The prevalence of HCV coinfection in HIV-infected individuals in Spain has decreased significantly in the last decade. Patients with active HCV infection mainly have genotypes 1a and 4, and a high percentage have liver cirrhosis.
Keywords: coinfection/epidemiology, hepatitis C/drug therapy/epidemiology, HIV infections/epidemiology, Spain/epidemiology
Abstract
Background. The purpose of this study was to assess the prevalence of anti-hepatitis C virus (HCV) antibodies (Abs) and active HCV infection in human immunodeficiency virus (HIV)-infected (HIV+) patients in Spain in 2015. This was a cross-sectional study.
Methods. The study was performed in 41 centers in 2015. Sample size was estimated for an accuracy of 2%, the number of patients from each hospital was determined by proportional allocation, and patients were selected using simple random sampling.
Results. The reference population was 35 791 patients, and the sample size was 1867 patients. Hepatitis C virus serostatus was known in 1843 patients (98.7%). Hepatitis C virus-Abs were detected in 695 patients (37.7%), in whom the main route of HIV acquisition was injection drug use (75.4%). Of these 695 patients, 402 had HCV RNA, 170 had had a sustained viral response (SVR) after anti-HCV therapy, and 102 cleared HCV spontaneously. Hepatitis C virus-ribonucleic acid results were unknown in 21 cases. Genotype distribution (known in 367 patients) was 1a in 143 patients (39.0%), 4 in 90 (24.5%) patients, 1b in 69 (18.8%) patients, 3 in 57 (15.5%) patients, 2 in 5 (1.4%) patients, and mixed in 3 (0.8%) patients. Liver cirrhosis was present in 93 patients (23.1%) with active HCV infection and in 39 (22.9%) patients with SVR after anti-HCV therapy.
Conclusions. The prevalence of HCV-Abs and active HCV infection in HIV+ patients in Spain is 37.7% and 22.1%, respectively; these figures are significantly lower than those recorded in 2002 and 2009. The predominant genotypes in patients with active HCV infection were 1a and 4. A high percentage of patients had cirrhosis. Cirrhosis is also common in patients with SVR after anti-HCV therapy.
Coinfection by hepatitis C virus (HCV) is one of the most common comorbid conditions in patients infected by the human immunodeficiency virus (HIV) [1]. Human immunodeficiency virus infection modifies the natural history of chronic hepatitis C, promoting faster progression to cirrhosis and end-stage liver disease [2] and increasing mortality in HIV/HCV-coinfected patients in the era of combined antiretroviral therapy [3, 4].
Recent data from the EuroSIDA cohort show that the prevalence of anti-HCV antibodies in HIV-infected individuals in Europe is 32.4%, and of those tested for HCV-ribonucleic acid (RNA), 70.5% are positive [5]. However, the prevalence of HCV antibody positivity varies across different European regions depending on the predominant route of acquisition of HIV. In Eastern and Southern Europe, where HIV infection is acquired mainly through injection drug use (IDU), 58% and 29% of patients, respectively, are also HCV antibody-positive. In Northern and Western Europe, where HIV infection is acquired mainly through sexual relations between men who have sex with men (MSM), 17% and 20% of patients, respectively, are HCV antibody-positive [5].
In Spain, the factors that determine the epidemiology of HIV/HCV coinfection have changed significantly in the last few years. These factors include the decline in IDU as a mechanism of transmission of HIV infection [6], the higher mortality rates in HIV/HCV-coinfected patients than in HIV-infected patients [4], the emergence of new HCV infections through high-risk sexual practices among MSM [7], and the availability of new, effective treatment against HCV infection.
We carried out this study to determine the prevalence of HCV infection in HIV-infected patients in Spain (including the percentage of patients with anti-HCV antibodies and the percentage of patients with detectable HCV-RNA) and to assess the characteristics of HIV-infected patients with active HCV infection. In addition, we compared the results of this study with those of 2 nationwide prevalence studies carried out by the “Grupo de Estudio del SIDA” ([GeSIDA] AIDS Study Group) of the “Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica” (Spanish Society of Infectious Diseases and Clinical Microbiology [SEIMC]) in 2002 [8] and 2009 [9].
SUBJECTS AND METHODS
Design and Sample Size Considerations
We performed a cross-sectional study in various medical centers throughout Spain. The reference population was all HIV-infected patients in active follow-up in the participating centers. Active follow-up was defined as at least 1 visit to the center in the previous 12 months.
Before the study was initiated, we estimated that the total number of patients in active follow-up at the participating centers and the prevalence of active HCV infection expected, which were 24 500 and 30%, respectively, according to the most recent survey carried out by GeSIDA [9]. Based on these figures, a confidence level of 95%, a design effect of 1.0, and accuracy for the sample size of 2.0%, we estimated that a sample of at least 1864 patients was needed.
Patient Selection
The number of patients to be included at each center was determined by proportional allocation, ie, the number of active patients at the center was proportional to the total number of active patients. The formula used to determine the sample size for each center was as follows: ni = (Ni/N) × n. Where ni was the sample size for the center i, Ni was the number of patients in active follow-up at the center i, N was the total number of patients in active follow-up, and n was the total size of the sample.
Patients were selected at each center by simple random sampling. First, personnel at each center identified all HIV-infected patients in active follow-up and generated a consecutive numerical list. Second, the staff of Fundación SEIMC/GeSIDA generated a random list of numbers corresponding to the patients to be included in the study.
The Institutional Ethics Committee of Hospital General Universitario Gregorio Marañón approved the study.
Variables and Statistical Analysis
The full list of variables collected is shown as Supplementary Material. In brief, we collected demographic data, HIV transmission category, Centers for Diseases Control and Prevention (CDC) disease category, current CD4/mm3, current HIV-RNA, whether the patients were on combined antiretroviral therapy (cART) and the regimen used, presence of HCV antibodies and, if applicable, presence of HCV-RNA, and presence of hepatitis B virus surface antigen (HBsAg).
In patients with HCV antibodies, information was obtained about anti-HCV therapy, and, if applicable, the regimens used and their outcomes. Patients receiving anti-HCV therapy at the time the study was performed were considered to be HCV-RNA–positive. In the case of patients with HCV antibodies and no HCV-RNA, we established whether this was due to spontaneous clearance or anti-HCV treatment.
In patients with HCV antibodies and positive HCV-RNA, we collected HCV genotype and subtype and IL28B subtypes. In patients positive for HCV-RNA and/or HBsAg, transient elastography results and the date the procedure was performed were recorded.
The presence of liver cirrhosis was investigated in all patients, as was the method of diagnosis: liver biopsy, transient elastography (liver stiffness >12.5 kPa), or diagnosis based on clinical/biological findings. Patients with prior or current episodes of ascites, hepatic encephalopathy, or variceal bleeding were considered to have decompensated liver disease. In patients with cirrhosis, current Child-Pugh and model for end-stage liver disease (MELD) scores were recorded. We also recorded whether patients had been diagnosed with hepatocellular carcinoma and whether liver transplantation had been performed.
All the information was entered into a common database at each institution using an online electronic case report form that satisfies Spanish data confidentiality requirements. All statistical analyses were performed using Stata (version 13.1; StataCorp, College Station, TX).
RESULTS
The study was carried out between June 1, 2015 and July 31, 2015. A total of 41 centers agreed to participate in the study. The reference population was 35 791 HIV-infected patients, and the sample size was 1867 patients.
Prevalence of Anti-Hepatitis C Virus Antibodies and Active Hepatitis C Virus Infection
Hepatitis C virus serostatus was known in 1843 (98.7%) patients, 695 of whom were HCV-seropositive; of these, 402 patients were HCV-RNA–positive, 170 were HCV-RNA–negative after sustained viral response (SVR) after anti-HCV therapy, 102 cleared HCV spontaneously, and HCV-RNA results were unknown in 21. Therefore, the prevalence of anti-HCV antibodies was 37.7% among tested patients (695 of 1843 patients whose serostatus was known), and the prevalence of active HCV infection was 22.1% (402 of 1822 patients with known HCV serostatus and with known HCV-RNA among those who tested positive for HCV antibodies).
Patients’ Characteristics
The characteristics of the 1867 patients included in the study are summarized in Table 1. No significant differences were found for sex between HCV-seronegative and HCV-seropositive patients; however, the latter were 5 years older than the former, on average. The frequency of injection drug use was significantly higher among HCV-seropositive patients than among HCV-seronegative patients, whereas the frequency of both transmission via heterosexual relations and sexual relations between MSM was significantly higher among HCV-seronegative than among HCV-seropositive patients. More HCV-seronegative patients than HCV-seropositive patients were in CDC category C.
Table 1.
Baseline Characteristics of the 1867 Patients Included in the Study
| Characteristic | HCV Antibodies |
P Valuea | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive |
|||||||||
| Unknown N = 24 | HCV-RNA Positive N = 402 | HCV-RNA Negative After Anti-HCV Rx N = 170 | Spontaneous Clearance of HCV-RNA N = 102 | HCV-RNA Unknown N = 21 | Total no. of HCV Seropositive Patients N = 695 | Negative N = 1148 | Total N = 1867 | ||
| Male sex, n (%) | 15 (62.5) | 305 (75.9) | 138 (81.2) | 60 (58.8) | 15 (71.4) | 518 (74.5) | 893 (77.8) | .11 | 1426 (76.4) |
| Age years, mean (SD) | 48 (13) | 50 (6) | 50 (6) | 51 (6) | 48 (6) | 50 (6) | 45 (12) | <.001 | 47 (10) |
| HIV transmission category, n (%) | |||||||||
| Injection drug use | 5 (20.8) | 308 (76.6) | 134 (78.8) | 67 (65.7) | 15 (71.4) | 524 (75.4) | 44 (3.8) | .001 | 573 (30.7) |
| Heterosexual relations | 9 (37.5) | 49 (12.2) | 9 (5.3) | 19 (18.6) | 3 (14.3) | 80 (11.5) | 369 (32.1) | 458 (24.5) | |
| Sexual relations between MSM | 4 (16.7) | 11 (2.7) | 7 (4.1) | 7 (6.9) | 3 (14.3) | 28 (4.0) | 623 (54.3) | 655 (35.1) | |
| Contaminated blood products | 0 | 6 (1.5) | 5 (2.9) | 2 (2.0) | 0 | 13 (1.9) | 7 (0.6) | 20 (1.1) | |
| Mother-to-child transmission | 2 (8.3) | 2 (0.5) | 1 (0.6) | 1 (1.0) | 0 | 4 (0.6) | 12 (1.0) | 18 (1.0) | |
| Other | 4 (16.7) | 26 (6.5) | 14 (8.2) | 6 (5.9) | 0 | 46 (6.6) | 93 (8.1) | 143 (7.7) | |
| CDC clinical category C, n (%) | 8 (33.3) | 134 (33.3) | 45 (26.5) | 35 (34.3) | 7 (33.3) | 221 (31.8) | 233 (20.3) | <.001 | 462 (24.7) |
| HBsAg, n (%) | |||||||||
| Negative | 7 (29.2) | 373 (92.8) | 165 (97.1) | 91 (89.2) | 18 (85.7) | 647 (93.1) | 1104 (96.2) | <.001 | 1758 (94.2) |
| Positive | 0 | 5 (1.2) | 2 (1.2) | 11 (10.8) | 1 (4.8) | 19 (2.7) | 36 (3.1) | 55 (2.9) | |
| Unknown | 17 (70.8) | 24 (6.0) | 3 (1.8) | 0 | 2 (9.5) | 29 (4.2) | 8 (0.7) | 54 (2.9) | |
| HDV antibodies (if HBsAg-positive) | |||||||||
| Negative | 0 | 4 (80.0) | 1 (50.0) | 4 (36.4) | 1 (100.0) | 10 (52.6) | 17 (47.2) | .028 | 27 (49.1) |
| Positive | 0 | 1 (20.0) | 0 | 5 (45.4) | 0 | 6 (31.6) | 3 (8.3) | 9 (16.4) | |
| Unknown | 0 | 0 | 1 (50.0) | 2 (18.2) | 0 | 3 (15.8) | 16 (44.4) | 19 (34.5) | |
| cART, n (%) | 23 (95.8) | 392 (97.5) | 168 (98.8) | 97 (95.1) | 19 (90.5) | 676 (97.3) | 1086 (94.6) | .007 | 1785 (95.6) |
| Type of cART regimen, n (%) | |||||||||
| 2 NRTI + 1 NNRTI | 9 (39.1) | 120 (30.6) | 57 (33.9) | 36 (37.1) | 6 (31.6) | 219 (32.4) | 548 (50.5) | <.001 | 776 (43.5) |
| 2 NRTI + 1 PI | 2 (8.7) | 98 (25.0) | 26 (15.5) | 17 (17.5) | 8 (42.1) | 149 (22.0) | 206 (19.0) | 357 (20.0) | |
| 2 NRTI + 1 integrase inhibitor | 4 (17.4) | 74 (18.9) | 33 (17.6) | 17 (17.5) | 1 (5.3) | 125 (18.5) | 145 (13.3) | 274 (15.3) | |
| PI-based monotherapy | 0 | 25 (6.4) | 19 (11.3) | 8 (8.2) | 0 | 52 (7.7) | 44 (4.0) | 96 (5.4) | |
| PI-based bitherapy | 5 (21.7) | 22 (5.6) | 12 (7.1) | 8 (8.2) | 2 (10.5) | 44 (6.5) | 39 (3.6) | 88 (4.9) | |
| Other | 3 (13.0) | 53 (13.5) | 21 (12.5) | 11 (11.3) | 2 (10.5) | 87 (12.9) | 104 (9.6) | 194 (10.9) | |
| Category of cART regimen, n (%) | |||||||||
| First-line therapy | 4 (17.4) | 34 (8.7) | 7 (4.2) | 9 (9.3) | 1 (5.3) | 51 (7.5) | 296 (27.3) | <.001 | 351 (19.7) |
| Switch not related to toxicity/failure | 9 (39.1) | 180 (45.9) | 80 (47.6) | 45 (46.4) | 8 (42.1) | 313 (46.3) | 406 (37.4) | 728 (40.8) | |
| Switch after failure | 4 (17.4) | 55 (14.0) | 21 (12.5) | 15 (15.5) | 5 (26.3) | 96 (14.2) | 102 (9.4) | 202 (11.3) | |
| Switch after toxicity | 6 (26.1) | 118 (30.1) | 57 (33.9) | 26 (26.8) | 5 (26.3) | 206 (30.5) | 254 (23.4) | 466 (26.1) | |
| Unknown | 0 | 5 (1.3) | 3 (1.8) | 2 (2.1) | 0 | 10 (1.5) | 28 (2.6) | 38 (2.1) | |
| HIV-RNA copies/mL, n (%) | |||||||||
| All patients | |||||||||
| <50 | 20 (83.3) | 357 (88.8) | 160 (94.1) | 93 (91.2) | 16 (76.2) | 626 (90.1) | 995 (86.7) | .023 | 1641 (87.9) |
| 50–200 | 0 | 21 (5.2) | 5 (2.9) | 4 (3.9) | 1 (4.8) | 31 (4.5) | 50 (4.4) | 81 (4.3) | |
| >200 | 4 (16.7) | 24 (6.0) | 5 (2.9) | 5 (4.9) | 4 (19.0) | 38 (5.5) | 103 (9.0) | 145 (7.8) | |
| Patients on cART | |||||||||
| <50 | 19 (82.6) | 351 (89.5) | 159 (94.6) | 89 (91.8) | 16 (84.2) | 615 (91.0) | 991 (91.3) | .98 | 1625 (91.1) |
| 50–200 | 0 | 21 (5.4) | 5 (3.0) | 4 (4.1) | 1 (5.3) | 31 (4.6) | 48 (4.4) | 79 (4.4) | |
| >200 | 4 (17.4) | 20 (5.1) | 4 (2.4) | 4 (4.1) | 2 (10.5) | 30 (4.4) | 47 (4.3) | 81 (4.5) | |
| CD4+ – T cells/µL, median (IQR) | |||||||||
| All patients | 581 (462–851) | 595 (379–817) | 595 (456–844) | 579 (442–767) | 518 (271–689) | 589 (405–804) | 660 (485–886) | <.001 | 630 (452–852) |
| Patients on cART | 583 (475–868) | 599 (382–812) | 597 (460–844) | 577 (442–716) | 546 (271–693) | 592 (416–803) | 668 (493–897) | <.001 | 638 (459–860) |
Abbreviations: cART, combination antiretroviral therapy; CDC, Centers for Disease Control and Prevention; HBsAg, hepatitis B virus surface antigen; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; IQR, interquartile range; MSM, men who have sex with men; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; RNA, ribonucleic acid; SD, standard deviation.
a P values for the comparisons between HCV-positive patients (N = 695) and HCV-negative patients (N = 1148) derived from the χ2 test for independence for categorical variables and the t test or the nonparametric Mann–Whitney U test for normally or nonnormally distributed continuous variables, respectively.
Hepatitis B virus surface antigen positivity was infrequent in both HCV-seronegative patients (3.1%) and HCV-seropositive patients (2.7%). Overall, 95.6% of patients were on cART. In comparison with HCV-seronegative patients, a small but significantly higher proportion of HCV-seropositive patients were on cART. The combination of 2 nucleoside reverse-transcriptase inhibitors plus 1 nonnucleoside reverse-transcriptase inhibitor was more common in HCV-seronegative patients than in HCV-seropositive patients; the combination of 2 nucleoside reverse-transcriptase inhibitors plus 1 integrase strand transfer inhibitor, and protease inhibitor-based therapies were more common in HCV-seropositive patients than in HCV-seronegative patients. In comparison with HCV-seropositive patients, a significantly higher proportion of HCV-seronegative patients were receiving a first-line cART regimen.
The proportion of patients with an HIV-RNA load <50 copies/mL was 87.9% overall and 91.1% in patients receiving cART. Among the latter, no significant differences were found between HCV-seropositive patients and HCV-seronegative patients. However, the CD4+ T-cell count was significantly higher in HCV-seronegative patients than in HCV-seropositive patients for the whole sample (660 vs 589 cells/µL; P < .001) and for patients on cART (668 vs 592 cells/µL; P < .001).
Liver Disease in Patients With Active Hepatitis C Virus Infection
The characteristics of liver disease in the 402 patients with active HCV infection are summarized in Table 2. The HCV genotype was unknown in 35 (8.7%) patients. Among the remaining 367 patients, the most common infecting genotypes were 1a (39.0%), 4 (24.5%), 1b (18.8%), and 3 (15.5%). Of the 167 patients tested for the IL28B polymorphism, 49 (29.3%) had a CC genotype. Five (1.2%) patients who were HCV-RNA–positive were also HBsAg-positive.
Table 2.
Characteristics of Liver Disease in 402 HCV RNA-Positive Patients
| HCV Genotype, n (%) | N = 402 |
| Unknown | 35 (8.7) |
| Known | 367 (91.3) |
| 1a | 143 (39.0) |
| 4 | 90 (24.5) |
| 1b | 69 (18.8) |
| 3 | 57 (15.5) |
| 2 | 5 (1.4) |
| Mixed | 3 (0.8) |
| IL28B polymorphism, n (%) | |
| Unknown | 235 (58.5) |
| Known | 167 (41.5) |
| CC | 49 (29.3) |
| CT | 89 (53.3) |
| TT | 29 (17.4) |
| Transient elastography (TE) results | |
| Patients with TE, n (%) | 345 (85.8) |
| Months from TE to study date, median (IQR) | 7.7 (3.5–15.5) |
| TE value – kPa, median (IQR) | 8.3 (5.9–13.0) |
| TE distribution according to cutoff values – kPa, n (%) | |
| <7.1 | 153 (44.3) |
| 7.1–9.5 | 41 (11.9) |
| 9.6–12.5 | 60 (17.4) |
| >12.5 | 91 (26.4) |
| FIB-4 results | |
| Patients with FIB-4, n (%) | 398 (99.0) |
| FIB-4 value, median (IQR) | 1.7 (1.2–2.7) |
| FIB-4 distribution according to cutoff values, n (%) | |
| ≤1 | 62 (15.6) |
| 1–3.25 | 265 (66.6) |
| ≥3.25 | 71 (17.8) |
| Anti-HCV therapy, n (%) | |
| Never | 233 (58.0) |
| Ongoing | 98 (24.4) |
| In the past | 71 (17.6) |
| Null response or partial response | 31 |
| Relapse | 22 |
| Discontinuation or interruption due to adverse events | 16 |
| Sustained viral responsea | 2 |
Abbreviations: FIB-4, fibrosis-4; HCV, hepatitis C virus; IQR, interquartile range; RNA, ribonucleic acid.
a Patients with HCV reinfection after successful treatment of hepatitis C.
Transient elastography was performed in 345 patients (85.8%) a median of 7.7 months before the study. The median liver stiffness value was 8.3 kPa. The distribution of liver stiffness by cutoff was as follows: <7.1 kPa (absent or mild liver fibrosis), 153 patients (44.3%); >9.5 kPa (advanced fibrosis), 151 patients (43.8%); and >12.5 kPa (cirrhosis), 91 patients (26.4%) [10]. In addition, the fibrosis-4 (FIB-4) score was available for 398 (99.0%) patients, of whom 71 (17.8%) had values >3.25 (indicative of advanced liver fibrosis) [11].
A total of 98 (24.4%) patients were receiving anti-HCV therapy during the study (68 interferon-free regimens and 30 interferon-based regimens), and 71 (17.6%) had previously received anti-HCV therapy (67 interferon-based regimens and 4 interferon-free regimens) (Supplementary Material). The distribution of patients who were naive for anti-HCV therapy according to liver stiffness was as follows: 113 of 153 (73.9%) for patients with liver stiffness <7.1 kPa, 49 of 151 (32.4%) for patients with liver stiffness >9.5 kPa, and 25 of 91 (27.5%) for patients with liver stiffness >12.5 kPa. Two (0.5%) patients with active HCV infection were considered reinfected after SVR with pegylated interferon plus ribavirin.
Liver Cirrhosis
Of the 1867 patients included in the study, 152 had cirrhosis, which was present in 93 of 402 (23.1%) patients with active HCV infection, 39 of 170 (22.9%) patients who cleared HCV after anti-HCV therapy, and 20 of the remaining 1295 (1.5%) patients.
A summary of the main features of liver cirrhosis in patients with active HCV infection and in those who cleared HCV infection after anti-HCV therapy is shown in Table 3. The most frequent method of diagnosis of cirrhosis in both groups was transient elastography.
Table 3.
Features of Liver Cirrhosis in Patients With Active HCV Infection and in Patients Who Cleared HCV Infection After Anti-HCV Therapy
| Feature | Active HCV Infection |
Clearance of HCV After Anti-HCV Therapy |
P Valuea |
|---|---|---|---|
| N = 402 | N = 170 | ||
| Liver cirrhosis, n (%) | 93 (23.1) | 39 (22.9) | .96 |
| Method of diagnosis (mutually exclusive), n (%) | .685 | ||
| Transient elastography | 82 (88.2) | 36 (92.3) | |
| Liver biopsy | 2 (2.1) | 1 (2.6) | |
| Clinical/biological diagnosis | 9 (9.7) | 2 (5.1) | |
| Decompensated cirrhosis, n (%) | 17 (18.3) | 2 (5.1) | .05 |
| Hepatocellular carcinoma, n (%) | 3 (3.2) | 1 (2.6) | .84 |
| Child-Pugh stage, n (%) | .497 | ||
| Stage A (5–6) | 74 (79.6) | 33 (84.6) | |
| Stage B (7–9) | 16 (17.2) | 6 (15.4) | |
| Stage C (10–15) | 3 (3.2) | 0 | |
| MELD score, median (IQR) | 8.5 (7.0–11.5) | 6.4 (6.4–8.6) | .01 |
| Serum albumin, median (IQR) | 4.0 (3.4–4.3) | 4.5 (4.0–4.6) | <.001 |
| FIB-4 | |||
| Patients with FIB-4, n (%) | 93 | 39 | |
| FIB-4 value, median (IQR) | 2.8 (1.6–4.8) | 1.9 (1.1–2.7) | .014 |
| FIB-4 distribution, n (%) | .073 | ||
| ≤1 | 11 (11.8) | 5 (12.8) | |
| 1–3.25 | 44 (47.3) | 26 (66.7) | |
| ≥3.25 | 38 (40.9) | 8 (20.5) | |
| Transient elastography | |||
| Patients with TE, n (%) | 87 (93.5) | 37 (94.8) | .943 |
| Months from last TE to study date, median (IQR) | 5.9 (2.7–15.9) | 11.5 (5.2–20.0) | .014 |
| Last TE value – kPa, median (IQR) | 19.0 (14.3–29.1) | 16.4 (12.1–21.4) | .071 |
| Last TE value distribution – kPa, n (%) | .019 | ||
| <7.1 | 0 | 3 (8.1) | |
| 7.1–9.5 | 1 (1.2) | 2 (5.4) | |
| 9.6–12.5 | 9 (10.3) | 5 (13.5) | |
| >12.5 | 77 (88.5) | 27 (73.0) | |
Abbreviations: FIB-4, fibrosis-4; HCV, hepatitis C virus; IQR, interquartile range; MELD, model for end-stage liver disease; TE, transient elastography.
a P values derived from the χ2 test for independence for categorical variables and the Mann–Whitney U test for continuous variables.
The proportion of patients with decompensated liver disease was significantly higher in patients with active HCV infection (18.3%) than in those who cleared HCV after anti-HCV therapy (5.1%). In addition, those with active HCV infection had significantly higher MELD scores, significantly lower concentrations of serum albumin, and significantly higher FIB-4 scores.
Most patients with cirrhosis were evaluated using transient elastography, and the median time from the date this was performed to the date data were collected was significantly shorter in patients with active HCV infection than in patients who cleared HCV after anti-HCV therapy (5.9 months vs 11.5 months, respectively). Higher stiffness values were found in patients with active HCV infection than in patients who cleared HCV after anti-HCV therapy, independently of whether the values were computed as continuous variables or as ordinal variables (according to well established cutoffs). However, statistical significance was only reached in the second comparison. Two patients with a prior diagnosis of liver cirrhosis and who cleared HCV after anti-HCV therapy were liver recipients.
Comparison With Studies Performed in 2002 and 2009
We compared the results of this study with those of 2 nationwide studies carried out by GeSIDA in 2002 and 2009 in a similar number of centers across the same geographical areas of Spain [8, 9].
The first study included 1260 patients who acquired HIV infection by IDU (55.2%) and sexual relations between MSM (17.2%). The HCV serostatus was known in 1216 (99.5%) patients, 739 (60.8%) of whom were HCV-seropositive. The presence of HCV-RNA was tested in 520 of the 739 HCV-seropositive patients, and the result was positive in 462 (88.8%); therefore, the estimated overall prevalence of active HCV infection was 54% [8].
The second study included 1548 patients who acquired HIV infection by IDU (44.0%) and sexual relations between MSM (24.1%). Hepatitis C virus serostatus was known in 1455 (99.8%) patients, 733 (50.2%) of whom were HCV-seropositive. The presence of HCV-RNA was tested in 698 of 733 HCV-seropositive patients, and the result was positive in 475 (68%); therefore, the estimated overall prevalence of active HCV infection was 34% [9].
Figure 1 summarizes the principal HIV transmission categories and the prevalence of HCV seropositivity and active HCV infection found in the nationwide cross-sectional studies carried out by GeSIDA in 2002, 2009, and 2015. The χ2 trend results showed a significant change in the mechanisms of HIV transmission categories (IDU and MSM) and in the seroprevalence of HCV and the prevalence of active HCV infection in 2002, 2009, and 2015 (P < .001 for all comparisons).
Figure 1.
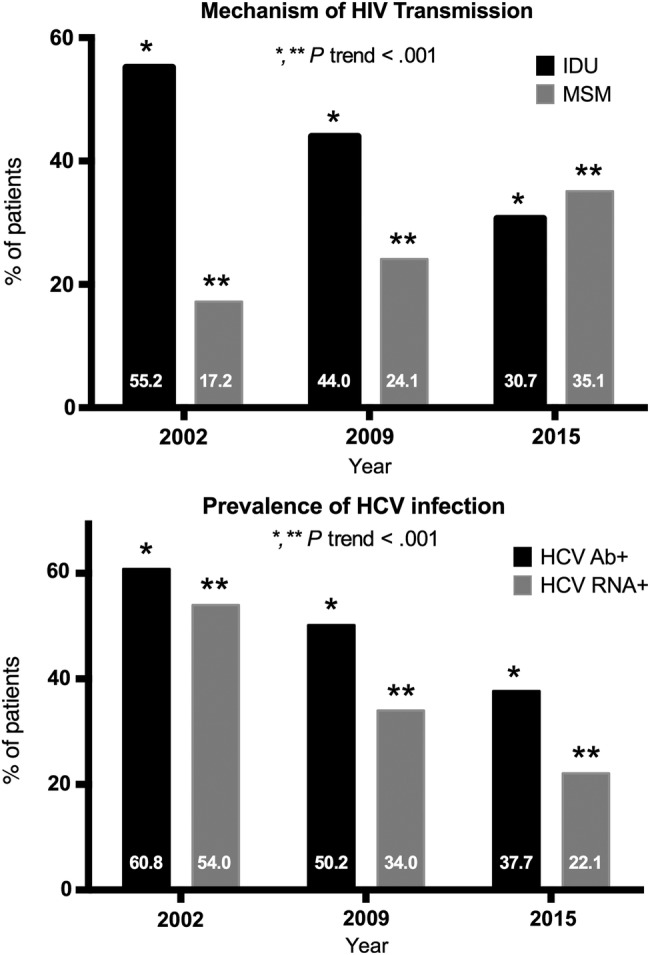
Principal human immunodeficiency virus (HIV) transmission categories and prevalence of hepatitis C virus (HCV) seropositivity and active HCV infection in the nationwide cross-sectional studies carried out by GeSIDA in 2002, 2009, and 2015. Abbreviations: HCV Ab+, presence of antibodies against HCV; HCV RNA+, detectable HCV-ribonucleic acid; IDU, injection drug use; MSM, men who have sex with men.
DISCUSSION
In this nationwide cross-sectional study, we found that the current seroprevalence of HCV and the prevalence of active HCV infection in Spain were 37.7% and 22.1%, respectively. These values are significantly lower than those found in similar studies performed by GeSIDA in 2002 and 2009 [8, 9].
The seroprevalence of HCV has fallen gradually from 60.8% in 2002 to 50.2% in 2009 to the current figure of 37.7% in 2015. This decline was mirrored by the decline in IDU as a route of HIV transmission during recent years: 55.2% in 2002, 44.0% in 2009, and 30.7% in 2015. This sharp decline in IDU as the predominant route of HIV transmission since 1997 is probably the main factor underlying the gradual decrease in the seroprevalence of HCV in Spain [6]. Other factors, such as the higher mortality rates found in HIV/HCV-coinfected patients compared with HIV-monoinfected patients during the cART era, may also have played a role [4].
Furthermore, we found that the prevalence of active HCV infection decreased markedly from 54.0% in 2002 to 34.4% in 2009 and to 22.1% in 2015, which is similar to the 18.9% reported in 2015 in a study carried out in Andalusia (Southern Spain) [12]. In addition to changes in routes of HIV acquisition and significant mortality among HIV/HCV-coinfected patients, the sharp reduction in active HCV infection can also be due to higher uptake of anti-HCV therapy in coinfected patients and the higher effectiveness of anti-HCV therapies during the last decade. In fact, the percentage of patients with current or past active HCV infection exposed to anti-HCV therapy increased from 23.0% in 2002 to 48.0% in 2009 and 59.3% in 2015. Moreover, the percentage of patients with detectable HCV RNA among those with positive anti-HCV antibody titers decreased from 87.0% in 2002 to 68.0% in 2009 and 57.8% in 2015.
Injection drug use was by far the predominant mode of HIV transmission in patients with HCV antibodies and in patients with active HCV infection. Transmission between MSM was minimal in both groups (4% and 2.7%, respectively). Although outbreaks of HCV infection among HIV-infected MSM who engage in high-risk sexual practices have been reported in Spain [7, 13], to date, this phenomenon has been restricted to specific areas of large cities such as Madrid and Barcelona. Therefore, sexually acquired HCV infection contributes little to the current burden of HIV/HCV coinfection in Spain. This observation might also explain the low frequency of patients with active HCV infection who were reinfected after SVR in this study (0.5%). In addition to the differences in the route of transmission between HCV-seronegative and HCV-seropositive patients, the latter were older, more frequently in CDC category C, and more frequently on cART. More than 90% of patients on cART had full suppression of HIV-RNA independently of their HCV serostatus; however, CD4+ T-cell counts were significantly lower in HCV-seropositive patients than in HCV-seronegative patients.
The most prevalent HCV genotypes among HIV/HCV-coinfected individuals in Spain are currently 1a and 4, which, together with genotype 3, reflect the predominance of IDU as the mode of transmission of HCV in HIV-infected patients. The frequency of genotypes 1b and 2—most commonly associated with blood transfusion and unsafe medical procedures—was accordingly somewhat lower [14]. The most conspicuous change in genotype distribution observed in the studies performed by GeSIDA was the relegation of genotypes 3 and 4 to second and third place after genotype 1 as the leading genotype. Genotype 3 caused 27% of infections in 2002 and 15.5% in 2015; in the case of genotype 4, the values for the same periods were 18% and 24.5%. We believe that the most likely explanation for this change is the selective pressure caused by interferon and ribavirin, which was the principal anti-HCV regimen used until 2012 in Spain and whose effectiveness in the context of HIV/HCV coinfection in real-life conditions has been less than half for genotypes 1 and 4 in comparison with genotypes 2 and 3 [15].
Fibrosis was staged using transient elastography in 85.8% of the 402 patients with active HCV infection. Liver stiffness indicative of absent or mild fibrosis (<7.1 kPa) was detected in 44.3% of patients. The proportion of patients who were naive for anti-HCV therapy was significantly higher in patients with no or mild fibrosis (three quarters) than in patients with advanced fibrosis (one third) or cirrhosis (one quarter), most likely owing to the common practice of postponement of anti-HCV therapy in patients with a low risk for progression because of fear of the side effects of interferon-based therapies or because of restrictive policies in the prescription of interferon-free direct antiviral agent-based therapies.
Liver cirrhosis, which is commonly confirmed using transient elastography, was identified in 23.1% of patients with active HCV infection, 22.9% of patients who cleared HCV after anti-HCV therapy, and 1.5% of the remaining patients not included in these 2 categories. In comparison with cirrhotic patients who cleared HCV after anti-HCV therapy, those with active HCV infection more frequently had decompensated disease, lower concentrations of serum albumin, and higher values in liver stiffness, MELD score, and FIB-4 score. It is noteworthy that of the 170 patients with cirrhosis who cleared HCV after anti-HCV therapy, 2 had decompensated disease and 1 had hepatocellular carcinoma at the time the study was carried out. This finding underscores the fact that despite the well known benefits of eradicating HCV in terms of reduced morbidity and mortality related and not related to liver disease [16, 17], a residual risk for liver-related events, especially hepatocellular carcinoma, persists in patients with HCV-related cirrhosis despite achievement of a SVR. This complication can arise up to 5 or more years after eradication of HCV [18]. The clinical implications of this observation are substantial, because hepatocellular carcinoma screening practices should continue for more than 5 years and probably for life in cirrhotic patients who achieve a SVR, thus greatly increasing the burden of HCV-related liver disease. This problem may be added to the list of reasons in favor of prioritizing anti-HCV treatment regardless of fibrosis stage in patients with HIV/HCV coinfection [19].
CONCLUSIONS
In conclusion, the results of this study show that the prevalence of HCV antibodies and active HCV infection in HIV-infected individuals in Spain has decreased significantly in the last decade. At present, patients with active HCV infection mainly have genotypes 1a and 4, and a high proportion have liver cirrhosis. Cirrhosis is also common in patients who have cleared HCV after anti-HCV therapy. Although this study reflects the experience of a single country, the findings provide strong arguments in favor of actively monitoring the burden of HIV/HCV coinfection on a larger scale. Such an approach is crucial in the context of new routes of HCV transmission and widespread use of direct-acting antiviral agents.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We are grateful to Herminia Esteban for administrative support during the performance of the study and to Thomas O′Boyle for writing assistance during the preparation of the manuscript.
Financial support. This study was supported by grant GLD14-00279 from the GILEAD Fellowship Programme (Spain). J. B. is an investigator from the Programa de Intensificación de la Actividad Investigadora en el Sistema Nacional de Salud (I3SNS) (Ref. no. INT15/00079).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
APPENDIX: The GeSIDA 8514 Study Group.
Hospital Gregorio Marañón, Madrid: L. Pérez-Latorre, P. Miralles, J. C. López, F. Parras, B. Padilla, T. Aldámiz, A. Carrero, C. Díez, F. Tejerina, and J. Berenguer. Hospital La Paz, Madrid: M. J. Núñez, F. Arnalich, J. R. Arribas, J. I. Bernardino, J. González-García, V. Hontañón, M. L. Martín-Carbonero, R. Montejano, M. L. Montes, V. Moreno, I. Pérez-Valero, C. Navarro, M. J. Núñez, E. Valencia, and J. González-García. Hospital Reina Sofía, Córdoba: Francisca Cuenca, A. Rivero-Román. Instituto de Salud Carlos III, Madrid: I. Jarrín. Hospital Ramón y Cajal, Madrid: M. J. Vivancos, S. Moreno, A. Moreno, J. L. Casado, M. J. Pérez-Elías, and C. Quereda. Hospital Vall d′Hebrón, Barcelona: A. Torrella, J. Navarro, N. Ramos, and M. Crespo. Hospital Clínico San Carlos, Madrid: M. Rodrigo, V. Estrada, J. Vergas, and M. J. Téllez. Hospital Santa Creu i Sant Pau, Barcelona: J. Muñoz, M. Gutiérrez, G. Mateo, J. M. Guardiola, and P. Domingo. Hospital Donostia, San Sebastián: M. Ibarguren, M. P. Carmona, F. Rodríguez-Arrondo, M. A. Goenaga, H. Azkune, M. A. Von Wichmann, and J. A. Iribarren. Hospital Doctor Peset, Valencia: J. Carmena and A. Artero. Hospital Virgen de la Victoria, Málaga: J. Ruiz, E. Nuño, R. Palacios, J. Santos, and M. Márquez. Hospital de la Princesa, Madrid: J. Sanz and I. Santos. Hospital Miguel Servet, Zaragoza: J. Moreno and P. Arazo. Hospital La Fe, Valencia: M. Montero, M. Tasias, S. Cuellar, E. Calabuig, M. Blanes, J. Fernández, J. López-Aldeguer, and M. Salavert. Hospital 12 de Octubre, Madrid: A. Hernando, L. Domínguez, O Bisbal, M. De Lagarde, M. Matarranz, Rafael Rubio, and F. Pulido. Hospital Virgen de las Nieves, Granada: C. García. Hospital Marques de Valdecilla, Santander: C. Armiñanzas, S. Echevarría, M. Gutiérrez-Cuadra, and C. Fariñas. Hospital General de Alicante, Alicante: L. Giner, S. Reus, E. Merino, V. Boix, D Torrús, I. Portilla, M. Pampliega, M. Díez, I. Egea, and J. Portilla. Hospital Universitario Basurto, Bilbao: OL Ferrero, S. Ibarra, I. López, M. de la Peña, Z Zubero, J. Baraia, and J. Muñoz. Hospital Príncipe de Asturias, Alcalá de Henares: J. de Miguel, A. Arranz, E. Casas, and J. Sanz. Hospital Clinico de Valencia, Valencia: A. Ferrer, and MJ. Galindo. Hospital San Pedro -CIBIR: Logroño: L. García, L. Pérez, and J.A. Oteo Hospital Fundación de Alcorcón, Alcorcón: M. Velasco, L. Moreno, R. Hervás, and J.E. Losa. Complejo Hospitalario Universitario de Granada, Granada: D Vinuesa, L. Muñoz, and J. Hernández-Quero, Hospital Universitari de Tarragona Joan XXIII, Tarragona: S. Veloso, J. Peraire, C. Viladés, M. Vargas, A. Castellano, and F. Vidal. Hospital San Eloy-OSI, Baracaldo: R. Silvariño. Hospital Virgen de la Cinta, Tortosa: AJ. Orti, E. Chamarro, and C. Escrig. Hospital Virgen de la Luz, Cuenca: P. Geijo. Hospital Virgen de la Concha, Zamora: A. Chocarro. Centro Sanitario Sandoval, Madrid: C. Rodríguez, T. Puerta, M. Raposo, M. Vera, and J. Del Romero. Hospital d′Olot i Comarcal de la Garrotxa, Olot: J. Bisbe. Hospital Son Llátzer, Palma de Mallorca: C. Cifuentes. Hospital de Sierrallana, Torrelavega: R. Teira. Hospital Universitari de Vic, Vic: J. Vilaró. Hospital Infanta Elena, Valdemoro: A. Vegas. Hospital Reina Sofía, Murcia: A. Cano, A. Alcaráz, A. Muñoz, and E. Bernal,. Hospital de Cabueñes, Gijón: M. Campoamor, MJ. Tuya, and B. de la Fuente Hospital Universitario de Torrejón: Torrejón de Ardoz: A. Gimeno, C. Montero, and S. Arponen Hospital de Mataró, Mataró: L. Force, and P. Barrufet. Hospital Universitario de Getafe, Getafe: G Gaspar. Hospital Rafael Méndez, Lorca: G Alonso, C. Toledo, AI Peláez, G Lara, I. Fernández, and MC Esteban.
Contributor Information
Collaborators: the GeSIDA 8514 Study Group, L. Pérez-Latorre, P. Miralles, J.C. López, F. Parras, B. Padilla, T. Aldámiz, A. Carrero, C. Díez, F. Tejerina, J. Berenguer, M.J. Núñez, F. Arnalich, J.R. Arribas, J.I. Bernardino, J. González-García, V. Hontañón, M.L. Martín-Carbonero, R. Montejano, M.L. Montes, V. Moreno, I. Pérez-Valero, C. Navarro, M.J. Núñez, E. Valencia, J. González-García, Francisca Cuenca, A. Rivero-Román, I. Jarrín, M.J. Vivancos, S. Moreno, A. Moreno, J.L. Casado, M.J. Pérez-Elías, C. Quereda, A. Torrella, J. Navarro, N. Ramos, M. Crespo, M. Rodrigo, V. Estrada, J. Vergas, M.J. Téllez, J. Muñoz, M. Gutiérrez, G. Mateo, J.M. Guardiola, P. Domingo, M. Ibarguren, M.P. Carmona, F. Rodríguez-Arrondo, M.A. Goenaga, H. Azkune, M.A. Von Wichmann, J.A. Iribarren, J. Carmena, A. Artero, J. Ruiz, E. Nuño, R. Palacios, J. Santos, M. Márquez, J. Sanz, I. Santos, J. Moreno, P. Arazo, M. Montero, M. Tasias, S. Cuellar, E. Calabuig, M. Blanes, J. Fernández, J. López-Aldeguer, M. Salavert, A. Hernando, L. Domínguez, O. Bisbal, M. De Lagarde, M. Matarranz, Rafael Rubio, F. Pulido, C. García, C. Armiñanzas, S. Echevarría, M. Gutiérrez-Cuadra, C. Fariñas, L. Giner, S. Reus, E. Merino, V. Boix, D. Torrús, I. Portilla, M. Pampliega, M. Díez, I. Egea, J. Portilla, O.L. Ferrero, S. Ibarra, I. López, M. de la Peña, Z. Zubero, J. Baraia, J. Muñoz, J. de Miguel, A. Arranz, E. Casas, J. Sanz, A. Ferrer, M.J. Galindo, L. García, L. Pérez, J.A. Oteo, M. Velasco, L. Moreno, R. Hervás, J.E. Losa, D. Vinuesa, L. Muñoz, J. Hernández-Quero, S. Veloso, J. Peraire, C. Viladés, M. Vargas, A. Castellano, F. Vidal, R. Silvariño, A.J. Orti, E. Chamarro, C. Escrig, P. Geijo, A. Chocarro, C. Rodríguez, T. Puerta, M. Raposo, M. Vera, J. Del Romero, J. Bisbe, C. Cifuentes, R. Teira, J. Vilaró, A. Vegas, A. Cano, A. Alcaráz, A. Muñoz, E. Bernal, M. Campoamor, M.J. Tuya, A. Gimeno, C. Montero, S. Arponen, L. Force, P. Barrufet, G. Gaspar, G. Alonso, C. Toledo, G. Lara, I. Fernández, and M.C. Esteban
References
- 1.Taylor LE, Swan T, Mayer KH. HIV coinfection with hepatitis C virus: evolving epidemiology and treatment paradigms. Clin Infect Dis 2012; 55:S33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham CS, Baden LR, Yu E et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001; 33:562–9. [DOI] [PubMed] [Google Scholar]
- 3.Chen TY, Ding EL, Seage GR III, Kim AY. Meta-analysis: increased mortality associated with hepatitis C in HIV-infected persons is unrelated to HIV disease progression. Clin Infect Dis 2009; 49:1605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenguer J, Alejos B, Hernando V et al. Trends in mortality according to hepatitis C virus serostatus in the era of combination antiretroviral therapy. AIDS 2012; 26:2241–6. [DOI] [PubMed] [Google Scholar]
- 5.Peters L, Mocroft A, Lundgren J et al. HIV and hepatitis C co-infection in Europe, Israel and Argentina: a EuroSIDA perspective. BMC Infect Dis 2014; 14:S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez Cachafeiro S, Del Amo J, Iribarren JA et al. Decrease in serial prevalence of coinfection with hepatitis C virus among HIV-infected patients in Spain, 1997–2006. Clin Infect Dis 2009; 48:1467–70. [DOI] [PubMed] [Google Scholar]
- 7.Montoya-Ferrer A, Fierer DS, Alvarez-Alvarez B et al. Acute hepatitis C outbreak among HIV-infected men, Madrid, Spain. Emerg Infect Dis 2011; 17:1560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Garcia JJ, Mahillo B, Hernandez S et al. [Prevalences of hepatitis virus coinfection and indications for chronic hepatitis C virus treatment and liver transplantation in Spanish HIV-infected patients. The GESIDA 29/02 and FIPSE 12185/01 Multicenter Study]. Enferm Infecc Microbiol Clin 2005; 23:340–8. [DOI] [PubMed] [Google Scholar]
- 9.González-García J, Navarro C, Condes E et al. [Evolución de la prevalencia de la coinfección por VHC, características de la hepatopatía y tratamiento específico en pacientes infectados por VIH en España. Estudio Gesida 57/07. Abstract # PO-41]. In: IV Congreso Nacional de GeSIDA. Toledo, Spain; 2012. [Google Scholar]
- 10.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol 2008; 48:835–47. [DOI] [PubMed] [Google Scholar]
- 11.Sterling RK, Lissen E, Clumeck N et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006; 43:1317–25. [DOI] [PubMed] [Google Scholar]
- 12.Rivero-Juarez A, Gutierrez-Valencia A, Castano M et al. Dimension of chronic hepatitis C virus in HIV-infected patients in the interferon-free era: an overview from south Spain. Eur J Clin Microbiol Infect Dis 2015; 34:2247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Rebollar M, Mallolas J, Perez I et al. Acute outbreak of hepatitis C in human immunodeficiency virus-infected patients. Enferm Infecc Microbiol Clin 2015; 33:3–8. [DOI] [PubMed] [Google Scholar]
- 14.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol 2008; 48:148–62. [DOI] [PubMed] [Google Scholar]
- 15.Berenguer J, Gonzalez-Garcia J, Lopez-Aldeguer J et al. Pegylated interferon {alpha}2a plus ribavirin versus pegylated interferon {alpha}2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. J Antimicrob Chemother 2009; 63:1256–63. [DOI] [PubMed] [Google Scholar]
- 16.Berenguer J, Alvarez-Pellicer J, Martin PM et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology 2009; 50:407–13. [DOI] [PubMed] [Google Scholar]
- 17.Berenguer J, Rodriguez E, Miralles P et al. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis 2012; 55:728–36. [DOI] [PubMed] [Google Scholar]
- 18.Morgan TR, Ghany MG, Kim HY et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010; 52:833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2015. J Hepatol 2015; 63:199–236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


