HCV was shed into the semen of one-third of HIV-infected MSM with recent or chronic HCV infection. Levels were plausibly sufficient to transmit HCV during unprotected anal intercourse, therefore condoms should be worn to prevent transmission.
Keywords: hepatitis C virus (HCV), HIV-infected MSM, sexual transmission, semen, shedding
Abstract
Background. The epidemic of sexually transmitted hepatitis C virus (HCV) infection among human immunodeficiency virus (HIV)-infected men who have sex with men (MSM) has been documented for over a decade. Despite this, there is no consensus as to the risk factors for sexual acquisition of HCV in these men.
Methods. We obtained paired semen and blood samples at 2-week intervals from HIV-infected MSM with recent and chronic HCV infection and quantified HCV in semen.
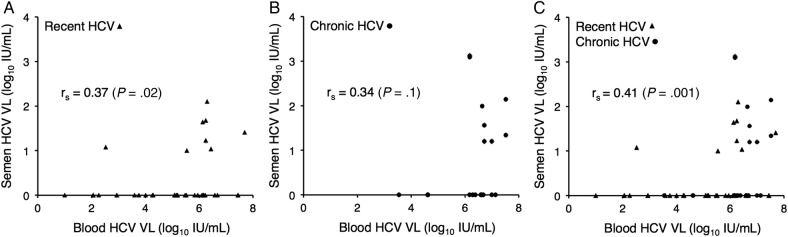
Results. Hepatitis C virus was quantified in 59 semen specimens from 33 men. Hepatitis C virus was shed in 16 (27%) of semen specimens from 11 (33%) of the men. Median HCV viral load (VL) in semen was 1.49 log10 IU/mL. Hepatitis C virus VL in blood was significantly higher at the time of HCV shedding in semen than when HCV shedding in semen was not detected (P = .002). Furthermore, there was a significant correlation between the HCV VL in blood and semen overall (rs = 0.41; P = .001), and in the subgroup with recent HCV infection (rs = 0.37; P = .02), but not in the subgroup with chronic HCV infection (rs = 0.34; P = .1).
Conclusions. One third of HIV-infected MSM coinfected with HCV shed HCV into their semen. Based on the HCV VL in semen in this study, an average ejaculate would deliver up to 6630 IU of virus into the rectum of the receptive partner. Therefore, our data strongly support that condoms should be used during anal intercourse among MSM to prevent transmission of HCV.
The epidemic of sexually transmitted hepatitis C virus (HCV) infection among human immunodeficiency virus (HIV)-infected men who have sex with men (MSM) has been well documented for over a decade [1]. Despite this, there is still no consensus as to what the risk factors are for sexual acquisition of HCV, probably because there are multiple routes of infection among MSM, and due to regional variability in sex-related practices [1]. We have previously shown that in New York City (NYC), the main sexual risk for HCV acquisition among HIV-infected MSM was receptive anal intercourse without a condom and with ejaculation of the insertive partner [2]. Witt et al [3], in a multisite US study that did not include NYC, also found unprotected receptive anal intercourse to be a significant risk factor for HCV acquisition. In contrast, a study in Germany [4] found no association with anal intercourse, and it emphasized that a subgroup of 7 (20.6%) HIV-infected MSM with recently acquired HCV reported frequent rectal trauma with bleeding, while Vanhommerig et al [5] in Amsterdam found that rectal trauma with bleeding was not associated with acquisition of HCV. Even if rectal trauma were an important risk factor for HCV acquisition, HCV would still need to be inoculated in the rectal mucosa. Because penises rarely bleed during insertive anal intercourse, a more likely source of HCV is semen.
That HCV is shed into the semen has been known for well over 2 decades [6]. The rates of shedding of HCV into semen have ranged widely among studies, between 5% and 57% [7–10]. Semen HCV viral load (VL) are significantly lower than the corresponding HCV VL in the blood [8, 11], as would be expected from a blood-semen barrier. With multiple large epidemiological studies of discordant, stable heterosexual couples showing no HCV transmissions [12–14], the general assumption has prevailed that the level of HCV in semen was insufficient to transmit HCV through sex. However, with the emerging international epidemic of HCV infection among sexually active HIV-infected MSM, and our epidemiological study results that strongly implicated semen as the source of HCV during receptive anal intercourse [2], we revisited this assumption. In this study, we enrolled HIV-infected MSM from our well characterized cohort of recently HCV-infected MSM and recruited additional men with chronic HCV infection to quantify HCV shedding in semen.
METHODS
Human immunodeficiency virus-infected MSM referred to the Mount Sinai Medical Center for the management of recent and chronic HCV infections were enrolled in this study. Written informed consent was obtained with approval of the Institutional Review Board of the Icahn School of Medicine at Mount Sinai (“Mount Sinai School of Medicine” at the time of enrollment of participants) in accordance with the Helsinki Declaration of 1975, as revised in 2000.
The date of clinical onset of HCV infection was defined as the date of the first-noted alanine aminotransferase (ALT) elevation, date of HCV antibody seroconversion, or date of first-noted HCV viremia, whichever came first. In this study, recent HCV was defined as the 1-year period after HCV seroconversion, whereas chronic HCV infection was defined as occurring after this 1-year period. We did not study seronegative acute HCV.
At the initial study visit, a detailed clinical history was obtained, including information about the likely route(s) of acquisition of HCV, and a physical was performed by one of us (D.S.F.), and medical records were obtained from the referring provider to ascertain the date of clinical onset of HCV infection. Testing for sexually transmitted infections (STIs) was performed for syphilis (rapid plasma reagin [RPR], Mount Sinai Clinical Laboratory), and testing for Chlamydia and Neisseria gonorrhea was performed on or within 1 week before the first semen specimen was collected, using nucleic acid testing on urine (APTIMA COMBO 2 Assay; Gen-Probe Inc, San Diego, CA).
Three paired blood and semen specimens were collected from participants at 2-week intervals. Hepatitis C virus VL in blood was measured using the COBAS AmpliPrep/COBAS TaqMan HCV Test (Roche Diagnostics; lower limit of quantification [LLOQ 43 IU/mL]). Collection and processing of semen was performed as previously described [15, 16]. In brief, home collection kits were provided to the participants at the first visit, which included sterile specimen containers, aliquots of sterile transport medium (80% RPMI 1640, 9% fetal bovine serum, 9% penicillin/streptomycin, and 2% nystatin, to be refrigerated at home), and printed instructions. Semen was collected at home by masturbation without lubrication into sterile containers, to which 1.7 mL of transport medium was immediately added. The specimens were brought to the clinic visit and processed within 4 hours of collection. After liquefaction at room temperature, seminal plasma and cells were separated by centrifugation at 700 x g for 12 minutes, and the seminal plasma was removed and frozen at −80℃. Ribonucleic acid (RNA) was isolated using the QIAmp Viral RNA Mini Kit (QIAGEN, Courtaboeuf, France), and HCV RNA was quantified using the (Abbott Molecular; m2000 RealTime System [LLOQ 12 IU/mL]). The determination of HCV VL was made for each semen specimen individually by adjusting for the total volume of the semen plus transport medium.
Statistical data analysis was performed using SPSS Statistics version 19.0. Pearson's χ2 test and Fisher's exact test were used for categorical variables as appropriate.
For continuous variables the Mann–Whitney U test was used, as was Spearman's rank-order correlation for 2 ranked continuous variables not normally distributed. Results with a P value of <.05 were considered statistically significant.
RESULTS
Baseline Characteristics
Thirty-three HIV-infected MSM with HCV coinfection were enrolled and provided at least 1 semen specimen between April 2013 and September 2014. Twenty-one (64%) were categorized as having recent HCV infection, 17 with primary HCV and 4 with reinfection after documented sustained virologic response (Table 1). The criteria for detection of HCV infection in those with primary HCV was a seroconversion interval of <6 months and ALT elevation >10 times the upper limit of normal (ULN) in 12 (71%); a seroconversion interval of <6 months and ALT elevation >3–5 times the ULN in 2; and a seroconversion interval of >6 months but with ALT elevation >10 times ULN in 3. The 4 reinfections were detected by either ALT elevation >10 times the ULN or detection of new HCV viremia within 6 months of a negative VL test. Twelve (36%) were categorized as having chronic HCV infection.
Table 1.
Baseline Characteristics of 33 HIV-Infected Men With Recent and Chronic HCV Infection
| Characteristic | Recent HCV n = 21 | Chronic HCV n = 12 | P Value |
|---|---|---|---|
| Median age (IQR), years | 36 (31–46) | 52 (38–55) | .007 |
| Race | |||
| White (%) | 12 (57) | 7 (58) | .6 |
| Black (%) | 4 (19) | 2 (17) | |
| Hispanic (%) | 5 (24) | 3 (25) | |
| HIV parameters | |||
| Median duration of HIV infection (IQR), years | 7 (4–12) | 10 (4–30) | .1 |
| Median CD4 count (IQR), cells/µL | 691 (537–808) | 710 (272–847) | .6 |
| Receiving cART (%) | 20 (95) | 12 (100) | .4 |
| Detectable HIV viremia (%) | 7 (33) | 8 (67) | .06 |
| STI | |||
| Reactive RPR (%) | 13 (62) | 2 (17) | .1 |
| Urethral STI test performed (%)a | 0/14 | 0/4 | – |
| HCV status | |||
| Primary infection (%) | 17 (81) | 12 (100) | .3 |
| Reinfection (%) | 4 (19) | 0 | |
| HCV genotype | |||
| 1a (%) | 20 (95) | 9 (75) | .3 |
| 1b (%) | 1 (5) | 1 (8) | |
| 2b (%) | 0 | 1 (8) | |
| 3a (%) | 0 | 1 (8) | |
| Route of HCV acquisition | |||
| Sex only (%) | 13 (62) | 6 (50) | .5 |
| Sex + IDU (%) | 8 (38) | 6 (50) | |
| Median ALT (IQR), U/L | 231 (87–492) | 62 (46–105) | .002 |
| Median blood HCV VL (IQR), log10 IU/mL | 5.52 (4.13–6.24) | 6.62 (6.21–6.93) | .006 |
Abbreviations: ALT, alanine aminotransferase; cART, combination antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; IQR, interquartile range; IU, international units; n, number; RPR, rapid plasma regain; STI, sexually transmitted infection; VL, viral load.
a Nucleic acid amplification testing for Chlamydia and Neisseria gonorrhea was performed in 14 (67%) patients with recent HCV and 4 (33%) patients with chronic HCV.
Men with recent HCV infection showed the expected differences of higher ALT levels and lower blood HCV VL than those with chronic HCV infection, and men with recent HCV infection were significantly younger (Table 1). All men in both groups reported receptive anal intercourse without a condom. Although almost half the men had also injected methamphetamine at least once, sharing of injection equipment was rare.
Blood and Semen Hepatitis C Virus Levels
A total of 63 semen specimens were collected from 33 men: 42 from men with recent HCV infection, and 21 from men with chronic HCV infection. Four (6%) specimens failed HCV VL quantification due to polymerase chain reaction inhibition, resulting in 59 evaluable semen specimens. Shedding of HCV into semen was detected in 11 (33%) of the men (Table 2) and in a total of 16 (27%) of the semen specimens (Table 3). Comparing men with and without shedding of HCV into semen, HCV VL in blood was significantly higher in the men with shedding both at baseline (P = .02) and at the time of HCV shedding (P = .002). There was no association between having a reactive RPR and detection of shedding of HCV into semen. None of the men tested positive for urethral Chlamydia or N gonorrhea.
Table 2.
Factors Associated With Detection of HCV Shedding Into Semen in 33 HIV-Infected Men With Recent and Chronic HCV Infection
| Characteristic | HCV Detected n = 11 | HCV not Detected n = 22 | P Value |
|---|---|---|---|
| Median age (IQR), years | 46 (34–54) | 40 (31–50) | .2 |
| HIV parameters | |||
| Median duration of HIV infection (IQR), years | 9 (4–12) | 9 (3–14) | 1.0 |
| Median CD4 count (IQR), cells/µL | 702 (469–837) | 691 (501–816) | .8 |
| Detectable HIV viremia (%) | 7 (64) | 8 (36) | .2 |
| Receiving cART (%) | 11 (100) | 21 (96) | 1.0 |
| Reactive RPR (%) | 6 (55) | 9 (41) | .4 |
| HCV status | |||
| Recent (%) | 6 (29) | 15 (71) | |
| Chronic (%) | 5 (42) | 7 (58) | .5 |
| Primary infection (%) | 9 (31) | 20 (69) | |
| Reinfection (%) | 2 (50) | 2 (50) | .6 |
| Time from HCV onset to first semen donation (IQR), median weeksa | 7 (3–11) | 8 (5–17) | .3 |
| Median ALT (IQR), U/Lb | 107 (61–523) | 138 (68–249) | .6 |
| Median blood HCV VL (IQR), log10 IU/mLb | 6.64 (6.18–7.00) | 5.64 (4.20–6.35) | .02 |
Abbreviations: ALT, alanine aminotransferase; cART, combination antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IU, international units; IQR, interquartile range; n, number; RPR, rapid plasma reagin; VL, viral load.
a In men with recent HCV only.
b Levels at baseline.
Table 3.
Factors Associated With Detection of HCV in 59 Semen Samples From HIV-Infected Men With Recent and Chronic HCV Infection
| Characteristic | HCV Detected n = 16 | HCV not Detected n = 43 | P Value |
|---|---|---|---|
| HCV status | |||
| Recent (%) | 8 (21) | 30 (79) | .2 |
| Chronic (%) | 8 (38) | 13 (62) | |
| Primary infection (%) | 14 (26) | 40 (74) | .6 |
| Reinfection (%) | 2 (40) | 3 (60) | |
| Time from HCV onset to semen donation (IQR), median weeksa | 8 (5–11) | 9 (6–17) | .4 |
| Median ALT (IQR), U/Lb | 107 (66–507) | 99 (60–222) | .3 |
| Volume of semen (IQR), mL | 1.5 (0.9–3.6) | 2.1 (1.2–2.8) | .8 |
| Semen HCV VL (IQR), log10 IU/mL | 1.49 (1.20–2.05) | – | – |
| Median blood HCV VL (IQR), log10 IU/mLb | 6.36 (6.18–6.93) | 5.47 (4.26–6.38) | .002 |
| Median paired blood and semen Δ HCV VL (IQR), log10 IU/mL | 5.08 (4.34–5.65) | – | – |
Abbreviations: ALT, alanine aminotransferase; Δ, difference; HCV, hepatitis C virus; n, number; IQR, interquartile range; IU, international units; VL, viral load.
a In men with recent HCV only.
b Levels at the time of semen donation.
The median HCV VL of the semen specimens in which HCV was detected was 1.49 log10 IU/mL. The median difference between blood and semen HCV VL was 5.08 log10 IU/mL. The relationship between the blood HCV VL and the detection of HCV shedding into semen is shown in Figure 1, and the correlation between the magnitude of blood and semen HCV VL is shown in Figure 2. It is interesting to note that there was a significant correlation between the HCV VL in blood and semen overall (rs = 0.41; P = .001) and in the recent HCV subgroup (rs = 0.37; P = .02), but not in the chronic HCV subgroup (rs = 0.34; P = .1) (Figure 2).
Figure 1.
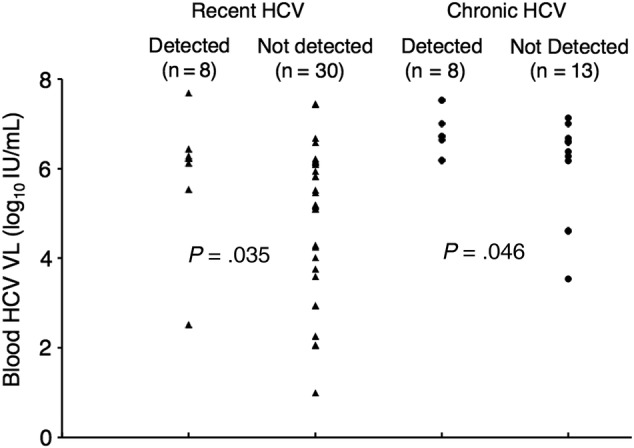
Detection of hepatitis C virus (HCV) in semen as a function of HCV viral load (VL) in blood. Triangles represent semen specimens from HIV-infected men with recent HCV infection, and closed circles represent semen specimens from HIV-infected men with chronic HCV infection.
Figure 2.
Correlation between hepatitis C virus (HCV) viral load (VL) in blood and semen. The recent HCV subgroup is shown in A; the chronic HCV subgroup is shown in B; and the combined groups are shown in C. Triangles represent semen specimens from men with recent HCV infection, and closed circles represent semen specimens from men with chronic HCV infection.
In the recent HCV subgroup, the median time between clinical onset of HCV and collection of semen specimens was 8 weeks; there was no association between detection of HCV shedding into semen and time between clinical onset of HCV and the collection of the semen specimen. Comparing the recent and chronic HCV subgroups, there were no significant differences in either the proportion of semen specimens in which HCV shedding was detected (21% and 38%, respectively; P = .2) or in the median semen HCV VL of those specimens (1.32 log10 IU/mL and 1.77 log10 IU/mL, respectively; P = .2), although the trend was toward both a higher proportion of detection and higher VL in those with chronic HCV infection.
Eleven men with recent HCV infection and 6 men with chronic HCV infection provided multiple semen specimens. Among those with recent HCV infection who provided multiple specimens, HCV shedding was detected in at least 1 specimen from 4 (36%) men, and 1 had HCV shedding detected in all specimens. Among those with chronic HCV infection who provided multiple specimens, HCV shedding was detected in at least 1 specimen in 4 (67%) men, and 3 had HCV shedding detected in all specimens.
DISCUSSION
Despite significant epidemiological [2, 3, 5, 17] and virological evidence [7–10], the role of HCV in semen in the sexual transmission of HCV among HIV-infected MSM still remains controversial. The phrases “traumatic sex” or “anal trauma” are commonly cited as necessary elements of HCV acquisition, but these factors have not been found consistently [1]. Such discordant results could have been due to insufficient statistical power to address the multiple risk factors, not asking the same question the same way in all studies, and especially important, due to regional differences in sex and drug practices. Schmidt et al [4] in Germany, found that rectal trauma with bleeding was associated with acquiring HCV in a subset of patients, yet Vanhommerig et al [5] in Amsterdam did not, nor have we (D.S.F., unpublished results). However, these discussions have largely skirted addressing the issue of the source of the HCV transmitted during sex to the anal-receptive partners.
It is interesting to note that this controversy over risk factors and source for sexually acquired HCV among MSM is reminiscent of the controversy that initially surrounded the source for sexually acquired hepatitis B virus (HBV) among MSM over 3 decades ago. Similar to HCV but in contrast to HIV, HBV is highly infectious through blood contact, and it does not infect cells present in the anogenital tract to directly mediate infection [18]. Early epidemiological studies strongly suggested that HBV was sexually transmitted among MSM, but the source of the virus was attributed to hypothesized bleeding of the penis during rough sex. However, the demonstration of HBV shedding into semen by Karayiannis et al [19] suggested HBV shedding into semen as the source of HBV transmission, rather than from the unrealistic possibility of bleeding of the penis. As a result, HBV from semen (and not blood) has now long been accepted as the source of sexually transmitted HBV. This acceptance has not, however, been the case with HCV, despite that the first publication clearly demonstrating shedding of HCV into semen was published almost 25 years ago [6] and the several epidemiological studies supporting this route in HIV-infected MSM [2, 3, 5, 17].
Based on the findings of our study, an average ejaculate [20] would deliver between 50 and 6630 IU of virus into the rectum of the receptive partner. Overall, this range of semen HCV VL is lower than those for either HBV or HIV, 2 other important viruses transmitted by semen [21, 22]. However, as few as 10–20 HCV particles delivered parenterally are required to establish infection [23]. Although HCV in semen does not appear to mediate HCV transmission in stable heterosexual discordant couples [12–14], these seemingly low HCV levels could play a significant role in sexual transmission of HCV when deposited into a rectum whose surface epithelial layer has been disrupted through anal intercourse.
A number of questions are raised by our results. Consistent with previous work [7, 8, 11], we have found that approximately one third of HIV-infected men with HCV infection shed HCV into their semen, and that this shedding is both qualitatively (ie, detected or not detected) and quantitatively related to the magnitude of blood HCV VL; however, less-so during chronic HCV infection. Shedding in semen was not always present when the blood HCV VL was high, nor was it always absent when the blood HCV VL was low, although these were significantly correlated, with 15 (94%) of 16 of specimens with detectable HCV shedding having a paired blood VL of >5 log10 IU/mL. Shedding in semen in individual men also varied qualitatively and quantitatively over the short collection period of this study (≤4 weeks), including shedding during very low-level blood HCV viremia in 2 semen specimens. Taken together, these observations suggest that although generally there may be a threshold that has to be reached for passage of HCV from blood to semen, the phenomenon is more complicated than a simple barrier that needs to be breached for HCV to enter seminal plasma. We have previously shown that HIV shedding in semen is affected by concomitant shedding of human herpes viruses (HHV) [24–27], and HHV may play a similar role in HCV shedding into semen. Studies are planned to collect these specimens and characterize these interactions.
Our study has a number of weaknesses. Testing for other STIs was performed in only just over half the men, but none were positive, so clearly STIs are not necessary for HCV shedding to occur. In addition, we were not able to obtain multiple semen specimens from all participants, which limited our ability to determine the variability of HCV shedding over time. Blood HCV VL was obtained on the day of semen collection from fewer men with chronic HCV infection than recent HCV infection. It is unlikely that this resulted in incorrect associations between blood VL and HCV shedding into semen, however, because blood VL rarely varies by more than 1 log10 IU/mL during chronic HCV infection [28, 29]. Ultimately, we believe the major limitation of this study was our inability to assess HCV shedding into semen during the ramp-up period of viremia in acute HCV infection, which occurs before HCV seroconversion [30]. This may be the period, similar to HIV infection [22], when HCV transmission linked to higher HCV shedding into semen is highest. Additional studies are needed to investigate the relationship between the timing of HCV acquisition and shedding of HCV into semen. In addition, we do not believe seminal HCV shedding is the source of HCV in all sexually acquired infections among MSM. For instance, in our experience, nonbloody fomite transmission (eg, penises, fists, sex toys) is likely to occur during group sex (D. S. F., unpublished observations, 2012), and we have found HCV in rectal fluid that may explain these transmissions (D. S. F., manuscript in preparation).
CONCLUSIONS
In summary, although more “traumatic” acts such as fisting or even vigorous penile insertion may increase susceptibility to HCV, we have demonstrated that semen often contains HCV and postulate that this semen contains sufficient virus to transmit infection to anal-receptive partners. The rectal mucosa changes generated by anal intercourse alone, without more significant trauma, may allow absorption of HCV from semen. The rectum in HIV-infected people in general may be more vulnerable to the penetration of HCV into the bloodstream due to the mucosal changes accompanying the persistent depletion of rectal CD4+ lymphocytes due to HIV infection [31]. With recent reports of sexual acquisition of HCV by MSM taking pre-exposure prophylaxis to prevent HIV infection [32] (D. S. F., unpublished results, 2014), however, pre-existing HIV infection is unlikely to be necessary for acquisition of HCV via the rectum. Therefore, our data strongly support that condoms should be used during anal intercourse among MSM to prevent HCV acquisition, regardless of HIV serostatus.
Acknowledgments
We thank the New York Acute Hepatitis C Referral Network who referred their patients to this study (Bisher Akil, MD; Juan Bailey, MD; Paul Bellman, MD; Daniel Bowers, MD; Krisczar Bungay, MD; Susanne Burger, MD; Ward Carpenter, MD; Rachel Chasan, MD; Robert Chavez, MD; Rita Chow, MD; Robert Cohen, MD; Patrick Dalton, MD; John Dellosso, MD; Adrian Demidont, DO; Stephen Dillon, MD; Eileen Donlon, NP; Terry Farrow, MD; Donald Gardenier, NP; Rodolfo Guadron, NP; Stuart Haber, MD; Lawrence Higgins, DO; Lawrence Hitzeman, MD; Ricky Hsu, MD; Shirish Huprikar, MD; Victor Inada, MD; Sneha Jacob, MD; Livette Johnson, MD; Barbara Johnston, MD; Donald Kaminsky, MD; Oscar Klein, MD; Jeffrey Kwong, NP; Jose Lares-Guia, MD; Eric Leach, NP; Randy Levine, MD; Irina Linetskaya, MD; Larisa Litvinova, MD; Amisha Malhotra, MD; William Mandell, MD; Martin Markowitz, MD; Gal Mayer, MD; Eddie Meraz, NP; Erik Mortensen, NP; Michel Ng, NP; Joseph Olivieri, MD; Charles Paolino, DO; Punyadech Photangtham, MD; George Psevdos, MD; Anita Radix, MD; Steven Rapaport, MD; Gabriela Rodriguez-Caprio, MD; William Shay, MD; Nirupama Somasundaram, NP; Lembitu Sorra, MD; Alicia Stivala, NP; Richie Tran, MD; Antonio Urbina, MD; Rona Vail, MD; Francis Wallach, MD; Wen Wang, MD; Susan Weiss, NP; and Melissa Wiener, MD). We also thank the patients who enthusiastically participated in this clinical research.
Financial support. This work was supported in part by the National Institutes of Allergy and Infectious Diseases (grants AI036214, AI100665; to D.M.S.) and the James B. Pendleton Charitable Trust (to D.M.S.).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Kaplan-Lewis E, Fierer DS. Acute HCV in HIV-infected MSM: modes of acquisition, liver fibrosis, and treatment. Curr HIV/AIDS Rep 2015; 12:317–25. [DOI] [PubMed] [Google Scholar]
- 2.Fierer DS, Uriel AJ, Carriero DC et al. . Sexual transmission of hepatitis C virus (HCV) among HIV-infected men who have sex with men (MSM), New York City, 2005–2010. MMWR Morb Mortal Wkly Rep 2011; 60:945–50. [PubMed] [Google Scholar]
- 3.Witt MD, Seaberg EC, Darilay A et al. . Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984–2011. Clin Infect Dis 2013; 57:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt AJ, Rockstroh JK, Vogel M et al. . Trouble with bleeding: risk factors for acute hepatitis C among HIV-positive gay men from Germany--a case-control study. PLoS One 2011; 6:e17781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanhommerig JW, Lambers FA, Schinkel J et al. . Risk factors for sexual transmission of hepatitis C virus among human immunodeficiency virus-Infected men who have sex with men: a case-Control study. Open Forum Infect Dis 2015; 2:ofv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liou TC, Chang TT, Young KC et al. . Detection of HCV RNA in saliva, urine, seminal fluid, and ascites. J Med Virol 1992; 37:197–202. [DOI] [PubMed] [Google Scholar]
- 7.Bourlet T, Lornage J, Maertens A et al. . Prospective evaluation of the threat related to the use of seminal fractions from hepatitis C virus-infected men in assisted reproductive techniques. Hum Reprod 2009; 24:530–5. [DOI] [PubMed] [Google Scholar]
- 8.Bradshaw D, Lamoury F, Catlett B et al. . A comparison of seminal hepatitis C virus (HCV) RNA levels during recent and chronic HCV infection in HIV-infected and HIV-uninfected individuals. J Infect Dis 2015; 211:736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leruez-Ville M, Kunstmann JM, De Almeida M et al. . Detection of hepatitis C virus in the semen of infected men. Lancet 2000; 356:42–3. [DOI] [PubMed] [Google Scholar]
- 10.Levy R, Tardy JC, Bourlet T et al. . Transmission risk of hepatitis C virus in assisted reproductive techniques. Hum Reprod 2000; 15:810–6. [DOI] [PubMed] [Google Scholar]
- 11.Briat A, Dulioust E, Galimand J et al. . Hepatitis C virus in the semen of men coinfected with HIV-1: prevalence and origin. AIDS 2005; 19:1827–35. [DOI] [PubMed] [Google Scholar]
- 12.Marincovich B, Castilla J, del Romero J et al. . Absence of hepatitis C virus transmission in a prospective cohort of heterosexual serodiscordant couples. Sex Transm Infect 2003; 79:160–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tahan V, Karaca C, Yildirim B et al. . Sexual transmission of HCV between spouses. Am J Gastroenterol 2005; 100:821–4. [DOI] [PubMed] [Google Scholar]
- 14.Vandelli C, Renzo F, Romano L et al. . Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. Am J Gastroenterol 2004; 99:855–9. [DOI] [PubMed] [Google Scholar]
- 15.Butler DM, Delport W, Kosakovsky Pond SL et al. . The origins of sexually transmitted HIV among men who have sex with men. Sci Transl Med 2010; 2:18re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianella S, Strain MC, Rought SE et al. . Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol 2012; 86:1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wandeler G, Gsponer T, Bregenzer A et al. . Hepatitis C virus infections in the Swiss HIV cohort study: a rapidly evolving epidemic. Clin Infect Dis 2012; 55:1408–16. [DOI] [PubMed] [Google Scholar]
- 18.Robinson WS. Hepatitis B virus and hepatitis D virus. In: Mandell GL, Bennet JE, Dolin R, eds. Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingstone; 2000: pp 1652–84. [Google Scholar]
- 19.Karayiannis P, Novick DM, Lok AS et al. . Hepatitis B virus DNA in saliva, urine, and seminal fluid of carriers of hepatitis B e antigen. Br Med J (Clin Res Ed) 1985; 290:1853–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rehan N, Sobrero AJ, Fertig JW. The semen of fertile men: statistical analysis of 1300 men. Fertil Steril 1975; 26:492–502. [DOI] [PubMed] [Google Scholar]
- 21.Jenison SA, Lemon SM, Baker LN, Newbold JE. Quantitative analysis of hepatitis B virus DNA in saliva and semen of chronically infected homosexual men. J Infect Dis 1987; 156:299–307. [DOI] [PubMed] [Google Scholar]
- 22.Pilcher CD, Tien HC, Eron JJ Jr.. et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis 2004; 189:1785–92. [DOI] [PubMed] [Google Scholar]
- 23.Katayama K, Kumagai J, Komiya Y et al. . Titration of hepatitis C virus in chimpanzees for determining the copy number required for transmission. Intervirology 2004; 47:57–64. [DOI] [PubMed] [Google Scholar]
- 24.Gianella S, Anderson CM, Vargas MV et al. . Cytomegalovirus DNA in semen and blood is associated with higher levels of proviral HIV DNA. J Infect Dis 2013; 207:898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gianella S, Morris SR, Anderson C et al. . Herpes viruses and HIV-1 drug resistance mutations influence the virologic and immunologic milieu of the male genital tract. AIDS 2013; 27:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gianella S, Morris SR, Vargas MV et al. . Role of seminal shedding of herpesviruses in HIV Type 1 Transmission. J Infect Dis 2013; 207:257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gianella S, Smith DM, Vargas MV et al. . Shedding of HIV and human herpesviruses in the semen of effectively treated HIV-1-infected men who have sex with men. Clin Infect Dis 2013; 57:441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen TT, Sedghi-Vaziri A, Wilkes LB et al. . Fluctuations in viral load (HCV RNA) are relatively insignificant in untreated patients with chronic HCV infection. J Viral Hepat 1996; 3:75–8. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto M, Chayama K, Kobayashi M et al. . Fluctuations of hepatitis C virus load are not related to amino acid substitutions in hypervariable region 1 and interferon sensitivity determining region. J Med Virol 1999; 58:247–55. [DOI] [PubMed] [Google Scholar]
- 30.Glynn SA, Wright DJ, Kleinman SH et al. . Dynamics of viremia in early hepatitis C virus infection. Transfusion 2005; 45:994–1002. [DOI] [PubMed] [Google Scholar]
- 31.Mehandru S, Poles MA, Tenner-Racz K et al. . Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLoS Med 2006; 3:e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volk JE, Marcus JL, Phengrasamy T, Hare CB. Incident hepatitis C virus infections among users of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis 2015; 60:1728–9. [DOI] [PMC free article] [PubMed] [Google Scholar]