ABSTRACT
Despite aggressive conventional therapy, lasting hemiplegia persists in a large percentage of stroke survivors. The aim of this article is to critically review the rationale behind targeting multiple sites along the motor learning network by combining robotic therapy with pharmacotherapy and virtual reality–based reward learning to alleviate upper extremity impairment in stroke survivors. Methods for personalizing pharmacologic facilitation to each individual’s unique biology are also reviewed. At the molecular level, treatment with levodopa was shown to induce long-term potentiation-like and practice-dependent plasticity. Clinically, trials combining conventional therapy with levodopa in stroke survivors yielded statistically significant but clinically unconvincing outcomes because of limited personalization, standardization, and reproducibility. Robotic therapy can induce neuroplasticity by delivering intensive, reproducible, and functionally meaningful interventions that are objective enough for the rigors of research. Robotic therapy also provides an apt platform for virtual reality, which boosts learning by engaging reward circuits. The future of stroke rehabilitation should target distinct molecular, synaptic, and cortical sites through personalized multimodal treatments to maximize motor recovery.
Key Words: Dopamine, Levodopa, Neuroplasticity, Robot-assisted Therapy, Stroke
Stroke is the third leading cause of death worldwide and a major cause of severe disability, with a projected total cost of $95 billion in the United States for 2015.1–3 With 80% of survivors experiencing upper limb paresis, it is the most common impairment following stroke. Less than half of patients with complete upper limb paralysis regain useful upper limb function after 6 mos, and complete functional recovery is regained in about one-tenth of these patients.4,5 This results in disability with independent activities of daily living. A meta-analysis showed that within the span of 6 mos post-stroke, rehabilitation should be provided to improve independence with activities of daily living.6 Despite treatment, a large number of patients continue to be permanently impaired. There is insufficient evidence to suggest that current treatments significantly impact motor recovery in the chronic phase. New therapies that enhance neuroplasticity beyond the critical 6-mo period are needed.
DOPAMINERGIC FACILITATION AND NEUROPLASTICITY
After ischemic brain damage, the spontaneous changes in regional brain activity, neuronal sprouting, and synaptic reorganization are globally referred to as neuroplasticity.7 This phenomenon denotes the central nervous system’s capacity to learn abilities, form or erase memories, and recover from injury. Evidence suggests that neuroplasticity is associated with motor and functional recuperation after stroke.8 Alternatively, disorganized neuroplasticity can lead to unsuccessful recovery.9
Harnessing organized neuroplasticity will require a solid understanding of its mechanisms so that drug targets can be trialed. Long-term potentiation (LTP) and long-term depression (LTD) are the most studied mechanisms of neuroplasticity.10 LTP is defined as the enhancement of synaptic strength11 through ionotropic12,13 and metabotropic14 glutamate receptors. On the other hand, LTD is defined as the decrease in synaptic strength.11 Although learning is mainly associated with LTP, some cortical regions, such as the cerebellum, rely on LTD for the development of new motor behaviors.15 Because both LTP and LTD explain one physiologic mechanism for motor learning, treatments that potentiate LTP and LTD could be targeted stroke rehabilitation.16
Enhancing LTP and LTD requires knowledge of the neurotransmitters that affect their formation. Several endogenous neurotransmitters, including noradrenaline,17 serotonin,18 acetylcholine,19 and dopamine,20 have been shown to regulate LTP. Although the rationale for targeting dopamine instead of the other aforementioned neurotransmitters is beyond the scope of this article, the biochemical evidence for dopaminergic modulation of LTP and LTD is robust.
One mechanism of LTP induction is mediated by a dopamine D1 receptor (D1R) signaling cascade that leads to acetylcholine release and subsequent induction of LTP.21 In addition, D1R induction also increases adenylyl cyclase activity, resulting in activation of the D1-PKA-DARPP32-PP1 complex, which is a key contributor to LTP.22 In cholinergic interneurons, activation of the dopamine D5 receptor is required for the induction of N-methyl-D-aspartate-independent LTP.20 It has also been suggested that the temporal interaction between D1Rs and metabotropic glutamate receptors may regulate the direction of synaptic plasticity (i.e., LTP or LTD), highlighting the importance of dopamine in neuroplasticity.23 The LTP-facilitating effect of dopamine follows an inverted U-shaped concentration curve.24,25 In healthy humans, doses of 100 mg of levodopa (a pharmacologic precursor of dopamine) generated a facilitatory effect, while doses of 25 and 200 mg were inhibitory.26
In addition to LTP, dopamine is also a modulator of LTD. Experiments have shown that dopamine affects LTD through several mechanisms. The dopamine D2 receptors (D2R) influence the endocannabinoid system, which is responsible for generating LTD.27 Furthermore, dopamine modulates cannabinoid receptors during the signaling cascade of LTP and LTD within many cerebellar synapses.28 Other studies demonstrated that dopamine also acts on D2Rs located on striatal interneurons to modulate LTD.29 Finally, dopamine D5 receptors located on nitric oxide synthase-positive striatal interneurons also mediate LTD.30
Taken together, this strong molecular evidence demonstrates dopaminergic modulation of neuroplasticity.
DOPAMINERGIC FACILITATION AND MOTOR LEARNING
Effective rehabilitation requires the formation of new motor memories, which is anatomically mediated by networks that connect the dorsolateral prefrontal cortex, primary motor cortex, striatum, and the cerebellum (see Fig. 1A). These structures produce motor drive, execute movements, instill reinforcement learning, and provide error-feedback learning, respectively. New motor memories are formed and pruned by the processes of LTP and LTD, which require dopaminergic signaling between the substantia nigra pars compacta and striatal medium spiny neurons in the putamen (see Fig. 1A). Within the motor loops of the basal ganglia,31 dopaminergic binding to D1Rs facilitate desired movements, whereas binding to D2Rs inhibit undesired movements.32–34
FIGURE 1.
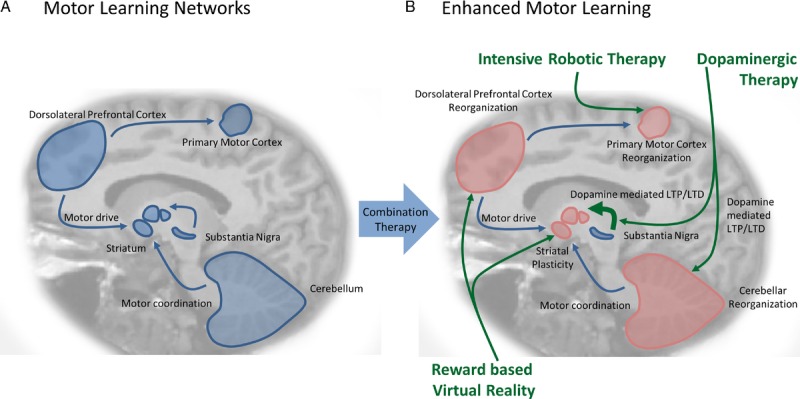
Enhancing motor learning. A, The motor learning network: Effective motor therapy requires the formation of new motor memories, which is anatomically mediated by networks that connect the dorsolateral prefrontal cortex, the striatum, and the cerebellum. These structures produce motor drive, coordinate motor drive while exerting postural control, and coordinate movements respectively. New motor memories are formed and pruned by the processes of LTP and LTD, which require dopaminergic signaling between the substantia nigra and striatal medium spiny neurons. B, Multifaceted approach to the enhancement of motor learning: Robotic therapy is an efficient medium for the delivery of intensive motor therapy and has been shown to induce primary motor cortex neuroplasticity in patients with stroke. Treatment with dopaminergics such as levodopa enhances neuroplasticity by inducing LTP and LTD in the striatum, nucleus accumbens, hippocampus and cerebellum. Virtual reality can be coupled with robotic therapy to deliver rewards during training and therefore encourage learning through the dorsolateral prefrontal cortex, orbital frontal cortex, and nucleus accumbens in the ventral striatum. These structures weigh the magnitude of rewards, process abstract rewards, and manage motivation plus reinforcement respectively.
In addition to its role in motor drive within the basal ganglia, the dopaminergic system also potentiates visuomotor integration,35,36 which is the coordination of perceptual and action-related information. At the receptor level, D1Rs are critical for proper visuomotor integration.37 This system is important for relating visualized environmental information with body position, thus enabling optimal movement planning and correction. Therefore, potentiating the coordination of motor drive and visuomotor integration through dopaminergic therapy may enhance recovery after stroke.
PHARMACOTHERAPIES AND MOTOR RECOVERY AFTER STROKE
Drugs that increase the availability of central nervous system neurotransmitters (dopamine, noradrenaline, serotonin, and acetylcholine) have been shown to exert a facilitatory effect on neuroplasticity. With this in mind, investigators have studied the effects of amphetamines, selective serotonin reuptake inhibitors, donepezil, psychostimulants such as methylphenidate, and dopaminergic agents on motor recovery after stroke. A detailed review of each agent’s effect on neuroplasticity is beyond the scope of this review. Of the aforementioned drugs, only levodopa has been shown to enhance the induction of LTP-like plasticity, practice-dependent plasticity, and motor recovery after stroke in human subjects. In addition, levodopa has a safe side effect profile and is not a controlled substance.
The most common side effect of levodopa-carbidopa (the most common medication for dopaminergic facilitation) is dyskinesia, followed by nausea, then hallucinations and dizziness. Although there is a significant risk of levodopa-induced dyskinesia in patients with Parkinson’s disease,38 the risk in patients with other conditions, such as stroke, is estimated to be much lower. In fact, levodopa has been used in numerous studies that focus on motor recovery in stroke survivors without any reports of dyskinesia and minor side effects.39–47 Levodopa has also been trialed for mood, cognition, aphasia, neglect, and restless leg syndrome, with no reports of significant dyskinesia.42,44,48,49 The literature suggests that treating stroke survivors with levodopa would unlikely cause levodopa-induced dyskinesia, unless there is comorbid basal ganglia damage or Parkinson’s disease.50
LEVODOPA THERAPY IN SUBACUTE AND CHRONIC STROKE SURVIVORS
Physical Therapy Combined with Levodopa in Subacute Stroke Survivors
Because levodopa has a relatively favorable side effect profile compared with other drugs that modulate neuroplasticity and because there is robust evidence behind dopamine’s mechanistic involvement in neuroplasticity, numerous research groups have investigated levodopa’s efficacy in poststroke motor recovery. Table 1 summarizes the studies carried out so far during the subacute stages of healing (<6 mos after stroke).
TABLE 1.
Human studies evaluating the effects of levodopa on hemiparesis during the first 6 mos after stroke onset
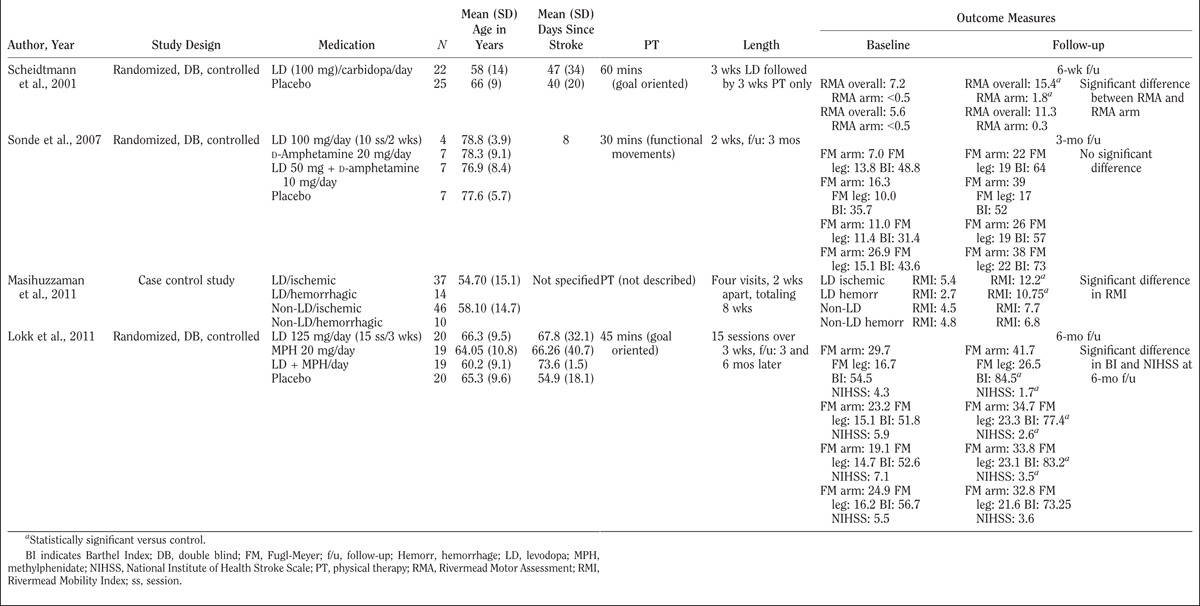
Scheidtmann et al.41 evaluated the effect of levodopa-carbidopa in combination with physical therapy on post-stroke subjects who were 3 wks to 6 mos post-stroke. The authors found that when 3 wks of levodopa-carbidopa was combined with 6 wks of physical therapy, functional motor outcomes were superior to a 6-wk course of physical therapy alone. Patients taking levodopa-carbidopa exhibited larger improvements in arm function, motor skills, and independent walking. This improvement lasted the full length of the study.
However, when Sonde et al.51 randomized patients who were 5 to 10 days post-stroke into groups combining a 2-wk course of physical therapy with either d-amphetamine, levodopa, d-amphetamine with levodopa, or placebo, they did not find any differences in Fugl-Meyer52 and Barthel Index53 scores. The most significant reasons for outcome differences between the Sonde et al. and the Scheidtmann et al. studies were stroke acuity and sample size.
Results similar to the ones reported by Scheidtmann et al.41 were obtained in a study carried out by Masihuzzaman et al.54 Stroke survivors were randomized to either physical therapy in combination with levodopa or physical therapy alone over 8 wks. Individuals who had an ischemic or hemorrhagic stroke were enrolled and their outcomes were stratified by etiology. The results showed larger improvements in the Rivermead Mobility Index55 in subjects who received physical therapy in combination with levodopa compared with those who received physical therapy alone. This observation was consistent among both the ischemic and hemorrhagic stroke groups.
In another study, Lokk et al.39 compared four groups of patients who were 15 to 180 days post-stroke. Patients were randomized to take a 3-wk course of goal-oriented physical therapy in conjunction with either levodopa, methylphenidate, levodopa plus methylphenidate, or placebo. Although the authors found no significant difference between the four groups’ Fugl-Meyer scores after 6 mos, they observed significantly different Barthel Indexes and National Institutes of Health Stroke Scale56 scores. This discrepancy in significance among the different outcome measures may reflect variability in ceiling effects. The average time of recruitment after stroke was approximately 2 mos. The average baseline Fugl-Meyer scores reported started relatively high, and all groups reached a high Fugl-Meyer ceiling by the time the study ended. Hence, it is not surprising that the authors were unable to identify significant differences across groups. Furthermore, there was high variability in pretrial motor abilities. This heterogeneity may have contributed to the inconsistent findings across different assessment scales.
It must be emphasized that the studies summarized above differ in many of their characteristics. These studies were marked by varying sample sizes, different outcome measures, diverse physical therapy durations, and a wide range of stroke acuity. The authors believe that an important source of heterogeneity among the results of these studies is stroke acuity at the time of enrollment. Stroke acuity is a variable that probably contributes the heaviest impact on significance because of the difficulty in distinguishing spontaneous endogenous recovery from the effects of the intervention. In addition, the study by Sonde et al.51 may have been underpowered. Finally, heterogeneity in lesion type and location may have been another contributor to loss of statistical power. After accounting for the above differences, one could formulate a general conclusion that the benefits of combining levodopa with physical therapy become more significant with higher dosages of physical therapy.
Physical Therapy Combined with Levodopa in Chronic Stroke Survivors
Given the challenge of distinguishing treatment benefit from endogenous recovery during the acute stages of stroke, several groups have focused on the chronic (>6 mos) phases of stroke (see Table 2).
TABLE 2.
Human studies evaluating the effects of levodopa in patients 6 mos after stroke onset
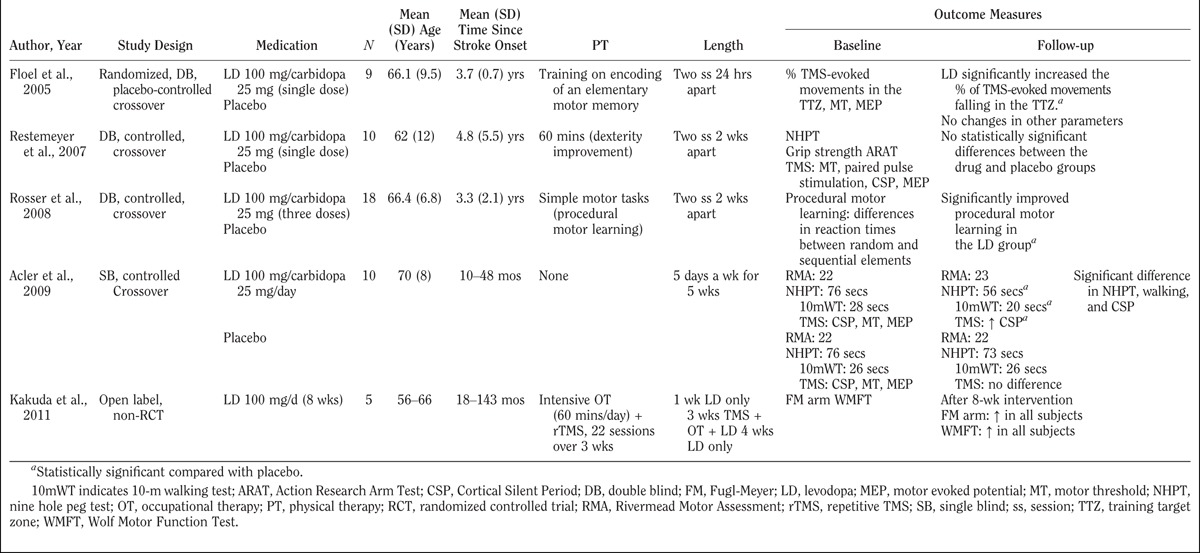
Floel et al.57 found that a single dose of levodopa could enhance the formation of training-dependent elementary motor memories. Individuals in the chronic phases post-stroke were randomized to levodopa versus placebo over a crossover design. Initially, the subjects were trained to move their thumb in the opposite direction of a transcranial magnetic stimulation (TMS)–induced basal thumb movement. Treatment with levodopa was associated with more frequent TMS-evoked movements into the trained direction when compared with placebo. There was no difference between the two groups’ movement and motor thresholds or motor evoked potential amplitudes.
In a double-blind, placebo-controlled, randomized crossover study, Rosser et al.47 demonstrated that levodopa can improve procedural motor learning in 18 patients with chronic motor dysfunction post-stroke. Procedural motor learning is defined as the ability to acquire motor skills or cognitive routines through regular exposure to a specific procedure constrained by invariant rules.58 The treatment group significantly outpaced the placebo group during completion of the training routine.
These two studies focused on the hypothesis that levodopa enhances the process of motor memory formation, first by improving elementary motor memory formation, then by enhancing the formation of procedural motor memories. These results are relevant because a primary objective in rehabilitation is to facilitate procedural motor learning by acquiring novel motor patterns.
In a crossover design, Restemeyer et al.46 evaluated the effects of a single dose of levodopa. The study used the 9 Hole Peg Test,59 the grip strength test, the Action Research Arm Test,60 and a neurophysiologic battery that measured motor threshold, paired pulse stimulation, and silent periods. A single dose of levodopa was administered before the training. After 1 hr, when peak serum level was achieved, cortical excitability was assessed. The study found no statistical difference between the levodopa group and the placebo group.
In another study, Acler et al.45 investigated the clinical and neurophysiologic outcomes of administering levodopa. This group found an improvement in 9 Hole Peg Test dexterity at the end of 5 wks. This enhancement correlated with a lengthening of the cortical silent period. After a stroke, the cortical silent period is shortened.61,62 This shortening may cause difficulties in focusing neuronal activity toward appropriate pathways. Thus, it seems that a lengthening of the cortical silent period would lead to a normalization of the cortical inhibition and result in better neuronal activity. This may stem from a possible levodopa-modulating effect on the sensorimotor integration process.
It is worth emphasizing that Acler et al.45 reached a different conclusion than Restemeyer et al.,46 perhaps because of the longer length of levodopa exposure: 5 wks versus one single dose, respectively. Furthermore, Acler et al.45 reached a different conclusion from Sonde et al.51 and Lokk et al.,39 likely because of the different criteria for subject selection. A single dose of levodopa may not be efficacious in inducing perceptible changes in cortical-excitability, or conversely, clinical outcomes may not be sensitive enough to detect the changes.
Finally, Kakuda et al.63 carried out a pilot study to assess the safety and potential benefits of combining intensive occupational therapy, low-frequency repetitive TMS, and the daily administration of 100 mg of levodopa in five post-stroke patients with upper limb hemiparesis over a period of 2 wks. The study reported significant improvements in motor function in all subjects without adverse effects.
The results of the above studies suggest that chronic stroke survivors can benefit from the coadministration of levodopa with physical therapy. They also suggest that a longer administration period of levodopa treatment allows for a higher likelihood of achieving significant benefits.
As a whole, the above studies demonstrated promising but admittedly mixed results. Arguably, stroke acuity is a major source of outcome inconsistency. This is likely because of the difficultly in teasing out spontaneous endogenous recovery from the effect of levodopa during the most acute stages of a stroke. However, stoke acuity is easily controlled for during study design. Although the lack of homogeneity in protocols, lesion type, outcome measures, and inadequate sample size all contributed to inconsistencies in the data, the authors believe that the uniformity of therapy is the most difficult variable to control for during stroke rehabilitation research.
ROBOT-ASSISTED UPPER LIMB THERAPY
In contrast to the interventions used in the above-mentioned studies, the data collected using robots are more precise than observational data from conventional therapy. Also, protocols are easier to standardize across multiple centers when compared with conventional therapy.64 Robotics can extend the applicability of high-intensity, task-oriented upper limb motor training to patients who would otherwise be excluded from traditional therapy. Because range of motion requirements for robots are less strict than those for constraint-induced movement therapy, patients who would otherwise be excluded from traditional therapy because of spasticity or motor weakness would have access to treatments via robot-assisted therapy. Patients who do not have enough motor strength to participate in repetitive task training can benefit from the gravity compensation provided by the robot. The virtual reality programs that are typically coupled to the robot can simulate training that is more functionally meaningful, such as picking fruit from a grocery store. This is important because functionally meaningful tasks are correlated with better motor memory.65,66 In addition, only minimal supervision is required from therapists. This can facilitate the implementation of home-based interventions. Finally, the economic cost of treating post-stroke upper limb impairment with robots is comparable with that of conventional therapy.67
End-Effector Systems
The most studied robot for upper limb rehabilitation is the MIT Manus system.68 The robotic arm can be programmed to facilitate or challenge the performance of arm-reaching movements. The system has undergone extensive clinical evaluation, including initial pilot studies69 and well-designed randomized clinical trials.70–73 Using this system, Lo et al.74 conducted the largest prospective randomized controlled trial performed so far with a focus on rehabilitation robotics. In the study, 127 chronic stroke survivors with moderate to severe upper limb impairments were treated with robot-assisted therapy (36 one-hour sessions over a period of 12 wks), intensive conventional therapy (matching robot-assisted therapy in dosage and intensity), or conventional therapy (i.e., usual care not dictated by the study protocol). Primary outcome measures were collected using the Fugl-Meyer motor test. The outcomes of robot-assisted therapy were found to be statistically comparable with intensive conventional therapy and superior to usual care.
Like the MIT Manus, the Mirror Image Motion Enabler75 is an end-effector system. However, the Mirror Image Motion Enabler system was specifically designed for motor training of bilateral movements. The Mirror Image Motion Enabler has also undergone significant clinical assessment.76–78 Clinical studies examined the potential benefits of motor training using the Mirror Image Motion Enabler system in 27 chronic stroke survivors,76 30 subjects in the subacute stage post-stroke,77 and 54 subjects in the acute stage post-stroke.78 The results of these clinical studies suggest that larger motor gains (as captured using the Fugl-Meyer motor test) can be achieved using robot-assisted therapy compared with conventional therapy but that the advantages are modest in size and are not retained over time.
Exoskeletons
A second category of robotic systems for rehabilitation is constituted by exoskeletons. In these systems, the robotic components are strapped to segments of the upper limb. With appropriate actuation, individual joint movements can be controlled. An example of an exoskeleton system is the ArmeoPower. The system is based on a design originally proposed by Riener et al.79 and has been recently tested in a prospective multicenter randomized clinical trial in chronic stroke survivors.80 In the study, 38 subjects were randomized to robot-assisted therapy, and 35 subjects, to conventional therapy. The intervention was based on administering 24 sessions of either robot-assisted or conventional therapy over a period of 8 wks. The study found larger motor gains (as measured using the Fugl-Meyer motor test) in the group randomized to robot-assisted therapy. However, the mean difference in Fugl-Meyer score change in response to the intervention between the two groups was modest. The study confirmed the results achieved in a previous pilot investigation by Brokaw et al.81
Actuated and Mechanically Passive Slings
A third category of robotic systems uses actuated or mechanically passive slings. An example is the Neurorehabilitation Robot.82 The system assists subjects in performing arm-reaching movements via actuation of the nylon wires of the sling strapped onto the subjects’ arm. The first pilot study on the Neurorehabilitation Robot system included 24 subjects in the acute stage post-stroke.83 The system was used as an adjunct to conventional therapy in an inpatient unit. Robot-assisted therapy consisted of 40 sessions lasting 20 to 25 mins delivered twice a day over approximately 3 wks. This pilot study showed significantly larger gains in the Fugl-Meyer motor test in the robot-assisted therapy group compared with controls. The results of the pilot study were confirmed in a follow-up study using 35 subjects.84 However, Masiero et al.85,86 did not observe greater gains when the Neurorehabilitation Robot system was used as an adjunct to conventional therapy, indicating that more research using these systems is required.
Passive Devices
Another genre of robotic devices, such as the ArmeoSpring, uses spring-based arm support systems. The ArmeoSpring was recently studied in subacute87,88 and chronic89 stroke survivors. Interestingly, although the unloading system affects the timing and amplitude of muscle activation involved in the control of upper limb movements, it does not affect the fundamental structure of physiologic muscle activation.90 These systems are simpler and less costly than traditional robotic systems, making their adoption more clinically feasible.
Recent systematic reviews91,92 of randomized clinical trials concluded that robot-assisted upper limb motor training enables improvements in motor function and activities of daily living. They have also shown that larger motor gains are often achieved with robot-assisted therapy as a replacement or adjunct to conventional therapy. These reviews also highlight the variety of robotic designs used in clinical studies. Although the above systems may differ mechanically, they remain similar in the fact that they all facilitate the performance of arm-reaching training. Other systems focus on distal functions such as the Bi-Manu-Track,93 which was designed to facilitate pronation-supination movements, and the Amadeo94 system, which was designed to facilitate grasp and release. Differences in robotic design make it difficult to compare results across studies. In addition, robotic systems for upper limb rehabilitation are typically combined with interactive games to motivate patients. Although the use of interactive games is beneficial to motor training95 (see “Virtual Reality-Based Reward Learning”), they add an additional source of variability.
Despite all of the advantages of robot-assisted therapy, the final treatment outcomes were only marginally superior to conventional therapy. Nonetheless, functional magnetic resonance imaging studies have shown that robot-assisted therapy can increase neuroplasticity (Fig. 1B) at the level of cortical reorganization.96 This makes robot-assisted therapy an ideal platform for boosting motor recovery with pharmacologic enhancement, which activates organized neuroplasticity at the synaptic level.21,97,98 Because these two treatments improve neuroplasticity at distinct levels (see Fig. 1B), their combination has the potential to augment each other and improve motor outcomes beyond the current conventional treatment. A technique with the potential to further compliment pharmacologic and robot-induced learning is virtual reality.
VIRTUAL REALITY–BASED REWARD LEARNING
Robotic therapy has the advantage of offering a highly suitable platform for the delivery of virtual reality–based reward learning during motor training. It is well known that reward enhances memory and learning.99 Virtual reality is an excellent medium to provide rewards in a rehabilitation environment. In fact, virtual and abstract rewards have been shown to be equally effective in engaging human reward circuits.99
Virtual reward–based learning relies on a network of both cortical and subcortical structures. Awareness of the neural anatomy may be important for clinicians to decide whether certain patients would benefit from reward-based training. The integrity of the orbitofrontal cortex is important for virtual and abstract reward processing.100 For instance, patients who have had damage to the prefrontal cortex may not be candidates for reward-based learning. Such patients are unable to process the magnitude of the rewards received and therefore cannot learn to choose the correct stimuli that will maximize their compensation.101 The reward processing system of the mesolimbic network has been shown via functional magnetic resonance imaging to be activated by virtual reality-based rewards.102 When subjects received unanticipated rewards for their learned behaviors in a virtual environment, the left hippocampus activated. Alternatively, the right hippocampus was activated when expected rewards were not delivered. Therefore, patients with hippocampal compromise may not be able to remember the association between reward and behavior that is necessary for reward-based learning.
Also, knowledge of the circuits that mediate reward-based learning could help clinicians decide which neurotransmitters to administer to enhance learning. The mesolimbic area uses dopamine in reward memory. The nucleus accumbens, amygdala, and hippocampus receive dopaminergic projections from the ventral tegmental area.103 Dopamine is especially important when reward delivery is predicated on behavior. When rewards are merely delivered at random, there is no dopaminergic firing or nucleus accumbens activation.104 Although rewards should be based on behavior, the evidence suggests that rewards should remain unpredictable to trainees. Ventral striatal dopaminergic neurons fire when rewards are not predicted; however, when predicted rewards are omitted, neuronal firing drops below baseline.105 The net quantity of a reward is less important than the expected quantity of a reward. Larger-than-expected rewards induced increases in dopaminergic release and LTP, whereas smaller-than-expected reward induced cessation of LTP and a decrease in dopamine level.106
Virtual Reality Specific to Stroke Rehabilitation
From a practical standpoint, virtual reality has been used as an adjunct to rehabilitation in the stroke population. (e.g., Yavuzer et al.107 and Saposnik et al.108). These studies indicate that the use of virtual reality rehabilitation is feasible for individuals with moderate motor impairments in the subacute and chronic phases post-stroke. One randomized controlled trial demonstrated that participation in virtual reality balance–related games was more effective than performing conventional exercises to maintain postural stability during walking.109 These results of improved postural stability are hypothesized to stem from adaptations of the neuromuscular system that are specific to virtual reality games.110
Furthermore, the repetitive practice of the same exercises during conventional rehabilitation leads to reduced engagement by patients.111 Creating a degree of fun can improve compliance and increase the patients’ attention span to spend more time on their rehabilitation program. Kafri et al.112 examined the effect of virtual reality on energy expenditure in patients after stroke. Participants displayed an improvement in activity and reported that they enjoyed the game. Virtual reality also provides immediate visual feedback and can therefore empower patients with a sense of control over their recovery.113,114 Also, it has been argued that the act of observing one’s own actions contributes to motor recovery by mirror motor neuron activation.115 Therefore, it is conceivable that patients who train with virtual reality may potentiate their functional improvement as they interact with the avatar presented on the screen.
Virtual reality reward–based training and robotic therapy are conveniently complimentary technologies with the potential to boost dopami nergic motor learning (Fig. 1B) and stroke recovery.
INDIVIDUALIZED TREATMENT FOR POST-STROKE REHABILITATION
Taken as a whole, the clinical studies that have tried to improve post-stroke motor recovery with levodopa-carbidopa have resulted in heterogeneous results. The authors believe that the heterogeneous results reported by these studies can be attributed to factors that are more biologically fundamental than sample size or protocol differences. It is the authors’ hypothesis that every stroke population can be divided into individuals who are either deficient in or have adequate dopaminergic signaling, aka dopaminergic tone. Perhaps, stroke survivors who respond to levodopa have a tendency toward dopamine-deficient pathology. Conversely, patients who are refractory to levodopa may be postsynaptically insensitive to dopamine or deficient in other neurotransmitter pathways such as noradrenaline or serotonin. In fact, dopamine-deficient states are not uncommon. Humans who carry the D2R polymorphism TAQ-IA express lower dopamine receptor density and lower dopaminergic tone and cannot learn from errors as efficiently as controls can.116 From a motor standpoint, age-related loss of skilled function can be rescued by levodopa administration.117
Stratifying Dopaminergic Tone
The authors propose that a more efficient way to improve motor function in stroke survivors is to start by profiling their dopaminergic balance. Health care professionals must tailor pharmacologic treatment to each individual. Dopamine deficiency could be objectively quantified through physical examination, neuropsychiatric testing, radiographic imaging, genetic polymorphisms, biomarkers, or TMS. In fact, Halstead finger tapping has been determined to be strongly correlated to dopaminergic deficiency measured by C11 raclopride binding to D2Rs in the striatum and cerebellum.118 Parkinsonian signs may not be suitable for determining dopaminergic deficiency as the movement disorder is confounded by deficiencies in other neurotransmitters119 and tauopathy.120 Certain neuropsychiatric tests also have strong correlation to “dopamine-sensitive tasks” such as the interference portions of the Stroop Color-Word Test.118,121 The written portion of the Symbol digit modalities test is also strongly correlated with dopaminergic deficiency. Positron emission tomography imaging is more expensive, time consuming, and invasive but, when coupled with C11 Raclopride, can identify dopaminergic deficiency in the striatum and cerebellum.122 Molecular biomarkers have been used to detect central dopamine deficiency states. Cerebral spinal fluid dihydroxyphenylacetic acid and dihydroxyphenylalanine concentrations were significantly lower in patients with Parkinson’s disease and multiple system atrophy than in controls.122 TMS has the ability to show that levodopa can increase the cortical silent period that is pathologically attenuated by stroke.45 A robust method for quantifying dopaminergic tone involves the summation of a set of dopamine-specific polymorphisms into a gene score. Pearson-Fuhrhop et al.123 showed that not only higher dopaminergic gene scores were associated with greater motor learning but, more importantly, also gene scores could predict how efficacious levodopa would be for certain subjects. Participants with low gene scores had low dopaminergic tone and therefore benefited from levodopa. Conversely, in subjects with high dopaminergic baselines, the addition of levodopa was detrimental to motor learning.123
It is the authors’ proposal that highly intensive robot-assisted therapy can be enhanced by levodopa if it is tailored toward stroke survivors who are most deficient in dopaminergic signaling (see Fig. 2). And it is the development of objective, noninvasive measures to accurately predict a patient’s potential response to levodopa that would represent a significant advancement in the field.
FIGURE 2.
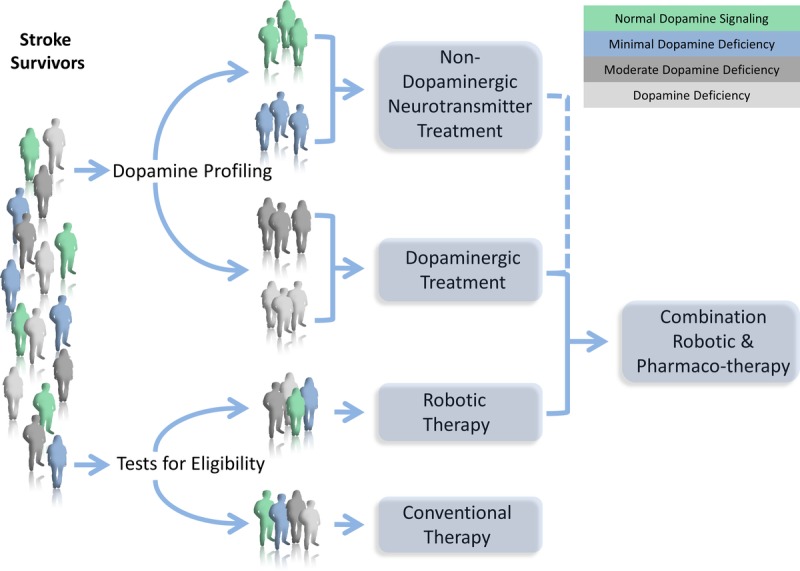
Individualized treatment for post-stoke rehabilitation. Every stroke population can theoretically be stratified into individuals who are either deficient in or have adequate dopaminergic signaling. Dopaminergic profiling could be objectively quantified by neuroimaging (lesion type, positron emission tomography), neuropsychiatric testing (Stroop color-word test, symbol digit modalities), physical examination (Halstead finger tapping), biomarkers (CSF dihydroxyphenylalanine/dihydroxyphenylacetate), dopaminergic gene polymorphisms (DAT, DRD1, DRD2, DRD3), and neuromodulation (TMS). There will likely be a gradient of baseline dopaminergic tone, and the most deficient patients would benefit from dopaminergic treatment. Patients who are profiled as non-dopamine-responders could be treated with nondopaminergic neurotransmitter treatment. The goal is to combine pharmacotherapy with robotic therapy to maximize outcomes. Eligibility for robotic therapy should include criteria such as Fugl-Meyer upper extremity scores, the modified Ashworth scale, the ability to follow commands, and the lack of limb pain.
Stroke survivors should also be screened for appropriateness for robot-assisted upper extremity training. Patients who would benefit most from robot-assisted therapy should have low Fugl-Meyer upper-limb scores, non-plegic arm spasticity worse than a Modified Ashworth of 3, minimal plegic arm pain, and intact command following.124,125 These qualifying patients should benefit from robotic therapy in combination with a pharmacotherapy that is tailored according to their biomarkers in order to maximize outcomes.
CONCLUSION
Despite aggressive conventional therapy, lasting disability remains in at least two-thirds of stroke survivors. Modern rehabilitation needs to improve upon conventional therapy by maximizing neuroplasticity, especially in the chronic phase after natural recovery has plateaued. The future of stroke rehabilitation needs to target motor recovery at multiple sites along the motor learning network by combining robotic therapy with pharmacotherapy and virtual reality-based reward learning. Furthermore, therapies need to be tailored to each individual’s unique biology. Only in this fashion will the product be more effective than the sum of each individual treatment.
ACKNOWLEDGMENTS
The authors thank Catherine Adans-Dester, BS, Massiel Dominguez, MD, Chiara Mancinelli, MS, Jorge Morales-Quezada, MD, PhD, Ryan McIntosh, BS, Nancy Torres, MD, and Sarah Javaheri, Esq.
Footnotes
Supported, in part, by grant Engineering for Neurologic Rehabilitation, NIH-NICHD, grant R24HD050821 and by grant entitled Improving Outcome Measurement for Medical Rehabilitation Clinical Trials, NIH-NICHD, grant R24HD065688. Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
REFERENCES
- 1. Norrving B, Kissela B: The global burden of stroke and need for a continuum of care. Neurology 2013; 80(3 suppl 2): S5– 12 [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014; 129: e28– 292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray CJ, Atkinson C, Bhalla K, et al. The state of US health, 1990–2010: Burden of diseases, injuries, and risk factors. JAMA 2013; 310: 591– 608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Langhorne P, Coupar F, Pollock A: Motor recovery after stroke: A systematic review. Lancet Neurol 2009; 8: 741– 54 [DOI] [PubMed] [Google Scholar]
- 5. Warlow CP, van Gijn J, Dennis MS, et al. Stroke: Practical Management. Malden, MA: Blackwell Publishing, 2008 [Google Scholar]
- 6. Kwakkel G, van Peppen R, Wagenaar RC, et al. Effects of augmented exercise therapy time after stroke: A meta-analysis. Stroke 2004; 35: 2529– 39 [DOI] [PubMed] [Google Scholar]
- 7. Nudo RJ: Postinfarct cortical plasticity and behavioral recovery. Stroke 2007; 38(2 suppl): 840– 5 [DOI] [PubMed] [Google Scholar]
- 8. Cheatwood JL, Emerick AJ, Kartje GL: Neuronal plasticity and functional recovery after ischemic stroke. Top Stroke Rehabil 2008; 15: 42– 50 [DOI] [PubMed] [Google Scholar]
- 9. Carey LM, Abbott DF, Egan GF, et al. Evolution of brain activation with good and poor motor recovery after stroke. Neurorehabil Neural Repair 2006; 20: 24– 41 [DOI] [PubMed] [Google Scholar]
- 10. Ziemann U, Meintzschel F, Korchounov A, et al. Pharmacological modulation of plasticity in the human motor cortex. Neurorehabil Neural Repair 2006; 20: 243– 51 [DOI] [PubMed] [Google Scholar]
- 11. Bliss TV, Cooke SF: Long-term potentiation and long-term depression: A clinical perspective. Clinics (Sao Paulo) 2011; 66(suppl 1): 3– 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park P, Volianskis A, Sanderson TM, et al. NMDA receptor-dependent long-term potentiation comprises a family of temporally overlapping forms of synaptic plasticity that are induced by different patterns of stimulation. Philos Trans R Soc Lond B Biol Sci 2014; 369: 20130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmitz D, Mellor J, Breustedt J, et al. Presynaptic kainate receptors impart an associative property to hippocampal mossy fiber long-term potentiation. Nat Neurosci 2003; 6: 1058– 63 [DOI] [PubMed] [Google Scholar]
- 14. Gubellini P, Saulle E, Centonze D, et al. Corticostriatal LTP requires combined mGluR1 and mGluR5 activation. Neuropharmacology 2003; 44: 8– 16 [DOI] [PubMed] [Google Scholar]
- 15. Hansel C, de Jeu M, Belmeguenai A, et al. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron 2006; 51: 835– 43 [DOI] [PubMed] [Google Scholar]
- 16. Whitlock JR, Heynen AJ, Shuler MG, et al. Learning induces long-term potentiation in the hippocampus. Science 2006; 313: 1093– 7 [DOI] [PubMed] [Google Scholar]
- 17. Tully K, Li Y, Tsvetkov E, et al. Norepinephrine enables the induction of associative long-term potentiation at thalamo-amygdala synapses. Proc Natl Acad Sci U S A 2007; 104: 14146– 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kojic L, Gu Q, Douglas RM, et al. Serotonin facilitates synaptic plasticity in kitten visual cortex: An in vitro study. Brain Res Dev Brain Res 1997; 101: 299– 304 [DOI] [PubMed] [Google Scholar]
- 19. Leung LS, Shen B, Rajakumar N, et al. Cholinergic activity enhances hippocampal long-term potentiation in CA1 during walking in rats. J Neurosci 2003; 23: 9297– 304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki T, Miura M, Nishimura K, et al. Dopamine-dependent synaptic plasticity in the striatal cholinergic interneurons. J Neurosci 2001; 21: 6492– 501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calabresi P, Picconi B, Tozzi A, et al. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 2007; 30: 211– 9 [DOI] [PubMed] [Google Scholar]
- 22. Calabresi P, Gubellini P, Centonze D, et al. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci 2000; 20: 8443– 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li C, Rainnie DG: Bidirectional regulation of synaptic plasticity in the basolateral amygdala induced by the D1-like family of dopamine receptors and group II metabotropic glutamate receptors. J Physiol 2014; 592(Pt 19): 4329– 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monte-Silva K, Liebetanz D, Grundey J, et al. Dosage-dependent non-linear effect of l-DOPA on human motor cortex plasticity. J Physiol 2010; 588(Pt 18): 3415– 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolomiets B, Marzo A, Caboche J, et al. Background dopamine concentration dependently facilitates long-term potentiation in rat prefrontal cortex through postsynaptic activation of extracellular signal-regulated kinases. Cereb Cortex 2009; 19: 2708– 18 [DOI] [PubMed] [Google Scholar]
- 26. Thirugnanasambandam N, Grundey J, Paulus W, et al. Dose-dependent nonlinear effect of l-DOPA on paired associative stimulation-induced neuroplasticity in humans. J Neurosci 2011; 31: 5294– 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kreitzer AC, Malenka RC: Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci 2005; 25: 10537– 45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grasselli G, Hansel C: Cerebellar long-term potentiation: Cellular mechanisms and role in learning. Int Rev Neurobiol 2014; 117: 39– 51 [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Kai L, Day M, et al. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 2006; 50: 443– 52 [DOI] [PubMed] [Google Scholar]
- 30. Centonze D, Grande C, Saulle E, et al. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci 2003; 23: 8506– 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wichmann T, DeLong MR: Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 1996; 6: 751– 8 [DOI] [PubMed] [Google Scholar]
- 32. Mink JW: The Basal Ganglia and involuntary movements: Impaired inhibition of competing motor patterns. Arch Neurol 2003; 60: 1365– 8 [DOI] [PubMed] [Google Scholar]
- 33. Nambu A: Seven problems on the basal ganglia. Curr Opin Neurobiol 2008; 18: 595– 604 [DOI] [PubMed] [Google Scholar]
- 34. Gerfen CR, Surmeier DJ: Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 2011; 34: 441– 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Colzato LS, van Wouwe NC, Hommel B: Feature binding and affect: Emotional modulation of visuo-motor integration. Neuropsychologia 2007; 45: 440– 6 [DOI] [PubMed] [Google Scholar]
- 36. Colzato LS, van Wouwe NC, Hommel B: Spontaneous eyeblink rate predicts the strength of visuomotor binding. Neuropsychologia 2007; 45: 2387– 92 [DOI] [PubMed] [Google Scholar]
- 37. Colzato LS, Hommel B: Cannabis, cocaine, and visuomotor integration: Evidence for a role of dopamine D1 receptors in binding perception and action. Neuropsychologia 2008; 46: 1570– 5 [DOI] [PubMed] [Google Scholar]
- 38. Factor SA: Current status of symptomatic medical therapy in Parkinson’s disease. Neurotherapeutics 2008; 5: 164– 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lokk J, Salman Roghani R, Delbari A: Effect of methylphenidate and/or levodopa coupled with physiotherapy on functional and motor recovery after stroke—A randomized, double-blind, placebo-controlled trial. Acta Neurol Scand 2011; 123: 266– 73 [DOI] [PubMed] [Google Scholar]
- 40. Gladstone DJ, Danells CJ, Armesto A, et al. Physiotherapy coupled with dextroamphetamine for rehabilitation after hemiparetic stroke: A randomized, double-blind, placebo-controlled trial. Stroke 2006; 37: 179– 85 [DOI] [PubMed] [Google Scholar]
- 41. Scheidtmann K, Fries W, Muller F, et al. Effect of levodopa in combination with physiotherapy on functional motor recovery after stroke: A prospective, randomised, double-blind study. Lancet 2001; 358: 787– 90 [DOI] [PubMed] [Google Scholar]
- 42. Leemann B, Laganaro M, Chetelat-Mabillard D, et al. Crossover trial of subacute computerized aphasia therapy for anomia with the addition of either levodopa or placebo. Neurorehabil Neural Repair 2011; 25: 43– 7 [DOI] [PubMed] [Google Scholar]
- 43. Mukand JA, Guilmette TJ, Allen DG, et al. Dopaminergic therapy with carbidopa l-DOPA for left neglect after stroke: A case series. Arch Phys Med Rehabil 2001; 82: 1279– 82 [DOI] [PubMed] [Google Scholar]
- 44. Seniow J, Litwin M, Litwin T, et al. New approach to the rehabilitation of post-stroke focal cognitive syndrome: Effect of levodopa combined with speech and language therapy on functional recovery from aphasia. J Neurol Sci 2009; 283: 214– 8 [DOI] [PubMed] [Google Scholar]
- 45. Acler M, Fiaschi A, Manganotti P: Long-term levodopa administration in chronic stroke patients: A clinical and neurophysiologic single-blind placebo-controlled cross-over pilot study. Restor Neurol Neurosci 2009; 27: 277– 83 [DOI] [PubMed] [Google Scholar]
- 46. Restemeyer C, Weiller C, Liepert J: No effect of a levodopa single dose on motor performance and motor excitability in chronic stroke: A double-blind placebo-controlled cross-over pilot study. Restor Neurol Neurosci 2007; 25: 143– 50 [PubMed] [Google Scholar]
- 47. Rosser N, Heuschmann P, Wersching H, et al. Levodopa improves procedural motor learning in chronic stroke patients. Arch Phys Med Rehabil 2008; 89: 1633– 41 [DOI] [PubMed] [Google Scholar]
- 48. Maric O, Zorner B, Dietz V: Levodopa therapy in incomplete spinal cord injury. J Neurotrauma 2008; 25: 1303– 7 [DOI] [PubMed] [Google Scholar]
- 49. von Scheele C, Kempi V: Long-term effect of dopaminergic drugs in restless legs: A 2-year follow-up. Arch Neurol 1990; 47: 1223– 4 [DOI] [PubMed] [Google Scholar]
- 50. Jenner P: Molecular mechanisms of l-DOPA-induced dyskinesia. Nat Rev Neurosci 2008; 9: 665– 77 [DOI] [PubMed] [Google Scholar]
- 51. Sonde L, Lokk J: Effects of amphetamine and/or l-DOPA and physiotherapy after stroke—A blinded randomized study. Acta Neurol Scand 2007; 115: 55– 9 [DOI] [PubMed] [Google Scholar]
- 52. Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient: 1, A method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13– 31 [PubMed] [Google Scholar]
- 53. Mahoney FI, Barthel DW: Functional evaluation: The Barthel Index. Md State Med J 1965; 14: 61– 5 [PubMed] [Google Scholar]
- 54. Masihuzzaman AM, Uddin MJ, Majumder S, et al. Effect of low dose levodopa on motor outcome of different types of stroke. Mymensingh Med J 2011; 20: 689– 93 [PubMed] [Google Scholar]
- 55. Kurtais Y, Kucukdeveci A, Elhan A, et al. Psychometric properties of the Rivermead Motor Assessment: Its utility in stroke. J Rehabil Med 2009; 41: 1055– 61 [DOI] [PubMed] [Google Scholar]
- 56. Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989; 20: 864– 70 [DOI] [PubMed] [Google Scholar]
- 57. Floel A, Breitenstein C, Hummel F, et al. Dopaminergic influences on formation of a motor memory. Ann Neurol 2005; 58: 121– 30 [DOI] [PubMed] [Google Scholar]
- 58. Rosenbaum DA, Carlson RA, Gilmore RO: Acquisition of intellectual and perceptual-motor skills. Annu Rev Psychol 2001; 52: 453– 70 [DOI] [PubMed] [Google Scholar]
- 59. Wang YC, Magasi SR, Bohannon RW, et al. Assessing dexterity function: A comparison of two alternatives for the NIH Toolbox. J Hand Ther 2011; 24: 313– 20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. van der Lee JH, Beckerman H, Lankhorst GJ, et al. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. J Rehabil Med 2001; 33: 110– 3 [DOI] [PubMed] [Google Scholar]
- 61. Ziemann U: TMS and drugs. Clin Neurophysiol 2004; 115: 1717– 29 [DOI] [PubMed] [Google Scholar]
- 62. Ziemann U, Reis J, Schwenkreis P, et al. TMS and drugs revisited 2014. Clin Neurophysiol ; 126: 2015– 68 [DOI] [PubMed] [Google Scholar]
- 63. Kakuda W, Abo M, Kobayashi K, et al. Combination treatment of low-frequency rTMS and occupational therapy with levodopa administration: An intensive neurorehabilitative approach for upper limb hemiparesis after stroke. Int J Neurosci 2011; 121: 373– 8 [DOI] [PubMed] [Google Scholar]
- 64. Loureiro RC, Harwin WS, Nagai K, et al. Advances in upper limb stroke rehabilitation: A technology push. Med Biol Eng Comput 2011; 49: 1103– 18 [DOI] [PubMed] [Google Scholar]
- 65. Bayona NA, Bitensky J, Salter K, et al. The role of task-specific training in rehabilitation therapies. Top Stroke Rehabil 2005; 12: 58– 65 [DOI] [PubMed] [Google Scholar]
- 66. Nudo RJ: Adaptive plasticity in motor cortex: Implications for rehabilitation after brain injury. J Rehabil Med 2003;(41 suppl): 7– 10 [DOI] [PubMed] [Google Scholar]
- 67. Wagner TH, Lo AC, Peduzzi P, et al. An economic analysis of robot-assisted therapy for long-term upper-limb impairment after stroke. Stroke 2011; 42: 2630– 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Krebs HI, Hogan N, Aisen ML, et al. Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng 1998; 6: 75– 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Aisen ML, Krebs HI, Hogan N, et al. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Arch Neurol 1997; 54: 443– 6 [DOI] [PubMed] [Google Scholar]
- 70. Volpe BT, Krebs HI, Hogan N, et al. A novel approach to stroke rehabilitation: Robot-aided sensorimotor stimulation. Neurology 2000; 54: 1938– 44 [DOI] [PubMed] [Google Scholar]
- 71. Daly JJ, Hogan N, Perepezko EM, et al. Response to upper-limb robotics and functional neuromuscular stimulation following stroke. J Rehabil Res Dev 2005; 42: 723– 36 [DOI] [PubMed] [Google Scholar]
- 72. Volpe BT, Lynch D, Rykman-Berland A, et al. Intensive sensorimotor arm training mediated by therapist or robot improves hemiparesis in patients with chronic stroke. Neurorehabil Neural Repair 2008; 22: 305– 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Conroy SS, Whitall J, Dipietro L, et al. Effect of gravity on robot-assisted motor training after chronic stroke: A randomized trial. Arch Phys Med Rehabil 2011; 92: 1754– 61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med 2010; 362: 1772– 83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Burgar CG, Lum PS, Shor PC, et al. Development of robots for rehabilitation therapy: The Palo Alto VA/Stanford experience. J Rehabil Res Dev 2000; 37: 663– 73 [PubMed] [Google Scholar]
- 76. Lum PS, Burgar CG, Shor PC, et al. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil 2002; 83: 952– 9 [DOI] [PubMed] [Google Scholar]
- 77. Lum PS, Burgar CG, Van der Loos M, et al. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: A follow-up study. J Rehabil Res Dev 2006; 43: 631– 42 [DOI] [PubMed] [Google Scholar]
- 78. Burgar CG, Lum PS, Scremin AM, et al. Robot-assisted upper-limb therapy in acute rehabilitation setting following stroke: Department of Veterans Affairs multisite clinical trial. J Rehabil Res Dev 2011; 48: 445– 58 [DOI] [PubMed] [Google Scholar]
- 79. Riener R, Nef T, Colombo G: Robot-aided neurorehabilitation of the upper extremities. Med Biol Eng Comput 2005; 43: 2– 10 [DOI] [PubMed] [Google Scholar]
- 80. Klamroth-Marganska V, Blanco J, Campen K, et al. Three-dimensional, task-specific robot therapy of the arm after stroke: A multicentre, parallel-group randomised trial. Lancet Neurol 2014; 13: 159– 66 [DOI] [PubMed] [Google Scholar]
- 81. Brokaw EB, Nichols D, Holley RJ, et al. Robotic therapy provides a stimulus for upper limb motor recovery after stroke that is complementary to and distinct from conventional therapy. Neurorehabil Neural Repair 2014; 28: 367– 76 [DOI] [PubMed] [Google Scholar]
- 82. Rosati G, Gallina P, Masiero S: Design, implementation and clinical tests of a wire-based robot for neurorehabilitation. IEEE Trans Neural Syst Rehabil Eng 2007; 15: 560– 9 [DOI] [PubMed] [Google Scholar]
- 83. Masiero S, Celia A, Armani M, et al. A novel robot device in rehabilitation of post-stroke hemiplegic upper limbs. Aging Clin Exp Res 2006; 18: 531– 5 [DOI] [PubMed] [Google Scholar]
- 84. Masiero S, Celia A, Rosati G, et al. Robotic-assisted rehabilitation of the upper limb after acute stroke. Arch Phys Med Rehabil 2007; 88: 142– 9 [DOI] [PubMed] [Google Scholar]
- 85. Masiero S, Armani M, Rosati G: Upper-limb robot-assisted therapy in rehabilitation of acute stroke patients: Focused review and results of new randomized controlled trial. J Rehabil Res Dev 2011; 48: 355– 66 [DOI] [PubMed] [Google Scholar]
- 86. Masiero S, Armani M, Ferlini G, et al. Randomized trial of a robotic assistive device for the upper extremity during early inpatient stroke rehabilitation. Neurorehabil Neural Repair 2014; 28: 377– 86 [DOI] [PubMed] [Google Scholar]
- 87. Prange GB, Kottink AI, Buurke JH, et al. The effect of arm support combined with rehabilitation games on upper-extremity function in subacute stroke: A randomized controlled trial. Neurorehabil Neural Repair 2015; 29: 174– 82 [DOI] [PubMed] [Google Scholar]
- 88. Bartolo M, De Nunzio AM, Sebastiano F, et al. Arm weight support training improves functional motor outcome and movement smoothness after stroke. Funct Neurol 2014; 29: 15– 21 [PMC free article] [PubMed] [Google Scholar]
- 89. Colomer C, Baldovi A, Torrome S, et al. Efficacy of Armeo(R) Spring during the chronic phase of stroke. Study in mild to moderate cases of hemiparesis. Neurologia 2013; 28: 261– 7 [DOI] [PubMed] [Google Scholar]
- 90. Coscia M, Cheung VC, Tropea P, et al. The effect of arm weight support on upper limb muscle synergies during reaching movements. J Neuroeng Rehabil 2014; 11: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mehrholz J, Hadrich A, Platz T, et al. Electromechanical and robot-assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev 2012; 6: CD006876. [DOI] [PubMed] [Google Scholar]
- 92. Norouzi-Gheidari N, Archambault PS, Fung J: Effects of robot-assisted therapy on stroke rehabilitation in upper limbs: Systematic review and meta-analysis of the literature. J Rehabil Res Dev 2012; 49: 479– 96 [DOI] [PubMed] [Google Scholar]
- 93. Hesse S, Schmidt H, Werner C, et al. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Curr Opin Neurol 2003; 16: 705– 10 [DOI] [PubMed] [Google Scholar]
- 94. Stein J, Bishop L, Gillen G, et al. Robot-assisted exercise for hand weakness after stroke: A pilot study. Am J Phys Med Rehabil 2011; 90: 887– 94 [DOI] [PubMed] [Google Scholar]
- 95. Laver K, George S, Thomas S, et al. Cochrane review: Virtual reality for stroke rehabilitation. Eur J Phys Rehabil Med 2012; 48: 523– 30 [PubMed] [Google Scholar]
- 96. Takahashi CD, Der-Yeghiaian L, Le V, et al. Robot-based hand motor therapy after stroke. Brain 2008; 131(pt 2): 425– 37 [DOI] [PubMed] [Google Scholar]
- 97. Lang N, Speck S, Harms J, et al. Dopaminergic potentiation of rTMS-induced motor cortex inhibition. Biol Psychiatry 2008; 63: 231– 3 [DOI] [PubMed] [Google Scholar]
- 98. Monte-Silva K, Kuo MF, Thirugnanasambandam N, et al. Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J Neurosci 2009; 29: 6124– 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. O’Doherty JP: Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr Opin Neurobiol 2004; 14: 769– 76 [DOI] [PubMed] [Google Scholar]
- 100. O’Doherty J, Kringelbach ML, Rolls ET, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 2001; 4: 95– 102 [DOI] [PubMed] [Google Scholar]
- 101. Bechara A, Damasio AR, Damasio H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994; 50: 7– 15 [DOI] [PubMed] [Google Scholar]
- 102. Marsh R, Hao X, Xu D, et al. A virtual reality-based fMRI study of reward-based spatial learning. Neuropsychologia 2010; 48: 2912– 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Swanson LW: The projections of the ventral tegmental area and adjacent regions: A combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 1982; 9: 321– 53 [DOI] [PubMed] [Google Scholar]
- 104. Vanni-Mercier G, Mauguiere F, Isnard J, et al. The hippocampus codes the uncertainty of cue-outcome associations: An intracranial electrophysiological study in humans. J Neurosci 2009; 29: 5287– 94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Schultz W: Behavioral dopamine signals. Trends Neurosci 2007; 30: 203– 10 [DOI] [PubMed] [Google Scholar]
- 106. Hong S, Hikosaka O: Dopamine-mediated learning and switching in cortico-striatal circuit explain behavioral changes in reinforcement learning. Front Behav Neurosci 2011; 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yavuzer G, Senel A, Atay MB, et al. “’Playstation eyetoy games” improve upper extremity-related motor functioning in subacute stroke: A randomized controlled clinical trial. Eur J Phys Rehabil Med 2008; 44: 237– 44 [PubMed] [Google Scholar]
- 108. Saposnik G, Mamdani M, Bayley M, et al. Effectiveness of Virtual Reality Exercises in STroke Rehabilitation (EVREST): Rationale, design, and protocol of a pilot randomized clinical trial assessing the Wii gaming system. Int J Stroke 2010; 5: 47– 51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rajaratnam BS, Gui Kaien J, Lee Jialin K, et al. Does the inclusion of virtual reality games within conventional rehabilitation enhance balance retraining after a recent episode of stroke? Rehabil Res Pract 2013; 2013: 649561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Singh DK, Rajaratnam BS, Palaniswamy V, et al. Participating in a virtual reality balance exercise program can reduce risk and fear of falls. Maturitas 2012; 73: 239– 43 [DOI] [PubMed] [Google Scholar]
- 111. Emery CA, Rose MS, McAllister JR, et al. A prevention strategy to reduce the incidence of injury in high school basketball: A cluster randomized controlled trial. Clin J Sport Med 2007; 17: 17– 24 [DOI] [PubMed] [Google Scholar]
- 112. Kafri M, Myslinski MJ, Gade VK, et al. Energy expenditure and exercise intensity of interactive video gaming in individuals poststroke. Neurorehabil Neural Repair 2014; 28: 56– 65 [DOI] [PubMed] [Google Scholar]
- 113. Betker AL, Szturm T, Moussavi ZK, et al. Video game-based exercises for balance rehabilitation: A single-subject design. Arch Phys Med Rehabil 2006; 87: 1141– 9 [DOI] [PubMed] [Google Scholar]
- 114. Lam YS, Man DW, Tam SF, et al. Virtual reality training for stroke rehabilitation. NeuroRehabilitation 2006; 21: 245– 53 [PubMed] [Google Scholar]
- 115. Johansson BB: Current trends in stroke rehabilitation: A review with focus on brain plasticity. Acta Neurol Scand 2011; 123: 147– 59 [DOI] [PubMed] [Google Scholar]
- 116. Klein TA, Neumann J, Reuter M, et al. Genetically determined differences in learning from errors. Science 2007; 318: 1642– 5 [DOI] [PubMed] [Google Scholar]
- 117. Floel A, Vomhof P, Lorenzen A, et al. Levodopa improves skilled hand functions in the elderly. Eur J Neurosci 2008; 27: 1301– 7 [DOI] [PubMed] [Google Scholar]
- 118. Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155: 344– 9 [DOI] [PubMed] [Google Scholar]
- 119. Francis PT, Perry EK: Cholinergic and other neurotransmitter mechanisms in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. Mov Disord 2007; 22(Suppl 17): S351– 7 [DOI] [PubMed] [Google Scholar]
- 120. Wills J, Jones J, Haggerty T, et al. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp Neurol 2010; 225: 210– 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Goldstein DS, Holmes C, Bentho O, et al. Biomarkers to detect central dopamine deficiency and distinguish Parkinson disease from multiple system atrophy. Parkinsonism Relat Disord 2008; 14: 600– 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Adamson J, Beswick A, Ebrahim S: Is stroke the most common cause of disability? J Stroke Cerebrovasc Dis 2004; 13: 171– 7 [DOI] [PubMed] [Google Scholar]
- 123. Pearson-Fuhrhop KM, Minton B, Acevedo D, et al. Genetic variation in the human brain dopamine system influences motor learning and its modulation by l-DOPA. PLoS One 2013; 8: e61197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hesse S, Hess A, Werner CC, et al. Effect on arm function and cost of robot-assisted group therapy in subacute patients with stroke and a moderately to severely affected arm: A randomized controlled trial. Clin Rehabil 2014; 28: 637– 47 [DOI] [PubMed] [Google Scholar]
- 125. Platz T, Pinkowski C, van Wijck F, et al. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin Rehabil 2005; 19: 404– 11 [DOI] [PubMed] [Google Scholar]


