Heterozygous loss-of-function mutations in the GNAL gene encoding the α subunit of the heterotrimeric G protein Golf (Gαolf) are known to cause isolated dystonia.1,2 Gαolf is enriched in the striatum where it couples D1 dopamine (D1R) and A2A adenosine (A2AR) receptors to the activation of adenylyl cyclase type 5 (AC5). Mutations in ADCY5, the gene encoding AC5, are also known to lead to chorea and dystonia.3,4 Previous functional studies of mutated Gαolf variants have revealed deficiencies in activation after D1R stimulation.1,5
Patients with heterozygous GNAL mutations typically exhibit an adult-onset focal cervical, laryngeal, and/or segmental dystonia.2 Such cases are typically either familial autosomal dominant or sporadic, resulting from de novo mutations. We describe a multiplex consanguineous Turkish family in which 2 affected children exhibited childhood-onset generalized dystonia. Affected patients were found to harbor a homozygous missense mutation in GNAL, representing biallelic mutations rather than the heterozygous GNAL mutations typically encountered.
We enrolled the patients in our ethics and institutional review board–approved research study after obtaining written informed consent. The index patient was born at term without complications. The mother took no medications during her pregnancy. Growth and early milestones were attained on time. The girl's parents first became concerned at age 1 year when she began to show evidence of exaggerated muscle tone. As the girl grew older, generalized dystonia affecting the head/neck, trunk, and limbs emerged. She experienced academic difficulties upon starting school, and was diagnosed with mild intellectual disability. At age 15 years, she exhibited generalized dystonia and impaired volitional movement. She experienced action-induced spasms and exacerbations of her baseline dystonia. Laboratory workup was unrevealing and her neuroimaging was unremarkable.
The girl's younger sister was also born at term without complications or prenatal exposures. She too met early motor and language milestones within the first year, but was noted to have hypertonia at age 1 year. She also lagged behind her peers in school and was diagnosed with mild intellectual disability. At age 11 years, she showed generalized dystonia that interfered with purposeful movements, with distressing dystonic spasms. Laboratory evaluations were nondiagnostic, and MRI of the brain was normal for age.
Given the family structure, we suspected an autosomal recessive disorder with identity by descent. Because autosomal recessive dystonia mutations have only recently been described,6 we began our studies suspecting a novel gene. We applied an approach of tandem homozygosity mapping and whole exome sequencing.
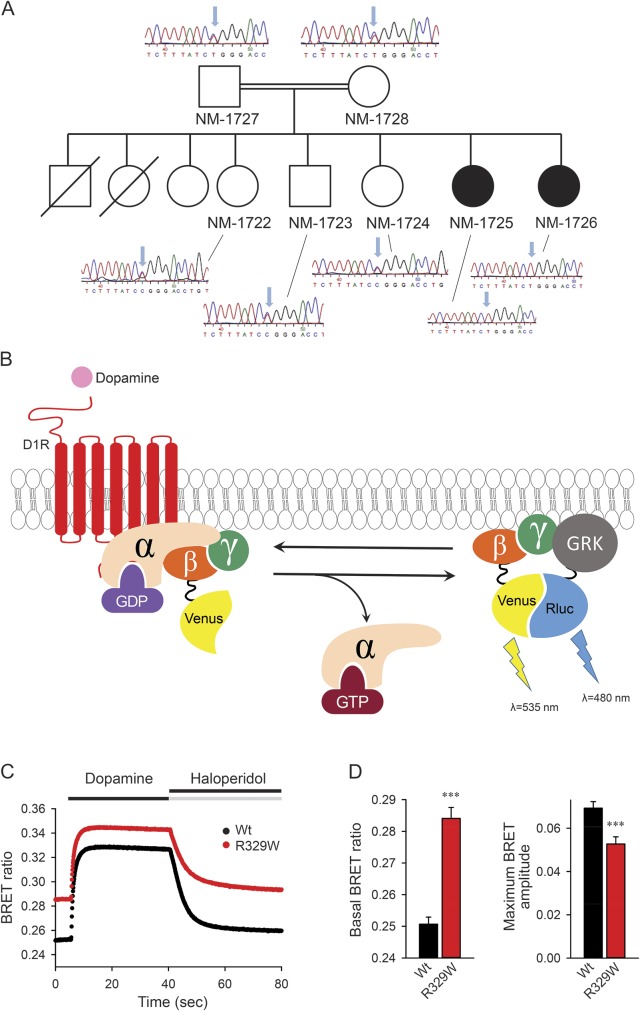
We identified 5 blocks of homozygosity ≥3 Mb shared by the affected sisters but absent from unaffected siblings. Whole exome sequencing was performed using the Complete Genomics platform (appendix e-1 at Neurology.org/ng), and revealed a novel homozygous c.1216C>T (p.R329W) missense variant in GNAL (NM_182978) within a prominent block of homozygosity on chromosome 18 (hg19; chr18:7,490,028-15,143,714). This variant was predicted to be deleterious across several algorithms (SIFT, PolyPhen-2, MutationTaster, and PhyloP) and affects a residue that is highly conserved across species. This variant was not observed in the Exome Variant Server and ExAc Browser databases, and segregated in a homozygous form with affected status in the family (figure, A). Both parents and several siblings were heterozygous but did not display any overt dystonia.
Figure. GNAL mutation and functional characterization.
(A) Family pedigrees. Filled symbols correspond to affected individuals. Empty symbols represent healthy individuals. Electropherograms show the missense mutation that leads to the (p.R293W) variant of GNAL. (B). Schematic of Gαolf functional coupling to D1R and bioluminescence resonance energy transfer (BRET) assay. Stimulation of the D1R by dopamine results in the dissociation of Gαolf from the heterotrimer. Released Gβγ subunits tagged with Venus become available for interaction with Nluc-tagged GRK reporter producing the BRET signal, which is determined by the change in the emission ratio at wavelengths 535 and 480 nm. (C) Time course. BRET signal changes upon stimulation of cells with dopamine and subsequent deactivation by haloperidol. (D) BRET ratios. Basal ratios calculated before the application of dopamine reflect the extent of the Gαolf–Gβγ heterotrimer formation, and changes in the BRET ratio from basal signal to maximal response reflect the amplitude of the response. Results represent the mean of quadruplicate wells from a typical experiment. Similar results were seen in 2 independent experiments. Error bars represent the SEM. An unpaired t test was performed to determine statistically significant differences. Asterisks indicate statistical significance from wild-type control: ***p < 0.001.
We suspected this missense change to be the probable cause of the patients' phenotype. To test this idea, we evaluated the ability of mutated Gαolf to be activated by the D1R upon reconstitution in HEK293T cells using our previously published bioluminescence resonance energy transfer (BRET) strategy1 (figure, B). In mutants, the basal BRET ratio is elevated, indicating a marked deficit in Gβγ binding and/or protein stability (figure, C). We further observed a markedly diminished activation of mutated Gαolf in response to D1R stimulation with dopamine (figure, D), indicating an additional partial loss of function.
Taken together, these findings suggest that our cases represent bona fide autosomal recessive GNAL-associated disease. The mutation seen in our patients seems to lead primarily to impaired Gαolf functional coupling to dopamine D1 receptors, differing from previously described mutations in GNAL that lead to a strict loss-of-function phenotype.1,5 It is likely that, although the coupling of Gαolf with dopamine D1 receptors is impaired, enough normal protein is present in heterozygotes to retain the ability to bind the receptor efficiently and maintain adequate biological activity. Thus, only biallelic (p.R329W) mutations impair Gαolf functional coupling enough to cause the observed phenotype. Our findings indicate that mutations in GNAL may manifest through either autosomal dominant or recessive modes of inheritance, with the present patients exhibiting generalized dystonia with onset in childhood.
Supplementary Material
Acknowledgments
Acknowledgment: The authors thank the patients and their family for their participation; without their support, this work would not have been possible.
Footnotes
Supplemental data at Neurology.org/ng
Author contributions: Dr. Masuho contributed to article conception, organization, and execution, performed functional experiments, and contributed to the review and critique of the manuscript. Dr. Fang contributed to article conception, organization, and execution, genetic analysis, and review and critique of the manuscript. Dr. Geng, Dr. Zhang, Dr. Jiang, Ms. Yarrow, and Dr. Burn contributed to genetic analysis and review and critique of the manuscript. Dr. Özgul, Dr. Yilmaz, Dr. Yalnızoğlu, and Dr. Yüksel contributed to patient phenotyping and review of the manuscript. Dr. Myers contributed to article conception, organization, and execution, genetic analysis and review and critique of the manuscript. Dr. Crotwell contributed to article conception, organization, and execution, genetic analysis, and review and critique of the manuscript. Dr. Padilla-Lopez contributed to article conception, organization, and execution, writing, review, and critique of the manuscript. Dr. Dursun contributed to patient phenotyping, article conception and organization, and review and critique of the manuscript. Dr. Martemyanov contributed to article conception, organization, and execution, oversaw functional experiments, and contributed to the review and critique of the manuscript. Dr. Kruer oversaw the project to completion, and contributed to article conception, organization, and execution, contributed to the genetic analysis, and contributed to the writing, review, and critique of the manuscript.
Study funding: Portions of this work were supported by the Shenzhen Municipal Government of China (CXB201108250094A), the NIH R01NS081282 (KAM), the Shields Award from the Child Neurology Foundation (MCK), the Dystonia Medical Research Foundation (MCK), and the Doris Duke Foundation CSDA2014112 (MCK).
Disclosure: Dr. Masuho reports no disclosures. Dr. Fang has been an employee of BGI-Shenzhen and has received research support from the Shenzhen Municipal Government of China. Dr. Geng has been an employee of BGI-Shenzhen and has received research support from the Shenzhen Municipal Government of China. Dr. Zhang has been an employee of BGI-Shenzhen and has received research support from the Shenzhen Municipal Government of China. Dr. Jiang has been an employee of BGI-Shenzhen and has received research support from the Shenzhen Municipal Government of China. Dr. Öment, Dr. Yılmaz, Dr. Yalnızoğlu, Dr. Yüksel, Ms. Yarrow, Dr. Myers, Dr. Burn, Dr. Crotwell, Dr. Padilla-Lopez, and Dr. Dursun report no disclosures. Dr. Martemyanov has served on the editorial board of the Journal of Biological Chemistry; and has received research support from NIH. Dr. Kruer has served as a grant reviewer for the Department of Defense, has served as an advisory committee member for Lundbeck, Inc., has served on the speakers' bureau of the Huntington Disease Society of America, receives grant support from Retrophin, Inc., has received research support from NIH/National Institute of Neurological Disorders and Stroke and the Doris Duke Charitable Foundation, and has been involved in legal proceedings as a consultant for HRSA National Vaccine Injury & Compensation Program. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Fuchs T, Saunders-Pullman R, Masuho I, et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet 2013;45:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vemula SR, Puschmann A, Xiao J, et al. Role of Gα(olf) in familial and sporadic adult-onset primary dystonia. Hum Mol Genet 2013;22:2510–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YZ, Matsushita MM, Robertson P, et al. Autosomal dominant familial dyskinesis and facial myokymia: single exome sequencing identifies a mutation in adenylyl cyclase 5. Arch Neurol 2012;69:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carapito R, Paul N, Untrau M, et al. A de novo ADCY5 mutation causes early-onset autosomal dominant chorea and dystonia. Mov Disord 2015;30:423–427. [DOI] [PubMed] [Google Scholar]
- 5.Kumar KR, Lohmann K, Masuho I, et al. A. Mutation in GNAL: a novel cause of craniocervical dystonia. JAMA Neurol 2014;71:490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zech M, Lam DD, Francescatto L, et al. Recessive mutations in the α3 (VI) collagen gene COL6A3 cause early-onset isolated dystonia. Am J Hum Genet 2015;96:883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.