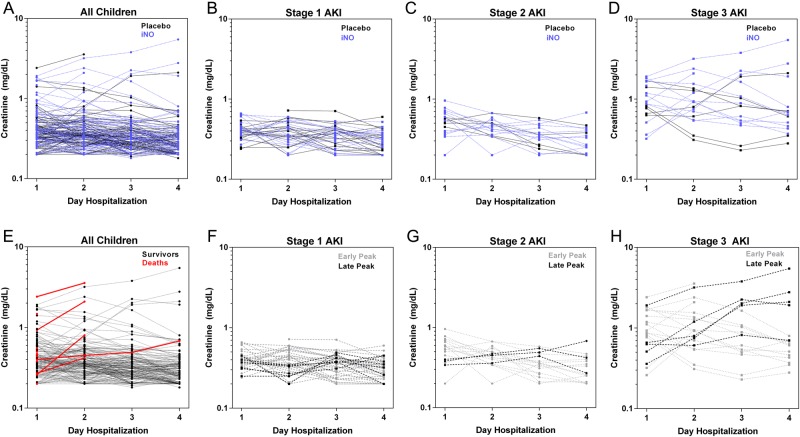
This study was nested within a randomized clinical trial evaluating iNO as an adjunctive therapy for SM where kidney function was monitored daily as a safety endpoint for the trial. Ten children had gas permanently discontinued for AKI: 9 with stage 3 disease and 1 with stage 2 disease. There were no differences in AKI between trial arms using the definition for clinical monitoring/gas withdrawal (P = .21) [23]. Children randomized to iNO were more likely to meet the definition of AKI than children in the placebo arm (iNO arm, n = 47 [54.0%] had AKI vs placebo, n = 34 [37.4%]: RR, 1.36; CI, 1.03–1.80; P = .026). We repeated the analysis focusing on children who developed AKI after starting treatment with iNO or placebo. Nineteen children (47.5%) in the iNO arm had worsening renal function after admission compared with 17 (38.6%) children in the placebo arm (P = .412). Ten children developed stage 3 AKI after admission with a peak Cr recorded after day 1: 4 (9.1%) in the placebo arm and 6 (15.0%) in the iNO arm (P = .404). The kinetics of Cr over hospitalization and across stages of AKI in children receiving iNO versus placebo are shown in Figure 2.
Figure 2.
Changes in creatinine over hospitalization according to outcome and stage of acute kidney injury (AKI). (A) Line graphs showing individual creatinine plots for each child according to trial arm with children randomized to placebo in black and children randomized to inhaled nitric oxide (iNO) in blue. Line graphs showing individual creatinine plots for children with AKI according to stage [(B) stage 1 AKI; (C) stage 2 AKI; (D) stage 3 AKI] and trial arm. (E) Line graphs showing individual creatinine plots for each child according to outcome with survivors shown in black and in-hospital deaths shown in red. (B–D) Line graphs showing individual creatinine plots for children with AKI according to stage [(F) stage 1 AKI; (G) stage 2 AKI; (H) stage 3 AKI] and the timing of creatinine peak, with early indicating the peak creatinine recorded on day 1 or 2 of hospitalization, and late indicating the peak creatinine recorded on day 3 or 4 of hospitalization.
To explore the evolution and resolution of AKI in malaria, we plotted the kinetics of Cr over hospitalization (Figure 2). Fifty percent of children had their Cr peak on day 1, 29% peaked on day 2, 14% peaked on day 3, and 7% peaked on day 4. There were no differences in the timing of Cr peak between children with or without AKI (P = .850), or across stages of AKI (P = .738). To evaluate the nature of renal injury/impairment in children with AKI, the BUN (mg/dL) to Cr (mg/dL) ratio was calculated at admission. Eighty-four percent of children had a BUN:Cr > 20 consistent with impaired renal perfusion (n = 64), whereas 8% had a BUN:Cr < 10 consistent with renal tubular damage. Because anemia can affect renal oxygenation, we assessed whether AKI was related to hemoglobin level or presence of severe anemia. There was no relationship between hemoglobin levels and eGFR or CysC levels, or the presence or stage of AKI (data not shown).
Biomarkers of Kidney Function Increase Across Stages of Acute Kidney Injury
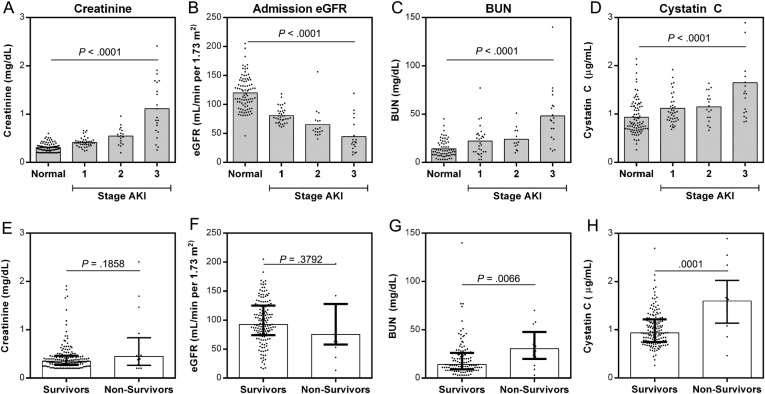
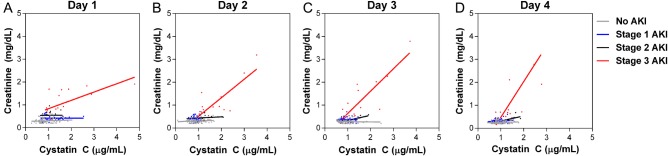
We evaluated biomarkers of kidney function (BUN and CysC) in children with or without AKI. Admission BUN and CysC were significantly higher in children with AKI compared with those without (BUN, P = .0086; CysC, P < .0001) with levels increasing across stages of AKI (BUN, P < .0001; CysC, P < .0001, test for trend) (Figure 3). Because both Cr and CysC are markers of GFR, we explored correlations between the markers over hospitalization stratified by severity of AKI. Creatinine and CysC were positively correlated over hospitalization (day 1, 2, 3, 4), with the strongest associations observed in children with stage 3 AKI (Figure 4).
Figure 3.
Biomarkers of renal function measured at admission across stages of acute kidney injury (AKI) and their association with acute mortality. (A–D) Scatter plots showing the median and distribution of renal biomarkers at admission in children with no AKI (normal), and AKI stratified by stage. The relationship between biomarkers and AKI was evaluated using a test for trend (Jonckheere-Terpstra Test for Ordered Alternatives). (E–H) Scatter plots showing the median, interquartile range, and distribution of renal biomarkers in survivors (n = 162) vs nonsurvivors (n = 16). Analysis was performed by Mann–Whitney U test. Abbreviations: BUN, blood urea nitrogen; eGFR, estimated GFR.
Figure 4.
Relationship between serum creatinine (Cr) and cystatin C (CysC) in children over hospitalization according to renal function. (A–D) Plots showing the relationship between Cr and CysC over hospitalization using linear regression stratified by acute kidney injury (AKI) stage.
Relationship Between Admission Biomarkers, Morbidity, and Mortality
There was an increase in mortality across stages of AKI (P = .004, linear-by-linear association) with mortality reaching 23.8% in children with stage 3 AKI (Table 1). The association between stage of AKI and mortality was seen in the placebo group only (P = .006) with 37.5% mortality in stage 3 AKI, indicating that this association was not an effect of iNO. The association between stage of AKI and mortality in the iNO group was not significant (P = .136) with mortality at 15.4% in stage 3 AKI. All children who died in-hospital with multiple Cr measurements had increasing Cr before death (n = 5). To explore the broader association between kidney function and outcome, we examined the association between levels of kidney biomarkers at admission and day 14 mortality. There was no difference in median Cr or eGFR between survivors and nonsurvivors. Median levels of both BUN and CysC at admission were higher in nonsurvivors (P = .0066 and P = .0001, respectively) (Figure 3). The ability of the biomarkers to predict day 14 mortality was further explored using ROC curves comparing the area under the curve. Both CysC and BUN had moderate discriminatory ability, with AUCs of 0.80 (95% CI, .65–.95) and 0.72 (95% CI, 0.57–0.87), respectively. Using the Youden index to identify biomarker cut-points, BUN > 21 mg/dL had a sensitivity of 78.6% (95% CI, 49.2–95.3) and a specificity of 67.2% (95% CI, 57.9–75.7), whereas CysC > 1.369 µg/mL had a sensitivity of 71.4% (95% CI, 41.9–91.6) and specificity of 85.5% (95% CI, 78.9–90.7) to predict death at day 14.
We looked at the association between AKI and all-cause mortality at 6 months postdischarge (Figure 1). Seven children died between day 14 and 6 months follow up (no AKI, n = 6; stage 1 AKI, n = 1). Admission levels of both CysC and BUN levels were elevated in children who died by 6 month follow up (P < .0001 and P = .009, respectively). Acute kidney injury was not associated with a significant increase in risk of all-cause mortality at 6 months (RR, 1.34; 95% CI, .82–2.19).
Acute kidney injury and stage of AKI was associated with increased duration of hospitalization (P = .001 and P = .002, respectively). This effect remained significant when stratified by trial arm (P < .0001 for both placebo and iNO arms). In the placebo arm, AKI was associated with neurodisability with 7 (25.0%) of children with AKI being diagnosed with neurodisability at discharge compared to 1 (1.9%) of children without AKI having neurodisability at discharge (P = .002). There was no difference in rates of neurodisability associated with AKI in children receiving iNO with 7.3% of children with AKI having neurodisability at discharge compared with 5.1% in children without AKI, P = 1.00. We investigated the association between renal biomarkers and neurodisability and found increased admission levels of Cr and lower admission eGFR was associated with neurodisability at discharge in the placebo group only (P = .005 and P = .003, respectively). Neither CysC nor BUN was associated with neurodisability at discharge in either group.
DISCUSSION
Although AKI is recognized as a relatively common complication in adults with SM, the incidence of AKI in children with malaria has not been systematically assessed with longitudinal assessment of Cr over hospitalization. Malaria is a major infection-related cause of AKI in malaria-endemic areas [6, 7, 24–26], but there are limited data on the incidence of AKI in pediatric SM with estimates ranging from 3% to 31% [6, 7, 27, 28]. In this study, we prospectively assessed the incidence of AKI in a cohort of children randomized to iNO or placebo as an adjunctive therapy for SM. Because iNO has been associated with an increased risk of developing AKI in critically ill adults [29], kidney function was monitored daily. We observed an increased risk (RR, 1.40; 95% CI, 1.03–1.90) of developing AKI associated with iNO therapy. Using the KDIGO guidelines to define and stage AKI in the cohort, 37.4% of children in the placebo arm had AKI with 58.8% stage 1, 17.6% stage 2, and 23.5% stage 3. There was an association between the severity of AKI and short-term mortality and neurodisability at discharge in the placebo arm only, despite a limited number of deaths in our population. Admission levels of CysC and BUN were associated with all-cause 14-day and 6-month mortality. Furthermore, all children who died in-hospital after the first day of admission had rising Cr before death, despite receiving intravenous fluids and effective antimalarial therapy. Our findings linking AKI to mortality are consistent with previous pediatric studies [4, 6–8, 30].
Blood urea nitrogen was identified as an independent predictor of mortality in a large cohort (n = 4148) of children with SM [4]. In this study, BUN was increased in AKI, across stages of AKI, and was associated with mortality. Blood urea nitrogen levels >21 mg/dL had a sensitivity and specificity of 78.6% and 67.2%, respectively, to predict short-term mortality (day 14). This threshold is comparable with the 20 mg/dL used to define uremia in previous reports [4].
Our data suggest hypoperfusion/renal ischemia may have contributed to AKI in the majority of children. Because BUN levels were only available at presentation, we cannot confirm whether pre-renal azotemia persisted after fluid administration. The recent FEAST trial suggests that bolus fluids may be harmful in African children with fever, compensated shock, and either respiratory distress or impaired consciousness [31], clinical features present in many of the children in the present study. Further studies will be required to define the indications for bolus fluid administration in the context of pre-renal azotemia in children with SM, because AKI in this population is also associated with increased mortality and bolus fluid administration may be helpful in correcting this condition. Alternatively, use of ionotropes to support intravascular volume may provide the necessary adjunctive therapy without requiring bolus fluid administration. Pathophysiologically, sequestration of parasitized erythrocytes in the microvasculature may contribute to kidney hypoperfusion. Post mortem studies have documented parasite sequestration in the kidneys in adults [3] and children [32] with SM. Cardiac dysfunction secondary to hypovolemia [33, 34] and severe anemia [35] may also contribute to poor kidney perfusion, although we did not see an association between anemia and AKI in this study. In adults with SM, biomarkers of inflammation (suPAR) and total parasite biomass (HRP2) were associated with AKI and the need for RRT [36], suggesting that both parasite and host factors contribute to AKI.
Prospective, blinded monitoring for AKI was performed during this randomized control trial using standardized US National Institute of Allergy and Infectious Diseases (NIAID) toxicity tables, because of concerns about nephrotoxicity reported previously [29], and was a criterion for treatment discontinuation [17]. Failure to detect this toxicity signal [17], which was graded using changes in the Cr level and age-specific thresholds, suggests that the NIAID tables may lack sensitivity and illustrates that ascertainment of AKI varies with definition [21]. Our data are consistent with meta-analyses showing a 40% increased risk of renal dysfunction associated with nitric oxide therapy [29, 37]. The majority of data on iNO and renal dysfunction come from studies of adults, and there are limited data on renal toxicity in pediatric populations. The association between iNO and AKI appears to be dose-dependent with high cumulative-dose iNO associated with increased risk of AKI, but medium and low cumulative doses were not [37]. Our data are consistent with high-dose iNO contributing to renal dysfunction. However, when we focused on the subset of children who had worsening renal function after starting iNO therapy, the significant effect was lost. Because children with SM are at risk for AKI, it is possible that additional oxidative stress from iNO in the form of reactive nitrogen species (eg, NO2) or elevated methemoglobin may exacerbate underlying renal dysfunction. Although there was an increase in incidence of AKI in the iNO group, the association between AKI and poor clinical outcomes (ie, neurodisability and mortality) was not significant in children receiving iNO, suggesting that iNO may have a beneficial effect that counteracts increasing Cr. Overall, these data suggest that caution is required in future studies involving iNO administration.
Without serial BUN assessments, we are unable to comment on the nature of kidney injury in the children that recorded increasing Cr over hospitalization. Although levels of Cr decreased by day 4 in most children, without a preinfection Cr measure, and longer follow up of patients postdischarge, we cannot ascertain whether patients fully recovered renal function. The WHO Handbook on Severe Malaria states that renal injury is reversible in survivors [38], but there is mounting evidence from other infections that this may not be the case [39, 40]. A meta-analysis examining outcomes after pediatric hemolytic-uremic syndrome found a 25% long-term risk of renal complications in survivors [40]. Even patients who fully recovered baseline renal function had an increased risk of developing renal complications in 5 to 10 years follow up [40].
Any AKI—including mild or reversible disease—is associated with an increased risk of mortality [19, 39]. Although Cr remains the clinical standard to assess renal function, Cr can overestimate GFR in patients with declining renal function in a rapidly evolving disease such as malaria [41]. In this study, admission levels of CysC were elevated in children with AKI and increased across stages of AKI. In adults with malaria, CysC was more frequently elevated than Cr, suggesting that it is a more sensitive marker of kidney injury/impairment than Cr [12]. Elevated CysC was also reported in 17% of Ghanaian children with uncomplicated P falciparum malaria [15]. In this study, elevated CysC predicted death, and it was better than Cr at discriminating between survivors and nonsurvivors (P = .0012, comparison of AUCs). Overall, CysC may be a more accurate measure of renal function, and a better prognostic marker than Cr, when Cr is not in steady state.
One of the strengths of this study was the longitudinal design with daily assessment of kidney function during hospitalization, which enabled us to identify acute changes in kidney function that developed or evolved after admission and detect smaller but significant changes in renal function. In low resource settings, serial Cr measures are often unavailable, so AKI is commonly reported based on a single measure at presentation. With 51.2% of children in the placebo arm having worsening renal function in the days after admission, this study represents an important advance providing a more accurate depiction of kidney injury/impairment than previous studies. Because children presented with severe disease, we lacked a premorbid assessment of renal function, so an estimated baseline “normal” Cr was calculated based on an eGFR of 120 mL/min per 1.73 m2 [21]. Additional studies are needed to define the best strategy to estimate baseline kidney function in settings where existing pediatric reference ranges may not accurately reflect population-specific normal ranges.
Overall, our results suggest that AKI is an underrecognized complication in pediatric SM and is associated with mortality. Although it is possible the increased mortality in AKI was related to severity of illness at presentation, AKI is recognized as an independent predictor of mortality in SM. Acute kidney injury in pediatric SM is likely multifactorial with dehydration, microvascular parasite sequestration, endothelial dysfunction, and severe anemia contributing to poor kidney perfusion and injury. By 2030, with more than 70% of patients living with end-stage renal disease expected to reside in low-income countries (18.1% prevalence in Uganda) [37], it is important to define whether repeated exposure to malaria or severe infections early in life may be contributing to increased susceptibility to CKD. Further studies will be required (1) to evaluate the short- and long-term prognosis of children surviving SM and (2) to evaluate whether interventions that improve microvascular perfusion (eg, by reducing parasite sequestration or improving endothelial dysfunction) can decrease AKI and improve outcomes in children with SM.
CONCLUSIONS
Acute kidney injury is a common complication in children with SM that develops or worsens in 50% of children after admission, highlighting the importance of serial Cr assessments in children admitted with SM. Additional studies are needed to define the long-term risk of CKD and mortality in children surviving SM.
Acknowledgments
We thank the patients and their families, the medical officers, the nurses and research assistants who cared for the patients and collected study data, and the medical superintendent of the Jinja Regional Referral Hospital.
Disclaimer. The funders had no role in study design, data collection, data analysis, data interpretation, writing of the report, or decision to submit the article for publication.
Authors' contributions. The study was conceptualized and designed by K. C. K. with input from A. L. C., M. H., and W. C. L. Patient recruitment and data collection were obtained by S. N., R. O. O., M. H., L. H., and A. L. C. Biomarker testing was performed by A. L. C. and K. R. B. Analysis was performed by A. L. C., with input from M. H., R. E. E., C. M., C. C. J., W. C. L., and K. C. K. A. L. C., R. E. E., and K. C. K. wrote the manuscript with input from all authors. All authors read and approved the final manuscript.
Financial support. This work was funded by a kind donation from Kim Kertland; the Tesari Foundation; the Sandra Rotman Centre for Global Health; the Association of Medical Microbiology and Infectious Disease Canada (Pfizer Post Residency Fellowship; to L. H.); and the Canadian Institutes of Health Research (MOP-115160, -13721, -136813, Canada Research Chair in Molecular Parasitology [to K. C. K.], Canada Research Chair in Infectious Diseases and Inflammation [to W. C. L.], Canadian Institute of Research Health [CIHR] Clinician-Scientist Training Award [to M. H.], and CIHR Post-Doctoral Research Award [to A. L. C.]).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Dondorp A, Nosten F, Stepniewska K et al. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005; 366:717–25. [DOI] [PubMed] [Google Scholar]
- 2.Trang TT, Phu NH, Vinh H et al. Acute renal failure in patients with severe falciparum malaria. Clin Infect Dis 1992; 15:874–80. [DOI] [PubMed] [Google Scholar]
- 3.Nguansangiam S, Day NP, Hien TT et al. A quantitative ultrastructural study of renal pathology in fatal Plasmodium falciparum malaria. Trop Med Int Health 2007; 12:1037–50. [DOI] [PubMed] [Google Scholar]
- 4.von Seidlein L, Olaosebikan R, Hendriksen IC et al. Predicting the clinical outcome of severe falciparum malaria in African children: findings from a large randomized trial. Clin Infect Dis 2012; 54:1080–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jallow M, Casals-Pascual C, Ackerman H et al. Clinical features of severe malaria associated with death: a 13-year observational study in the Gambia. PLoS One 2012; 7:e45645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor K, Gupta S. Malarial acute kidney injury in a paediatric intensive care unit. Trop Doct 2012; 42:203–5. [DOI] [PubMed] [Google Scholar]
- 7.Imani PD, Odiit A, Hingorani SR et al. Acute kidney injury and its association with in-hospital mortality among children with acute infections. Pediatr Nephrol 2013; 28:2199–206. [DOI] [PubMed] [Google Scholar]
- 8.Waller D, Krishna S, Crawley J et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis 1995; 21:577–87. [DOI] [PubMed] [Google Scholar]
- 9.Chertow GM, Burdick E, Honour M et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16:3365–70. [DOI] [PubMed] [Google Scholar]
- 10.KDIGO. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; Supplement 2:1–138. [Google Scholar]
- 11.Zhang Z, Lu B, Sheng X, Jin N. Cystatin C in prediction of acute kidney injury: a systemic review and meta-analysis. Am J Kidney Dis 2011; 58:356–65. [DOI] [PubMed] [Google Scholar]
- 12.Gunther A, Burchard GD, Slevogt H et al. Renal dysfunction in falciparum--malaria is detected more often when assessed by serum concentration of cystatin C instead of creatinine. Trop Med Int Health 2002; 7:931–4. [DOI] [PubMed] [Google Scholar]
- 13.Vinge E, Lindergard B, Nilsson-Ehle P, Grubb A. Relationships among serum cystatin C, serum creatinine, lean tissue mass and glomerular filtration rate in healthy adults. Scand J Clin Lab Invest 1999; 59:587–92. [DOI] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burchard GD, Ehrhardt S, Mockenhaupt FP et al. Renal dysfunction in children with uncomplicated, Plasmodium falciparum malaria in Tamale, Ghana. Ann Trop Med Parasitol 2003; 97:345–50. [DOI] [PubMed] [Google Scholar]
- 16.Volpon LC, Sugo EK, Carlotti AP. Diagnostic and prognostic value of serum cystatin C in critically ill children with acute kidney injury. Pediatr Crit Care Med 2015; 16:e125–31. [DOI] [PubMed] [Google Scholar]
- 17.Hawkes MT, Conroy AL, Opoka RO et al. Inhaled nitric oxide as adjunctive therapy for severe malaria: a randomized controlled trial. Malar J 2015; 14:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shephard MD. Point-of-care testing and creatinine measurement. Clin Biochem Rev 2011; 32:109–14. [PMC free article] [PubMed] [Google Scholar]
- 19.Kellum JA, Lameire N; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013; 17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Muñoz A, Schneider MF et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zappitelli M, Parikh CR, Akcan-Arikan A et al. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol 2008; 3:948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–45. [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basu G, Chrispal A, Boorugu H et al. Acute kidney injury in tropical acute febrile illness in a tertiary care centre--RIFLE criteria validation. Nephrol Dial Transplant 2011; 26:524–31. [DOI] [PubMed] [Google Scholar]
- 25.Esezobor CI, Ladapo TA, Lesi FE. Clinical profile and hospital outcome of children with severe acute kidney injury in a developing country. J Trop Pediatr 2015; 61:54–60. [DOI] [PubMed] [Google Scholar]
- 26.Olowu WA, Adelusola KA. Pediatric acute renal failure in southwestern Nigeria. Kidney Int 2004; 66:1541–8. [DOI] [PubMed] [Google Scholar]
- 27.Zaki SA, Shenoy P, Shanbag P et al. Acute renal failure associated with malaria in children. Saudi J Kidney Dis Transpl 2013; 24:303–8. [DOI] [PubMed] [Google Scholar]
- 28.Kunuanunua TS, Nsibu CN, Gini-Ehungu JL et al. Acute renal failure and severe malaria in Congolese children living in Kinshasa, Democratic Republic of Congo. Nephrol Ther 2013; 9:160–5. [DOI] [PubMed] [Google Scholar]
- 29.Adhikari NK, Burns KE, Friedrich JO et al. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. BMJ 2007; 334:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maitland K, Levin M, English M et al. Severe P. falciparum malaria in Kenyan children: evidence for hypovolaemia. QJM 2003; 96:427–34. [DOI] [PubMed] [Google Scholar]
- 31.Maitland K, Kiguli S, Opoka RO et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med 2011; 364:2483–95. [DOI] [PubMed] [Google Scholar]
- 32.Seydel KB, Milner DA, Kamiza SB et al. The distribution and intensity of parasite sequestration in comatose Malawian children. J Infect Dis 2006; 194:208–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrhardt S, Mockenhaupt FP, Anemana SD et al. High levels of circulating cardiac proteins indicate cardiac impairment in African children with severe Plasmodium falciparum malaria. Microbes Infect 2005; 7:1204–10. [DOI] [PubMed] [Google Scholar]
- 34.Yacoub S, Lang HJ, Shebbe M et al. Cardiac function and hemodynamics in Kenyan children with severe malaria. Crit Care Med 2010; 38:940–5. [DOI] [PubMed] [Google Scholar]
- 35.Nguah SB, Feldt T, Hoffmann S et al. Cardiac function in Ghanaian children with severe malaria. Intensive Care Med 2012; 38:2032–41. [DOI] [PubMed] [Google Scholar]
- 36.Plewes K, Royakkers AA, Hanson J et al. Correlation of biomarkers for parasite burden and immune activation with acute kidney injury in severe falciparum malaria. Malar J 2014; 13:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruan SY, Huang TM, Wu HY et al. Inhaled nitric oxide therapy and risk of renal dysfunction: a systematic review and meta-analysis of randomized trials. Crit Care 2015; 19:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanifer JW, Jing B, Tolan S et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health 2014; 2:e174–81. [DOI] [PubMed] [Google Scholar]
- 39.Heung M, Chawla LS. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens 2012; 21:628–34. [DOI] [PubMed] [Google Scholar]
- 40.Garg AX, Suri RS, Barrowman N et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 2003; 290:1360–70. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA 2015; 313:837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]