Abstract
Background. Cryptococcal meningitis has a high mortality in human immunodeficiency virus (HIV)-infected persons in Africa. This is preventable with early screening and preemptive therapy. We evaluated the prevalence of cryptococcal disease by antigen testing, possible associated factors, and outcomes in HIV-infected patients being managed in a tertiary hospital in Lagos, Nigeria.
Methods. Sera were collected from 214 consenting HIV-infected participants with CD4+ counts <250 cells/mm3, irrespective of their antiretroviral therapy (ART) status, between November 2014 and May 2015. A cryptococcal antigen (CrAg) lateral flow assay was used for testing. Pertinent clinical data were obtained from patients and their case notes.
Results. Of the 214 participants, females (124; 57.9%) outnumbered males. Mean age was 41.3 ± 9.4 (standard deviation) years. The majority (204; 95.3%) were ART experienced. The median CD4+ cell count was 160 cells/mm3 (interquartile range, 90–210). The overall seroprevalence of cryptococcal antigenemia was 8.9% (19 of 214); 6 of 61 (9.8%) in those with CD4+ cell counts <100 cells/mm3, 4 of 80 (5.0%) in the 100–200 group, and 9 of 73 (12.3%) in 200–250 cells/mm3 group. Among ART-naive patients, 1 of 10 (10%) was CrAg positive. Twenty-seven of 214 (12.6%) had associated oral thrush. Potential baseline meningitis symptoms (3 of 214 [1.4%] experienced neck pain or stiffness and 21 of 214 [9.8%] experienced headache) were common in the study group, but the result was not statistically significant in relation to CrAg positivity. Two of 19 (10.5%) CrAg-positive patients died, 10 of 19 (52.6%) were lost to follow up, and 7 of 19 (36.8%) were alive. Empirical fluconazole was routinely given to those with low CD4 counts <100 cells/mm3, which was unrelated to CrAg positivity (P = .018).
Conclusions. We report a prevalence of 8.9% cryptococcal antigenemia in a setting where first-line antifungals are not readily available. We recommend CrAg screening for HIV-infected patients, even for patients on ART.
Keywords: antiretroviral therapy, cryptococcal antigenemia, fluconazole, HIV infection, sub-Saharan Africa
With approximately 35.3 million individuals living with human immunodeficiency virus (HIV) and an estimated peak of 2.3 million HIV-associated deaths in 2012 [1], sub-Saharan Africa continues to struggle with a high prevalence of HIV and consequent opportunistic infections. Cryptococcal meningitis (CM) is one of the most common of these and the most common cause of meningitis among HIV-infected adults [2, 3]. A recent review by Veltman et al [4] found higher rates of CM (19%–68%) than tuberculosis meningitis (1%–36%). Nigeria has an estimated population of 3.2 million HIV-infected persons, second only to South Africa in terms of country burden.
Asymptomatic cryptococcosis is subclinical but heralds clinically obvious disease including CM by weeks to months [5–7]. Rates of asymptomatic cryptococcosis are inversely proportional to CD4 count [3, 8–11]. Reports from sub-Saharan Africa demonstrated that patients with CD4 ≤ 100 cells/µL have a cryptococcal antigen (CrAg) seroprevalence between 2.2% and 21.0% or up to 11.5% in studies, including only asymptomatic, antiretroviral therapy (ART)-naive HIV-infected patients [6, 7, 9–16].
Treatment of cryptococcosis is still below standard in most African settings [17], given the limited access to ART, poor diagnostics including lumbar puncture, the poor availability of first-line antifungal drugs used in treatment of CM, and muted uptake of recommendations for management of the increased intracranial pressure. Therefore, cryptococcal-associated mortality in Africa remains disappointingly high, ranging from 20% to 50% over 10 weeks from presentation, even in settings with available first-line drugs [18]. Limited diagnostic resources in many hospitals across sub-Saharan Africa, including an inability to perform fungal cultures or CrAg testing [19], is all too common.
Nigeria has an estimated 3.2 million HIV-infected patients, the second largest country burden in the world, after South Africa. In Benin City, a seroprevalence of CrAg of 12.7% amongst ART-naive patients [9] was used to make an estimate of 57 866 cases of cryptococcosis in Nigeria [20]. In this study, we evaluated the CrAg prevalence in HIV-infected patients in Lagos, with CD4+ count <250 cells/mm3, irrespective of their ART status.
METHODS
Study Population
This was a prospective cross-sectional study at the US President's Emergency Plan for AIDS Relief (PEPFAR) clinic, Lagos University Teaching Hospital. The clinic has over 5000 registered HIV-infected patients. Lagos is a cosmopolitan city with an estimated population of 18 million people. All consecutive consenting HIV-infected adults with CD4+ count of 250 cells/mm3 or less were recruited into the study irrespective of their ART status. The exclusion criteria were nonconsenting patients, CD4+ counts >250 cells/mm3, patients diagnosed with cryptococcosis and/or meningitis, and <18 years of age. For very sick patients, informed consent was obtained from the next of kin/surrogate if patients were too weak to give consent. Ethical approval was obtained from the institutional ethics review board. The study period was between November 2014 and May 2015.
Data Collection
A structured questionnaire was used to collect data on sociodemographic, medical history, and laboratory results. Other pertinent data such as clinical examination reports, final clinical diagnosis, drug history (including ART and antifungal medications), and viral load results were obtained from patients' case files. Participants' personal details were coded and stored in a locked file.
Sample Collection and Processing
Venous blood (5 mL) was collected from each patient into an ethylenediaminetetraacetic acid vacutinized tube. The CD4+ cell count was done first (following laboratory standard operating procedures) using the Partec Cyflow Counter (Partec, Münster, Germany). The CrAg lateral flow assay (LFA) testing was performed on the residual sample according to manufacturer's instructions (Immuno-Mycologics, Norman, OK). The LFA uses immunochromatographic test strips that have been impregnated with monoclonal antibody against capsular polysaccharide antigens common to pathogenic Cryptococcus spp [21]. Samples were stored at 2–80C for up to 72 hours if there was a delay in testing.
The Test Results Were Communicated to the Managing Clinicians to Assist in Management of the Participants
Outcome was categorized as either (1) dead or lost to follow up (LTFU) or (2) still in the PEPFAR program and currently on treatment. Patients were classified as LTFU if, at the time of conclusion of the study, at least 2 months had elapsed since the patient's last scheduled pick-up date and they did not return later [22].
Statistical Analysis
All analyses were performed using SPSS 20.0 (IBM Corp, Armonk, New York, TX) program. Medians and frequencies (percentage) were used to describe patients' characteristics. Fisher's exact test was used to compare categorical variables where appropriate. Student's t test was performed to assess the differences between means. Binary logistic regression was used to determine factors associated with positive serum CrAg. For strength of association, adjusted odds ratios and a P value of ≤.05 was considered significant.
RESULTS
A total of 214 HIV-infected outpatients attending the PEPFAR clinic were recruited. None were suspected of having clinical meningitis at sample collection, and none had been managed for cryptococcosis previously. Of these, 95.3% (204) were ART experienced (Table 1). Antiretroviral therapy naive accounted for 4.7% (10), and 2.3% (5) were ART defaulters. Females (124; 57.9%) outnumbered males (90; 42.1%) with a 1.4:1 ratio. The median age of the studied population was 40 years (interquartile range [IQR], 35–48) with a range of 19–74 years. The median CD4+ cell count was 160 cells/mm3 (IQR, 90–210). The median viral load in the 164 patients in whom it was done was 6132 (IQR, 200–116 179) with a range of 20–3.7 million copies/mL (Table 1). The duration of ART use ranged from <1 to 118 month at the conclusion of the study (Table 2).
Table 1.
Overview of Study Population Demographics and Clinical Characteristics
| Variables | CD4 Ranges (Cells/mm3) |
||
|---|---|---|---|
| <100 (n = 61) | 100–200 (n = 80) | 200–250 (n = 73) | |
| Gender | |||
| Male, n (%) | 27 (30.0%) | 32 (35.6%) | 31 (34.4%) |
| Female, n (%) | 31 (27.4%) | 48 (38.7%) | 42 (33.9%) |
| Age | |||
| Median (IQR) | 40 (37–45.7) | 40 (34–48) | 42.5 (34–51.3) |
| Viral load (copies/mL) | |||
| Median (IQR) | 46 512 (4660–158 000) | 2503.9 (110–88 569) | 1108 (78.3–11 345) |
| CD4+ cell count | |||
| Median (IQR) | 53 (29–83) | 155.5 (131–185.5) | 227 (210–247.5) |
| ART experienced, | |||
| N (%) | 60 (98.4%) | 73 (91.3%) | 71 (97.3%) |
| Fluconazole use, N (%) | 33 (54%) | 28 (35%) | 15 (20.5%) |
| Neck stiffness/pain, | |||
| N (%) | 0 (0.0%) | 1 (33.3%) | 2 (67.7%) |
| Oral thrush, | |||
| N (%) | 9 (42.9%) | 8 (38.1%) | 4 (19.0%) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range.
Table 2.
Cryptococcal Antigenemia-Positive Patients to Date Clinical Characteristics and Outcomes in Relation to Antiretroviral Therapy
| Serial No. | Date Last Seen | Outcomea | Current CD4 Count (Cells/mm3) | Last Viral Load (Copies/mL) | Duration of ART at Conclusion of Study (Months) | Current ART Regimen |
|---|---|---|---|---|---|---|
| 1 | Dec 4, 2015 | Currently in treatment | 796 | Undetectable | 37 | TDF/3TC/EFC |
| 2 | Apr 20, 2015 | Lost to follow up | 119 | 1 279 204 | 23 | TDF/3TC/EFC |
| 3 | May 5, 2015 | Died | 33 | 171 481 | 40 | TDF/3TC/ATV/r |
| 4 | Dec 23, 2015 | Currently in treatment | 214 | Undetectable | 8 | TDF/3TC/EFC |
| 5 | Oct 11, 2015 | Lost to follow up | 211 | 25 | 60 | TDF/3TC/ATV/r |
| 6 | Nov 8, 2014 | Lost to follow up | 107 | 233 867 | 20 | TDF/3TC/EFC |
| 7 | Dec 10, 2015 | Currently in treatment | 404 | Undetectable | 53 | TDF/3TC/EFC |
| 8 | Jan 12, 2016 | Current in treatment | 493 | Undetectable | 97 | AZT/3TC/NVP |
| 9 | Sept 20, 2015 | Lost to follow up | 291 | 33 | 14 | 3TC/ABC/EFV |
| 10 | Dec 21, 2015 | Lost to follow up | 352 | 25 | 117 | TDF/3TC/EFC |
| 11 | Jan 6, 2016 | Currently in treatment | 135 | 30 468 | 60 | TDF/3TC/ATV/r |
| 12 | Sept 26, 2015 | Lost to follow up | 115 | 85 290 | 19 | TDF/3TC/EFC |
| 13 | Jan 13, 2016 | Currently in treatment | 297 | 25 | 98 | TDF/3TC/ATV/r |
| 14 | Jan 7, 2016 | Currently in treatment | 294 | 2077 | 118 | TDF/3TC/ATV/r/AZT |
| 15 | Sept 26, 2015 | Lost to follow up | 221 | 144 000 | 33 | AZT/3TC/NVP |
| 16 | Sept 7, 2015 | Lost to follow up | 228 | 33 101 | 97 | AZT/3TC/NVP |
| 17 | Jan 15, 2015 | Lost to follow up | 85 | 301 266 | 22 | TDF/3TC/EFC |
| 18 | Aug 31, 2015 | Lost to follow up | 31 | 206 382 | 63 | TDF/3TC/ATV/r |
| 19 | May 20, 2015 | Died | 4 | 474 966 | <1 | TDF/3TC/EFC |
Abbreviations: ABC, abacavir; ART, antiretroviral therapy; ATV/r, atazanavir/ritonavir; AZT, zidovudine; EFV, efavirenz; NVP, nevirapine; 3TC, lamivudine; TDF, tenofovir.
a Patients were classified as lost to follow up if, at the time of conclusion of study, at least 2 months had elapsed since the patient's last scheduled pick-up date and they did not later return. Currently in treatment refers to ART treatment.
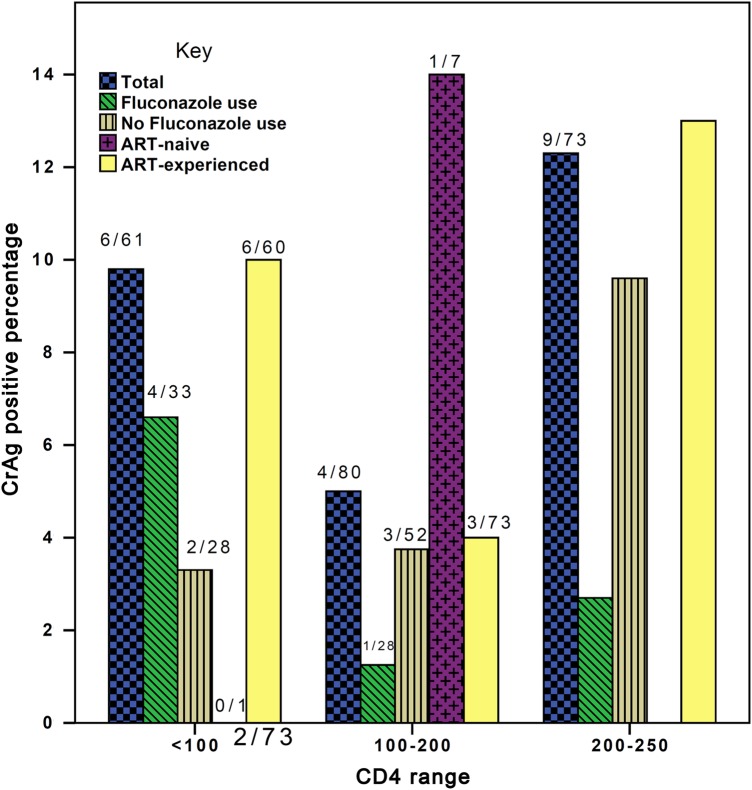
The overall prevalence of cryptococcal antigenemia was 8.9% (19 of 214) (Figure 1). The distribution of CrAg positive across the categorized CD4+ cell groups was 6 of 61 (9.8%) in those with CD4+ cell counts <100 cells/mm3, 4 of 80 (5.0%) in the 100–200 group, and 9 of 73 (12.3%) in 200–250 cells/mm3 group (Figure 1). The majority of the CrAg-positive patients were ART-experienced, whereas of the 10 ART-naive patients, 1 (10%) was CrAg positive with a CD4 cell count of 190. There was no statistical significance between CrAg-positive and CrAg-negative patients in HIV viral load (Mann–Whitney U test).
Figure 1.
Cryptococcal antigen (CrAg) positivity, fluconazole use, and antiretroviral therapy (ART) relationship.
Twenty-seven (12.61%) patients had oral thrush, and 12 of 21 (57.1%) of these patients were on fluconazole therapy. The standard preventative dose used is 200 mg daily for patients with CD4+ count <100 cells/mm3, and this is the policy in the center of study. The occurrence of oral thrush was not statistically related to CrAg positivity. Other comorbidities such as diabetes mellitus (n-1) and tuberculosis (n-2) were rare. Amongst the CrAg-positive patients, 3 (14.3%) had headache, (unrelated to lower CD4+ cell count; P = .055) and 3 patients had neck stiffness or pain (unrelated to CrAg positivity).
Overall, 7 of 19 (36.8%) of the CrAg-positive patients were on prophylactic fluconazole. Thirty-three of 61 (54%) patients with CD4+ cell count <100 cells/mm3 were on prophylactic fluconazole compared with 15 of 73 (20.5%) of those with >200 cells/mm3, whereas the 100–200 CD4+ cell count group accounted for 28 of 80 (35%) (Table 1) (P < .001). There was variability in the practice of prophylactic fluconazole administration in the studied population. Fluconazole use was more common in low CD4 count patients (P = .001), and yet 4 of 6 (67%) patients with a CD4 < 100 with a positive CrAg were on fluconazole compared with those with a CD4 > 100, of whom only 3 of 13 (23%) were on fluconazole (P = .13).
Of the 19 patients with positive CrAg results, 2 (10.5%) died of CM. All patients were observed until at least 2 months after their scheduled drug pick-up (Table 2). Ten of 19 (52.6%) were LTFU, and the remaining patients are alive and currently on ART treatment. None of the CrAg-negative patients developed CM.
The CrAg positivity results were communicated to the managing clinicians. However, no intervention measures were carried out because there is no local policy or guideline for dealing with this because CrAg testing is not routinely done in Nigeria. Lumbar puncture was performed in at least 1 of the patients with CM who was treated with high-dose (800 mg daily) fluconazole only because of nonavailability of amphotericin B and flucytosine; the patient had it for only 3 days before demise.
DISCUSSION
Our study revealed an overall prevalence of cryptococcal antigenemia of 8.9% amongst HIV-infected outpatients with CD4+ counts <250 cells/mm3, irrespective of their ART status, This result is particularly disturbing because the World Health Organization (WHO) recommends that screening for asymptomatic CrAg should be limited to ART-naive patients with CD4 counts <100 cells/mm3 [2, 9–11, 14–17]. Among the study participants in the <100 CD4+ cell count group, cryptococcal antigenemia was found in 9.8%. This prevalence is lower than the 12.7% found in Benin City, Nigeria [9]; however, that study was conducted among ART-naive patients. Among 10 ART-naive patients in our study, only 1 was CrAg positive, and that patient was in the 100–200 CD4 group; further studies will be required in this patient group.
In our study, 8.9% of patients were CrAg positive, and this is higher than the rates found in other sub-Saharan African studies such as Ghana (2.2%), Uganda (5.8%), and Tanzania (3.7%) [4]; this rate is also lower than those from Kenya (11.5%), Ethiopia (11%), and Uganda (19%) [4]. A number of reasons have been proffered for these disparities such as seasonal variations [9, 15, 23, 24]. Cryptococcus spp are acquired through inhalation, and variable intensity of local environmental presence is also a likely cause [23, 25]. Although there are few pigeons in Lagos, lack of town planning allows for poultry farms in residential areas and chickens freely roam the streets.
CD4+ cell count values in this study are probably valid because the test was conducted in the AIDS Prevention Initiative of Nigeria (APIN) laboratory, which is an offshoot of the Harvard School of Public Health PEPFAR project, and the scientists there were trained by Centers for Disease Control and Prevention personnel. The CD4+ cell count test utilizes control beads as an internal control. The laboratory does quality control with College of American Pathologists, UK NeQas, and other international agencies. In the 100–200 CD4+ group, we demonstrated a surprisingly high prevalence, comparable to that from a similar study from Ethiopia [11], which demonstrated a 14.6% prevalence of cryptococcal antigenemia. We concur with their suggestion that a likely reason for the high prevalence of cryptococcal antigenemia among persons with a CD4 > 100 is our inclusion of patients already on ART, and that cryptococcal infection occurred at a lower CD4 count before ART initiation, but they remained antigenemic as their CD4 count improved. Guidance on the management of CrAg+ patients on ART with a rising CD4 count not taking fluconazole is missing from current recommendations.
In our study site, prophylactic fluconazole has been adopted for patients with low CD4+ cell counts in view of the absence of any diagnostic test for cryptococcosis, the high mortality associated with CM, and the nonavailability of the recommended first-line drugs (amphotericin B and flucytosine) in Nigeria. A randomized clinical trial in Uganda revealed that fluconazole was highly effective and safe in the prevention of invasive cryptococcal disease, with a protective effect that occurred both before the start and in the first few months of ART [26]. It is remarkable that no deaths occurred in the fluconazole group. Others have found benefit with this strategy too, albeit at higher doses of fluconazole than we use in Lagos [27, 28]. Adoption of this preventive strategy in Lagos is one option, but not fully effective and fluconazole resistance is a concern yet to be documented in cryptococcal isolates in Nigeria but seen in Candida spp [29]. Fluconazole use was more common in low CD4 count patients as per local protocol, and 4 of 6 (67%) patients with a CD4 < 100 with a positive CrAg were on fluconazole and did not develop meningitis. In the absence of CrAg screening, this preventative strategy would seem to be justified.
An alternative strategy is routine clinical screening for CrAg, giving fluconazole to those who are positive, as advocated by WHO [17]. At this time, the point-of-care test, which we used, is affordable, easy to do, requires minimal infrastructure, and has excellent sensitivity and specificity. Screening for serum CrAg has been demonstrated to be highly sensitive, specific, and cost effective in preventing death in HIV-infected patients with CD4 counts of <100 cells/mm3 [30]. Currently, no center in Nigeria routinely screens for CrAg regardless of the CD4+ count.
Two of the patients that died developed CM in less than 3 weeks after CrAg positivity, consistent with findings by French et al [5], who noted that CrAg positivity preceded symptoms of CM by a median duration of 22 days. The Cryptococcal Optimal ART Timing (COAT) study shows that early administration of ART to patients with CM (which may be asymptomatic when early) do worse than if ART is delayed [31]. This is a major argument for proactive screening before starting ART. However, our study further buttresses the beneficial effect of ART in preventing clinical cryptococcosis infection despite cryptococcal antigenemia.
A significant limitation of this study was the limited clinical follow up of patients, although it was designed as a cross-sectional study and not a longitudinal one. Some of the patients who were LTFU might, in fact, have died without knowledge of the PEPFAR program. Not all patients had viral load assays done, thus limiting our full understanding of the impact of ART on CrAg positivity. The remarkably low number of ART-naive patients recruited surprised us, and this reflects several issues related to patient access to HIV testing and adherence. There is also the challenge of fluidity of patient clinic attendance due to phobia of stigmatization.
CONCLUSIONS
Despite Nigeria's population of 160 million, 3.2 million of whom are living with HIV infection, none have access to first-line drugs for management of CM. It is thus imperative that the public health importance of screening for cryptococcal antigenemia in the “at-risk” group be given urgent attention and included in the national guidelines in the management of HIV-infected Nigerians.
Supplementary Material
Acknowledgments
This study would not have been possible without the hard work and determination of the following people: data manager Hameed Adelabu at the US President's Emergency Plan for AIDS Relief (PEPFAR) clinic in Lagos University Teaching Hospital, Lagos, Nigeria; and Phil Foden, an excellent biostatistician who worked tirelessly to analyze the data generated.
Financial support. This study was supported in part by the Medical Education Partnership Initiative in Nigeria (MEPIN) project funded by the Forgarty International Center, the Office of AIDS Research, and the National Genome Research Institute of the National Institutes of Health, the Health Resources and Service Administration, and the Office of the U.S. Global AIDS Coordinator (award number R24TW008878).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 2014; 384:258–71. [DOI] [PubMed] [Google Scholar]
- 2.Meya D, Rajasingham R, Nalintya E et al. Preventing cryptococcosis—shifting the paradigm in the era of highly active antiretroviral therapy. Curr Trop Med Rep 2015; 2:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenney J, Smith RM, Chiller TM et al. Prevalence and correlates of cryptococcal antigen positivity among AIDS patients--United States, 1986–2012. MMWR Morb Mortal Wkly Rep 2014; 63:585–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Veltman JA, Bristow CC, Klausner JD. Meningitis in HIV-positive patients in sub-Saharan Africa: a review. J Int AIDS Soc 2014; 17:19184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French N, Gray K, Watera C et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 2002; 16:1031–8. [DOI] [PubMed] [Google Scholar]
- 6.Liechty CA, Solberg P, Were W et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health 2007; 12:929–35. [DOI] [PubMed] [Google Scholar]
- 7.Jarvis JN, Lawn SD, Vogt M et al. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis 2009; 48:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Micol R, Lortholary O, Sar B et al. Prevalence, determinants of positivity, and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J Acquir Immune Defic Syndr 2007; 45:555–9. [DOI] [PubMed] [Google Scholar]
- 9.Osazuwa F, Dirisu JO, Okuonghae PE, Ugbebor O. Screening for cryptococcal antigenemia in anti-retroviral naïve AIDS patients in Benin City, Nigeria. Oman Med J 2012; 27:228–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyella J, Meya D, Bajunirwe F, Kamya MR. Prevalence and factors associated with cryptococcal antigenemia among severely immunosuppressed HIV-infected adults in Uganda: a cross-sectional study. J Int AIDS Soc 2012; 15:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alemu AS, Kempker RR, Tenna A et al. High prevalence of cryptococcal antigenemia among HIV-infected patients receiving antiretroviral therapy in Ethiopia. PLoS One 2013; 8:e58377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tassie JM, Pepper L, Fogg C et al. Systematic screening of cryptococcal antigenemia in HIV-positive adults in Uganda. J Acquir Immune Defic Syndr 2003; 33:411–2. [DOI] [PubMed] [Google Scholar]
- 13.Mamoojee Y, Shakoor S, Gorton RL et al. Short Communication: low seroprevalence of cryptococcal antigenaemia in patients with advanced HIV infection enrolling in an antiretroviral programme in Ghana. Trop Med Int Health 2011; 16:53–6. [DOI] [PubMed] [Google Scholar]
- 14.Meyer AC, Kendi CK, Penner JA et al. The impact of routine cryptococcal antigen screening on survival among HIV-infected individuals with advanced immunosuppression in Kenya. Trop Med Int Health 2013; 18:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rugemalila J, Maro VP, Kapanda G et al. Cryptococcal antigen prevalence in HIV-infected Tanzanians: a cross-sectional study and evaluation of a point-of-care lateral flow assay. Trop Med Int Health 2013; 18:1075–9. [DOI] [PubMed] [Google Scholar]
- 16.Magambo KA, Kalluvya SE, Kapoor SW et al. Utility of urine and serum lateral flow assays to determine the prevalence and predictors of cryptococcal antigenemia in HIV-positive outpatients beginning antiretroviral therapy in Mwanza, Tanzania. J Int AIDS Soc 2014; 17:19040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. WHO Production Services; 2011. [PubMed] [Google Scholar]
- 18.Letang E, Müller MC, Ntamatungiro AJ et al. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis 2015; 2:ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen DB, Zijlstra EE, Mukaka M et al. Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop Med Int Health 2010; 15:910–7. [DOI] [PubMed] [Google Scholar]
- 20.Oladele RO, Denning DW. Burden of serious fungal infection in Nigeria. West Afr J Med 2014; 33:107–14. [PubMed] [Google Scholar]
- 21.Lindsley MD, Mekha N, Baggett HC et al. Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis 2011; 53:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meloni ST, Chang C, Chaplin B et al. Time-dependent predictors of loss to follow-up in a large HIV treatment cohort in Nigeria. Open Forum Infect Dis 2014; 1:ofu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogaerts J, Rouvroy D, Taelman H et al. AIDS-associated cryptococcal meningitis in Rwanda (1983–1992): epidemiologic and diagnostic features. J Infect 1999; 39:32–7. [DOI] [PubMed] [Google Scholar]
- 24.Hansen J, Slechta ES, Gates-Hollingsworth MA et al. Large-scale evaluation of the immuno-mycologics lateral flow and enzyme-linked immunoassays for detection of cryptococcal antigen in serum and cerebrospinal fluid. Clin Vaccine Immunol 2013; 20:52–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuroki M, Phichaichumpon C, Yasuoka A et al. Environmental isolation of Cryptococcus neoformans from an endemic region of HIV-associated cryptococcosis in Thailand. Yeast 2004; 21:809–12. [DOI] [PubMed] [Google Scholar]
- 26.Parkes-Ratanshi R, Wakeham K, Levin J et al. Primary prophylaxis of cryptococcal disease with fluconazole in HIV-positive Ugandan adults: a double-blind, randomised, placebo-controlled trial. Lancet Infect Dis 2011; 11:933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smitson CC, Tenna A, Tsegaye M et al. No association of cryptococcal antigenemia with poor outcomes among antiretroviral therapy-experienced HIV-infected patients in Addis Ababa, Ethiopia. PLoS One 2014; 9:e85698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapoor SW, Magambo KA, Kalluvya SE et al. Six-month outcomes of HIV-infected patients given short-course fluconazole therapy for asymptomatic cryptococcal antigenemia. AIDS 2015; 29:2473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pam VK, Akpan JU, Oduyebo OO et al. Fluconazole susceptibility and ERG11 gene expression in vaginal candida species isolated from Lagos Nigeria. Int J Mol Epidemiol Genet 2012; 3:84–90. [PMC free article] [PubMed] [Google Scholar]
- 30.Meya DB, Manabe YC, Castelnuovo B et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulware DR, Meya DB, Muzoora C et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370:2487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.