Summary
To maintain a symbiotic relationship between the host and its resident intestinal microbiota, appropriate mucosal T cell responses to commensal antigens must be established. Mice acquire both IgG and IgA maternally; the former has primarily been implicated in passive immunity to pathogens while the latter mediates host-commensal mutualism. Here we report the surprising observation that mice generate T cell independent and largely Toll-like receptor (TLR) dependent IgG2b and IgG3 antibody responses against their gut microbiota. We demonstrate that maternal acquisition of these antibodies dampens mucosal T follicular helper responses and subsequent germinal center B cell responses following birth. This work reveals a feedback loop whereby T cell independent, TLR-dependent antibodies limit mucosal adaptive immune responses to newly acquired commensal antigens and uncovers a broader function for maternal IgG.
Introduction
Establishing and maintaining a symbiotic relationship with the gut microbiota is critical to the health of coelomic animals. Gut commensal bacteria facilitate digestion, prevent infection, and enhance immune function (Belkaid and Hand, 2014; Hooper et al., 2012). The host employs many mechanisms to maintain this beneficial relationship including production of antimicrobial factors and immunosuppressive cytokines and selective differentiation of specialized immune cells(Belkaid and Hand, 2014; Hooper et al., 2012). Dysregulated immune responses resulting from breakdowns in these homeostatic mechanisms underlie the development of intestinal inflammation and can lead to pathologies such as Crohn’s disease and ulcerative colitis (Neurath, 2014).
The differentiation of CD4+ T helper (Th) cells within the gut is carefully controlled such that T cells specific for many commensal species are either biased toward a regulatory T cell (Treg) fate or remain ignorant of their cognate antigens (Hand et al., 2012; Lathrop et al., 2011). Nevertheless, commensal-specific T cell responses occur in several circumstances. Colonization with microbes that adhere to the intestinal epithelium, such as segmented filamentous bacterium (SFB), is sufficient to elicit effector Th17 cells (Atarashi et al., 2015; Ivanov et al., 2009). Changes in the inflammatory environment, such as infection with Toxoplasma gondii, can also promote Th1 cell responses to commensal antigens (Hand et al., 2012). While these examples demonstrate that commensal species can elicit inflammatory T cells, such responses are not the norm. The mechanisms that limit T cell activation to commensal antigens, however, remain poorly understood.
Following birth, the virgin immune system must appropriately respond to colonization of the gut by complex and dynamic microbial species before many of the mechanisms that promote intestinal homeostasis throughout life are fully established. Lactating mothers nourish neonatal mammals with breast milk rich in factors that compensate for the immature intestinal immune system of their offspring. For example, acquisition of TGF-β and IL-10 from breast milk functions to limit bacterial translocation and promote tolerogenic responses to the microbiota (Garofalo et al., 1995; Letterio et al., 1994). Mothers also influence immunity in their offspring through the provision of antibodies. Acquisition of maternal IgG antibodies both in utero and via breast milk helps protect neonates against pathogens (Niewiesk, 2014). Likewise, ingestion of IgA can mediate passive immunity to enteric infections (Lamm, 1997) and reinforce appropriate anti-commensal immune responses in offspring (Macpherson et al., 2008). Thus, maternal antibodies may educate the neonatal immune system on newly acquired commensal species thereby facilitating appropriate effector T cell (Teff) responses to the microbiota during early life.
IgA is the dominant antibody isotype produced within the gut and mouse mammary glands (Macpherson et al., 2008; Weisz-Carrington et al., 1977). Dimeric IgA produced in the gut lamina propria (LP) is transported to the lumen by the polymeric Ig receptor where it limits microbial access to the intestinal epithelium (Cerutti and Rescigno, 2008). IgA-secreting cells are generated in the presence or absence of T cell help. Activation of innate immune receptors such as TLRs by microbial ligands promotes T-independent (TI) IgA responses resulting in the production of low affinity antibodies specific for a diverse array of commensal species (Fagarasan et al., 2010; Pabst, 2012). In contrast, high affinity T-dependent IgA responses are elicited by enteric pathogens and a subset of microbes with unique properties, such as SFB (Bunker et al., 2015; Pabst, 2012). These two pools of IgA aid in the broad recognition of microbes that colonize the intestine and help preserve the intestinal barrier. Based on analysis of antibody responses to a subset of commensal species in healthy mice and observations that mammals with defects in intestinal homeostasis develop IgG responses to commensal antigens, it has been proposed that the commensal specific antibody response is entirely restricted to IgA (Harmsen et al., 2012; Macpherson et al., 2000; Slack et al., 2009).
Here we report that healthy mice generate a broad, T cell independent IgG response to a diverse array of commensal species. We identify breast milk as a primary source of anti-commensal IgG antibodies early in life and demonstrate that maternally acquired anti-commensal IgG helps dampen Th cell driven immune responses to newly encountered microbes.
Results
Identification of Anti-Commensal IgG2b and IgG3 antibodies
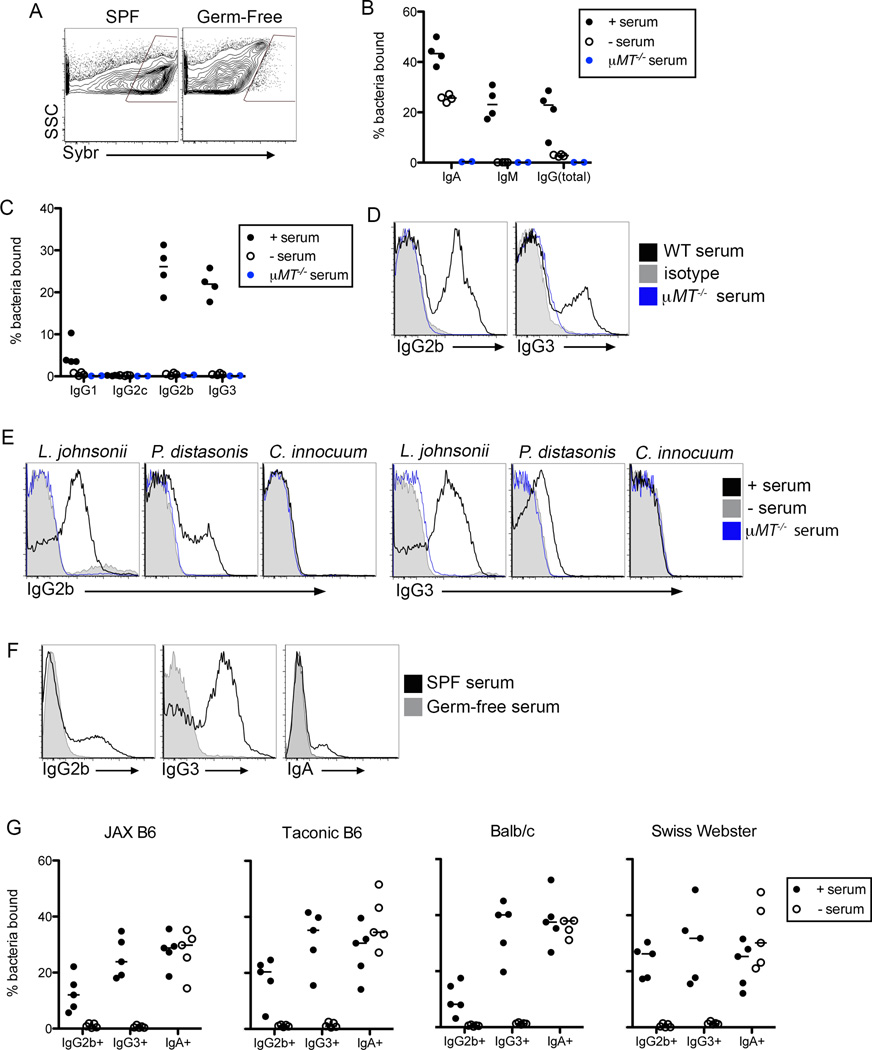
To characterize the antibody response to the microbiota, we developed a high-throughput flow cytometry assay we termed mFLOW for ‘microbiota flow cytometry’. Using feces as a source of microbiota we stained bacteria with sera from the same mouse. This assay varies from a method in which IgA bound microbes are isolated from intestinal contents (Bunker et al., 2015; Palm et al., 2014; van der Waaij et al., 1994); addition of sera to intestinal contents allows profiling of the complete antibody response to the microbiota, rather than restricting analysis to antibodies present in the intestinal lumen already bound to commensals. We used the DNA dye SYBR Green to preferentially stain microbes in the feces of colonized (specific pathogen free; SPF) mice, but not fecal particulates in germ-free (GF) animals, allowing us to gate definitively on (SYBRhi) bacteria (Figure 1A) (Maurice et al., 2013). We then used isotype specific secondary antibodies to characterize the anti-commensal antibody response. As expected, a fraction of microbes stained positive for IgA and IgM (Figure 1B). Surprisingly, IgG also bound a significant proportion of the microbiota (Figure 1B). As in previous reports, we observed minimal binding of microbes by IgG1 and IgG2c antibodies; instead, the majority of anti-commensal IgG antibodies were of the IgG2b and IgG3 isotypes (Figure 1C)(Slack et al., 2009).
Figure 1. IgG Antibodies are Generated Against the Microbiota in Healthy Mice.
(A) mFLOW analysis of SYBR Green incorporation by intestinal contents of SPF and GF mice. Gate indicates events present in SPF but not GF animals, hereafter referred to as SYBRhi bacteria. (B) mFLOW analysis, gated on SYBRhi bacteria, of the fraction of fecal bacteria bound by total IgG, IgM and IgA in the presence or absence of WT or µMT−/− serum. (C) Similar analysis as described in (B), gated on SYBRhi bacteria, performed with secondary antibodies against the indicated mouse IgG isotypes. (D) mFLOW analysis of IgG2b and IgG3 staining of SYBR+ bacteria following incubation with WT or µMT−/− serum, or mouse IgG2b or IgG3 isotype control antibodies. (E) mFLOW analysis of IgG2b or IgG3 binding to pure cultures of commensal bacterial isolates. (F) mFLOW analysis of IgG2b, IgG3 or IgA staining of SYBR+ microbes from SPF-mice following incubation with SPF or GF sera, as indicated. (G) Serum and feces were isolated from the indicated mouse strains maintained in distinct facilities: Berkeley, Jackson Laboratories (JAX), and Taconic Biosciences (Taconic). Graphs depict the frequencies of SYBR+ bacteria bound by the indicated antibody isotypes as measured by mFLOW. Bars on graphs indicate medians; symbols represent individual mice. Data are representative of 2–6 experiments, with 3–5 mice per group. See also Figure S1.
Although IgA bound microbes were detected in the absence of serum, we did not observe IgG-bound microbes, consistent with a lack of transport of IgG antibodies across the intestinal epithelium (Figure 1B)(Bunker et al., 2015; Palm et al., 2014). Immunofluorescent staining of intestinal sections confirmed these results (Figure S1A). We ruled out antigen-independent binding of IgG antibodies by bacterial proteins that bind to the IgG constant regions (Fc) by performing mFLOW with monoclonal IgG2b and IgG3 isotype antibodies that did not stain commensal bacteria by mFLOW (Figure 1D). The presence of antibodies was required for this response, as sera from B cell-deficient µMT−/− mice did not result in detectable IgG2b+ or IgG3+ events (Figures 1B–D).
To confirm that antibody-bound particles measured by mFLOW were microbes, we showed that IgG2b and IgG3 antibodies from the sera of healthy mice could stain pure cultures of several bacteria strains isolated from feces of mice in our colony. For example, we detected IgG2b and IgG3 antibodies specific for Lactobacillus johnsonii and Parabacteroides distasonis, but not Clostridium innocuum (Figure 1E). We did not observe binding of IgG2b or IgG3 to pathogenic bacteria, such as Salmonella enterica serovar Typhimurium and Citrobacter rodentium, or to a non-pathogenic Escherichia coli isolate (Figure S1B). We generated several microbe-specific IgG3 hybridomas from wild-type (WT) mice that recapitulated commensal-specific staining (Figure S1C). Finally, IgG from sera of GF animals did not stain SYBR+ microbes from SPF mice (Figure 1F), indicating that anti-commensal IgG antibodies are not ‘natural’ antibodies observed in mice housed under GF conditions in the absence of microbiota (Bos et al., 1989).
Both the host genetic background and microbial variation in SPF facilities can influence IgA responses to the microbiota (Fransen et al., 2015; Moon et al., 2015). By mFLOW, we observed similar anti-commensal IgG2b and IgG3 responses in C57BL/6 mice housed at UC Berkeley, Jackson Labs, and Taconic (Figure 1G). Additionally, anti-commensal IgG responses were detectable in inbred and outbred mouse strains (Figure 1G). These results demonstrate that generation of systemic anti-commensal IgG2b and IgG3 antibodies is a general aspect of immunity to the microbiota.
IgG Antibodies Recognize a Broad Spectrum of Commensal Bacteria
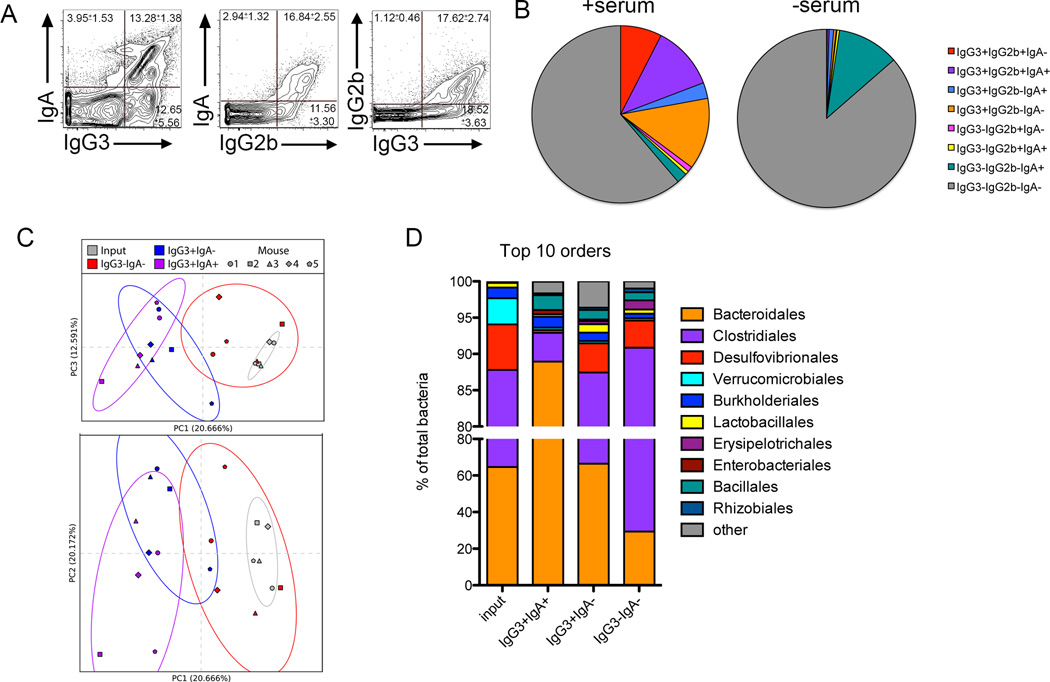
We compared the overlap between bacteria bound by IgA, IgG2b and IgG3. The majority of IgA-bound microbes also stained positive for IgG3 and IgG2b (Figure 2A, 2B). Additionally, IgG3 bound some commensals not bound by IgG2b or IgA, indicating that IgG3 may expand the spectrum of microbes recognized by the immune system. We next sorted populations of antibody-bound bacteria and identified bacterial species by 16S rRNA-based profiling. As IgG3 bound all microbes bound by IgG2b (Figure 2A, 2B) we confined our analysis to IgG3+IgA-, IgG3+IgA+, IgG3-IgA- sorted groups and compared these to input samples. After clustering and classifying 97% identity operational taxonomic units (OTUs; Supplementary Methods), we used Qiime 1.8.0 (Caporaso et al., 2010) to perform principal coordinate analysis (PCoA) on the unweighted UniFrac distances (Lozupone and Knight, 2005; Lozupone et al., 2011; Lozupone et al., 2007) among our samples. The antibody-bound microbial fractions (both IgG3+IgA+ and IgG3+IgA-) clustered away from unbound (IgG3-IgA-) and input populations with little overlap, indicating that these fractions contain taxonomically divergent species of microbes (Figure 2C). The sorted and input fractions are best differentiated by principle component (PC) 1 and PC3, whereas PC2 seems to account for variation among individual mice. The microbial species diversity (Shannon diversity index) also varied between the separate fractions, with an overall decrease from the input to the selectively bound fractions (Figure S2A). While there is little variation of microbial composition between cohoused littermate animals, there can be considerable differences between mice housed in different enclosures (Figure S2B). Regardless of the input microbiomes, sorting based on antibody binding consistently segregated unbound bacteria away from antibody-bound microbes (compare Figure 2C, sort B and Figure S2C, sort C). IgG3, and by correlation IgG2b, therefore bind discrete groups of commensal bacteria regardless of differences in composition of bacteria colonizing the gut.
Figure 2. Broad Recognition of Fecal Microbes by IgG2b and IgG3.
(A) mFLOW analyses of IgG2b, IgG3, and IgA binding of SYBR+ fecal microbiota isolated from WT mice. Numbers in each quadrant indicate the mean percentage of events (± SD) of all samples within the experiment. n=5. (B) Pie chart depicting fraction of microbiota bound by indicated antibody isotypes. Fractions shown represent the average of four mice. (C) Unweighted UniFrac PC plot of input and sorted fecal bacterial fractions of cohoused mice. Shapes represent different mice. Colors refer to sorted and input populations as indicated in legend. Ellipses indicate 2 standard deviations from the mean of the selected data points. (D) Taxonomic distribution of bacteria in the sorted samples compared with the input (unsorted) fraction is shown. Relative abundances classified at the Order level are reported for the 10 most abundant taxa. All data shown are representative of 3–4 experiments, with 3–5 mice per group. See also Figure S2.
We examined the breadth of microbes bound by IgG3 antibodies by comparing the relative abundance of bacterial genera in the sorted fractions. IgG3 antibodies bound genera from all bacterial orders present in the input samples, indicating that these antibodies recognize a diverse spectrum of commensals (Figure 2D). Further, IgG3 antibodies enhanced commensal recognition beyond that of IgA as demonstrated by the enrichment of genera in the Clostridiales and Desulfovibrionales orders in the IgG3+IgA- fraction as compared to the IgG3+IgA+ fraction. In a separate sorting experiment using mice colonized with distinct microbiota (sort C, see Figure S2B) commensal species bound by IgG3 changed, with OTUs belonging to the order Bifidobacteriales and Lactobacillales being enriched in the IgG3+IgA- fraction as compared to the IgG3+IgA+ fraction (Figure S2D), emphasizing the dynamism of the anti-commensal IgG response.
Characterization and Localization of IgG2b and IgG3 Antibody Secreting Cells
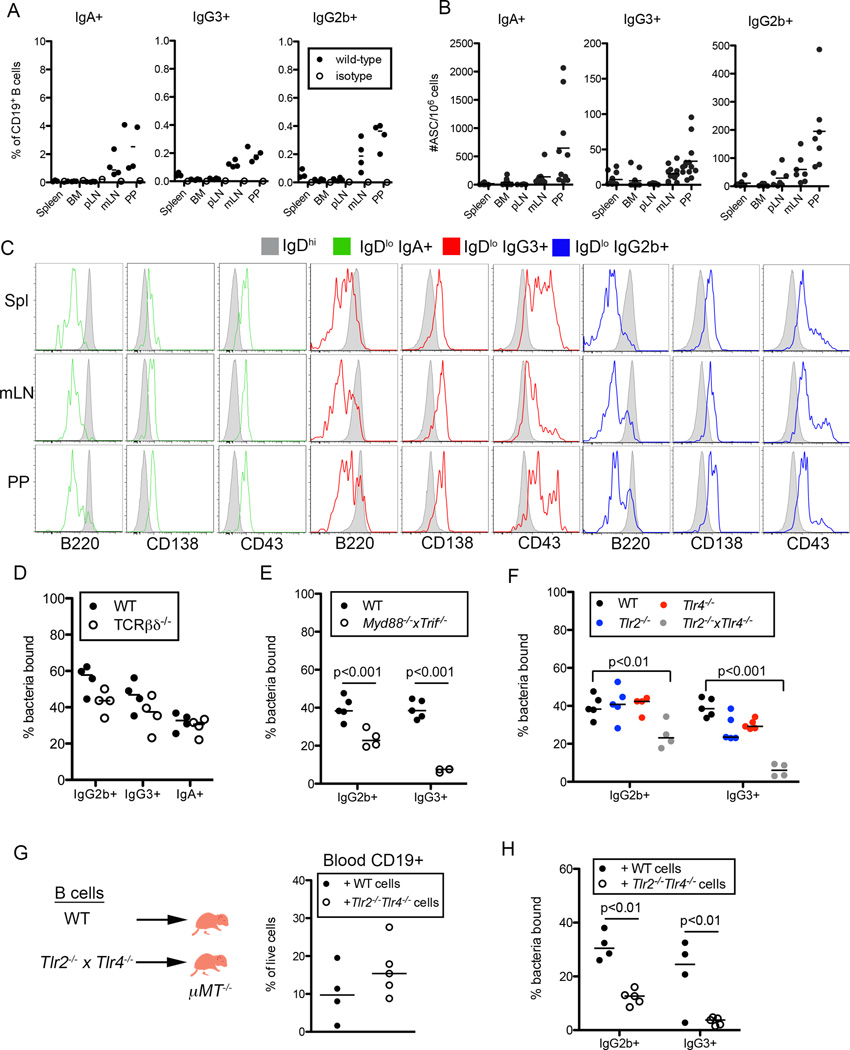
We next characterized the B cells producing IgG3 and IgG2b antibodies and found that, like IgA+ B cells, the frequencies and numbers of IgG2b+ and IgG3+ B cells were elevated in the mesenteric lymph nodes (mLN) and Peyer’s patches (PP) relative to other lymphoid organs (Figure 3A, S3A). Additionally, IgG2b and IgG3 secreting cells could be detected at mucosal sites such the mLN, PP and gut LP, albeit at lower frequencies than IgA+ B cells (Figures 3B, 3SB). In comparison to IgDhi naïve B cells, IgG3+, IgG2b+ and IgA+ B cells expressed markers characteristic of plasma cells, including decreased B220 levels and high CD138 expression (Figure 3C). Further, these B cells expressed elevated levels of the B-1 cell associated marker CD43.
Figure 3. Characterization of Anti-Commensal IgG2b and IgG3 Response.
(A, B) Frequencies out of CD19+ cells (A) and numbers (B) of IgG2b, IgG3, and IgA expressing B cells in different tissues of WT mice, as measured by flow cytometry (A) or ELISpot (B). (C) Representative flow cytometric analysis of indicated marker expression by IgA+, IgG3+, or IgG2b+ CD19+ B cells as compared to IgDhi CD19+ B cells in different tissues of WT mice. (D) Frequencies of SYBR+ bacteria bound by IgG2b, IgG3 and IgA antibodies following incubation with serum isolated from WT or Tcrb−/− mice. (E,F) Frequencies of SYBR+ bacteria bound by IgG2b and IgG3 antibodies following incubation (E) with serum isolated from WT or Myd88−/−Trif−/− mice or (F) with serum isolated from WT, Tlr2−/−, Tlr4−/− or Tlr2−/−Tlr4−/− mice. (G,H) (G) Cartoon depicts experimental design. Graph displays frequencies of CD19+ B cells present in the blood of transferred mice at 10 weeks of age. (H) Frequencies of SYBR+ bacteria bound by IgG2b and IgG3 antibodies as measured by mFLOW following incubation with serum isolated from indicated transferred animals. Bars on graphs indicate medians; symbols represent individual mice. Data in (A) and (C) are representative of 3 experiments with 4 mice per group. Data in (B) are combined from 4 independent experiments. Data in (D–H) are representative of 3–6 experiments with 3–6 mice per group. Statistical significance was determined using two-way ANOVA. Bonferroni post-tests were used for pairwise comparisons. See also Figure S3.
Generation of Anti-Commensal IgG Antibodies is T cell Independent and Largely TLR Dependent
Both IgG3 and IgG2b can be produced in the absence of T cell help (Figure S3C)(Markowitz et al., 1993). To determine if production of anti-commensal IgG2b and IgG3 required T cells we performed mFLOW using samples from Tcrb−/− and Tcrb−/−Tcrd−/− mice. The fraction of bacteria bound by IgG2b, IgG3, and IgA was equivalent between T-cell deficient and sufficient mice indicating that T cells were not required for this response (Figure 3D, S3D).
TI antibody responses fall into two groups based on antigen type: TI-II antigens are generally polyvalent, repetitive structure, whereas TI-I antigens have mitogenic properties and are often ligands for TLRs. TLRs respond to a variety of microbe-derived ligands including those expressed by the gut microbiota and require MyD88 and TRIF to signal. Generation of anti-commensal IgG2b and IgG3 was severely impaired in Myd88−/−Trif−/− mice compared to co-housed controls (Figure 3E). Serum IgG3 titers are reduced in Myd88−/−Trif−/− mice (Figure S3E) (Pasare and Medzhitov, 2005); however, normalizing IgG3 titers to WT levels prior to incubation with commensals did not restore anti-commensal IgG3 staining (Figure S3F), confirming that signaling through MyD88 and TRIF is required for production of anti-commensal IgG3. Notably, we did not observe defects in anti-commensal IgG responses in mice lacking components of other innate immune pathways, including Caspase1−/−Caspase11−/−, Nod1−/−Nod2−/− or Dectin1−/− strains (Figure S3G).
Next, we focused on determining the contribution of particular TLRs. The chaperone protein Unc93b1 is required for the function of TLRs 3, 5, 7, 8, 9, 11, 12, and 13 (Lee et al., 2013; Tabeta et al., 2006). Mice expressing a non-functional Unc93b1 allele (Unc93b13d/3d) generated WT levels of anti-commensal IgG antibodies (Figure S3H). In contrast, mice lacking both TLR2 and TLR4 displayed a significant reduction in anti-commensal IgG2b responses and a complete lack of anti-commensal IgG3 staining (Figure 3F). The fraction of microbiota bound by IgG3 was partially reduced when stained with sera from either Tlr2−/− or Tlr4−/− animals, while anti-commensal IgG2b responses were unaffected (Figure 3F). Thus, both TLR2 and TLR4, receptors for bacterial lipopeptides and lipopolysaccharide (LPS), respectively, participate in the generation of anti-commensal IgG2b and IgG3.
To determine the requirement for TLR signaling on B cells for anti-commensal IgG responses, we reconstituted B cell deficient µMT−/− pups with WT or Tlr2−/−Tlr4−/− B cells at day 1 post birth. At 10 weeks of age, we observed equivalent reconstitution of WT and knockout cells in the blood, spleen, mLN and PP of recipient mice (Figure 3G, S3I), yet mice that received Tlr2−/−Tlr4−/− B cells exhibited diminished anti-commensal IgG2b and IgG3 responses (Figure 3H). Importantly, we observed normal anti-commensal IgM production and IgG3 responses following immunization with the TI-II antigen NP-Ficoll, indicating that Tlr2−/−Tlr4−/− B cells were functional (Figure S3J, S3K). Thus, TLR2 and TLR4 signaling on B cells is required for optimal generation of anti-commensal IgG antibodies, demonstrating that, through TLRs, commensal-derived products drive the differentiation of naïve B cells into plasma cells that produce IgG2b and IgG3 antibodies targeting gut microbial antigens.
Maternal Transmission of Microbiota-Specific IgG Antibodies
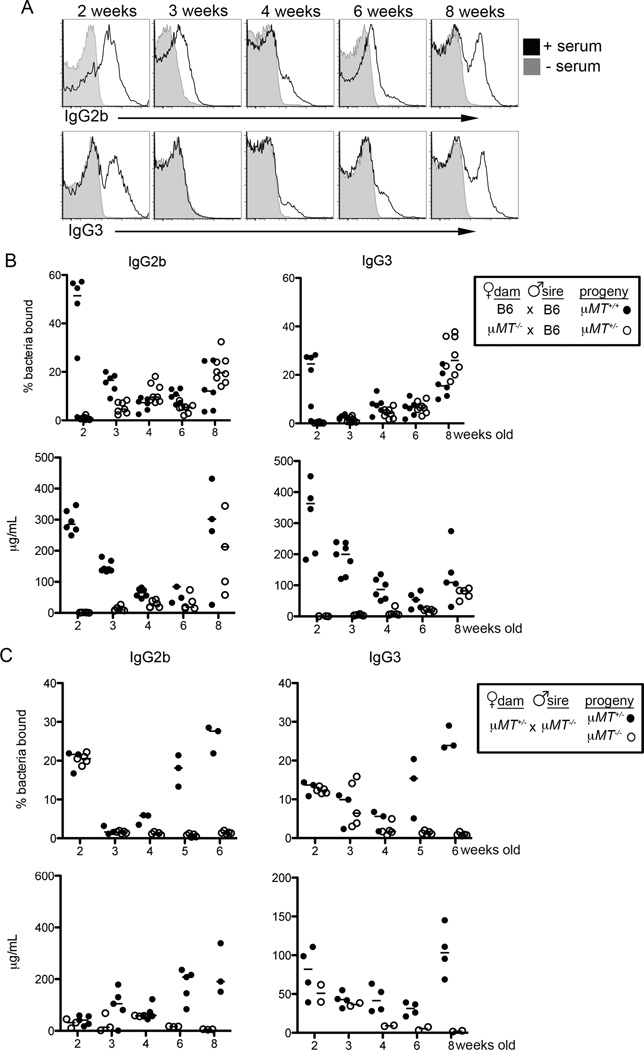
We detected anti-commensal IgG2b and IgG3 responses in mice as early as two weeks of age (Figure 4A). These responses waned at three and four weeks of age and increased again in older mice. The presence of these antibodies in young mice suggested that, like IgA, they are maternally derived. Indeed, pups acquire maternal IgG antibodies through breast milk as well as in utero via the neonatal Fc receptor (FcRN) (Roopenian and Akilesh, 2007). To determine whether anti-commensal IgG antibodies are acquired maternally, we first bred B cell-deficient µMT−/− dams with WT sires to generate offspring that can generate functional anti-commensal B cell responses, but lack maternally-derived antibodies. In contrast to offspring born to WT dams, anti-commensal IgG2b and IgG3 responses were reduced at two and three weeks of age in pups born to µMT−/− dams (Figure 4B, top). These results correlate with serum IgG2b and IgG3 titers in these animals, and indicate that anti-commensal IgG responses are mediated primarily by maternally-derived antibodies at early time points (Figure 4B, bottom). In addition to their presence in the circulation, IgG2b, IgG3 and IgA antibodies of maternal origin could be detected in the stomach and small intestinal contents of young mice (Figure S4A). Further, IgG2b and IgG3 bound bacteria were present in the stomach and intestinal lumen of two-week-old mice in the absence of added sera (Figure S4B, S4C).
Figure 4. Maternal Transmission of Anti-Commensal IgG Antibodies.
(A) Representative histograms of SYBR+ bacteria bound by serum IgG2b (upper) or IgG3 (lower) from WT mice of the indicated age. (B) mFLOW analysis (upper) of SYBR+ bacteria bound by IgG2b and IgG3 antibodies following incubation with serum and total serum titers (lower) of mice that can or cannot acquire maternal antibodies at the indicated timepoints. (C) mFLOW analysis (upper) of the frequency of SYBR+ bacteria bound by IgG2b and IgG3 antibodies following incubation with serum and total serum titers (lower) of µMT+/− and µMT−/− littermate animals at the indicated timepoints. Bars on all graphs indicate medians; symbols represent individual mice. Data are representative of 2–6 independent experiments with 3–6 mice per group. See also Figure S4.
To corroborate these results we mated µMT+/− dams with B cell-deficient µMT−/− sires so that approximately half of the resulting offspring lack B cells and therefore any antibodies in these mice are of maternal origin. As expected, maternal IgM was not transferred to B-cell deficient offspring, as evidenced by the negligible serum IgM titers and IgM-bound bacteria in µMT−/− pups (Figure S4D) (Kane and Acquah, 2009). In contrast, commensal-specific, maternally-derived IgG3 and IgG2b antibodies were readily detected by mFLOW and ELISA analysis in offspring at two to three weeks of age (Figure 4C). Thus, commensal-specific IgG antibodies are maternally acquired and coordinate with IgA to facilitate immune recognition of the microbiota.
Maternal Antibodies Regulate Mucosal Helper T cell Activation
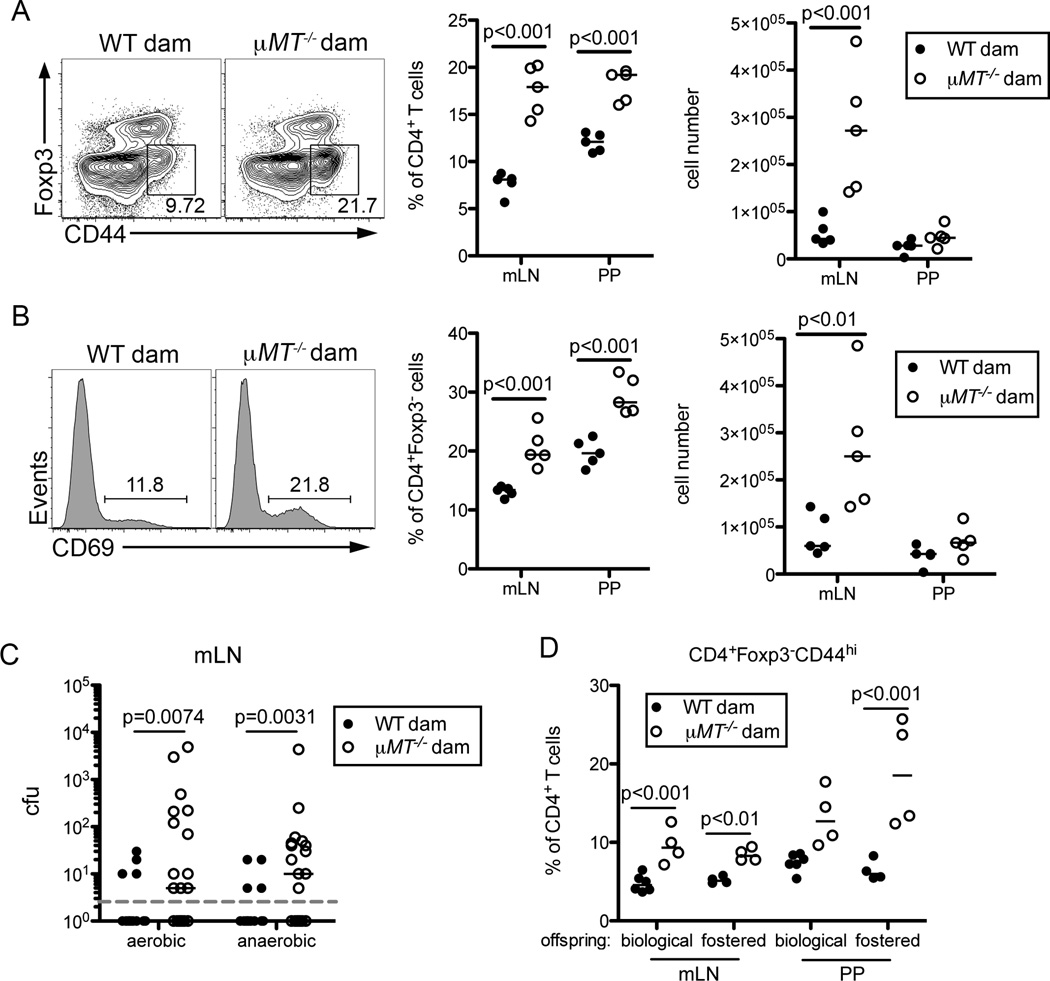
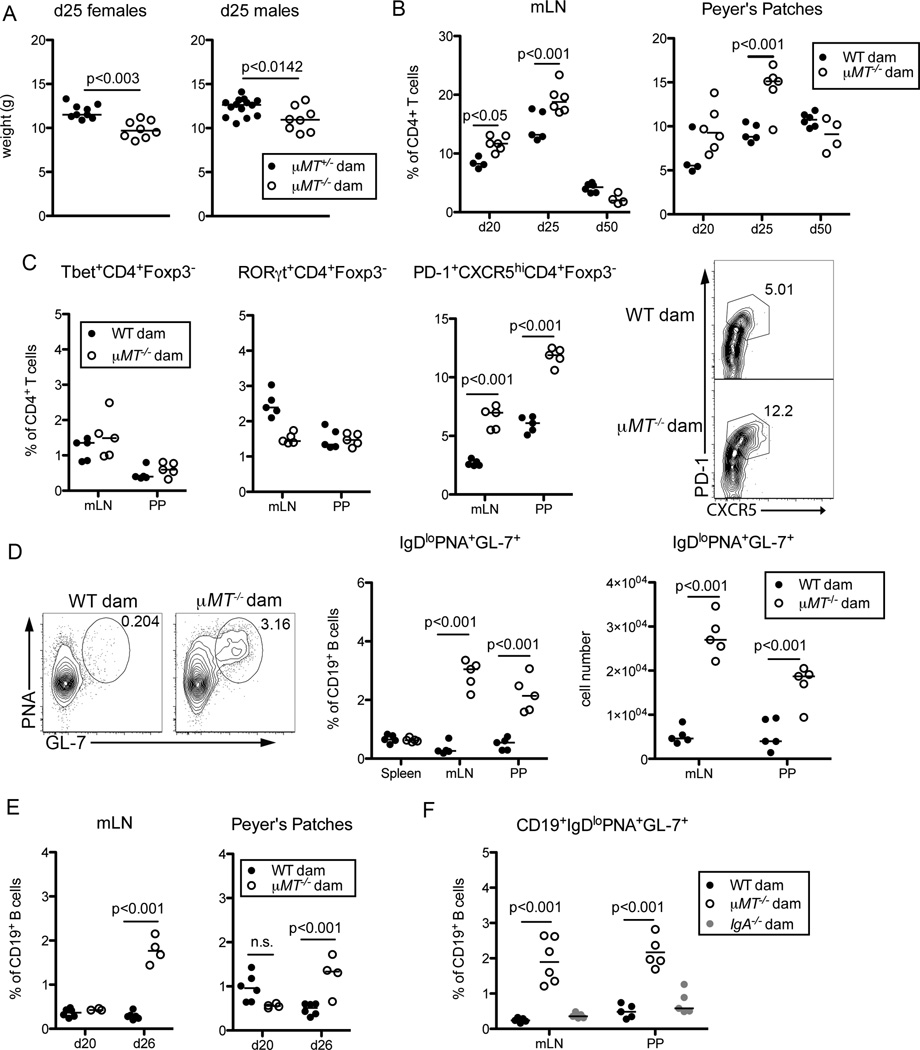
During the weaning period, the shift in diet coupled with the reduced concentrations of immunoregulatory factors from breast milk drive changes in the composition of the microbiota (Laukens et al., 2016). To determine if maternally-derived commensal-specific antibodies help establish appropriate immune responses in the neonatal gut, we compared offspring of B-cell deficient and B cell sufficient dams at d25–26 post birth. We did not observe any gross differences in CD8 Teff, Tregs, γδ T cells, NK cells, dendritic cells or neutrophils at this time (Figure S5A and data not shown). However, we did detect an increase in both the frequency and number of CD4+Foxp3−CD44hi T effector (Teff) cells isolated from the mLN and PP of mice lacking maternal antibodies compared to controls (Figure 5A). This was accompanied by an expansion of CD69+ CD4+ T cells in maternal-antibody deficient mice, indicating these T cells had recently experienced TCR-mediated activation (Figure 5B).
Figure 5. Regulation of Mucosal T cell Responses by Maternal Antibodies.
(A) Representative flow cytometric analyses (left) of CD4+Foxp3−CD44hi Teff cells present in the mLN of mice sufficient or deficient in maternal antibodies at d25 of age. Dot plots gated on CD4+CD8− live cells. Graphs display frequencies (middle) and cell numbers (right) of CD4+Foxp3−CD44hi Teff cells present in the mLN and PP of indicated offspring. (B) Representative histograms (left) of CD69 expression by gated CD4+Foxp3− live cells present in the mLN of mice as in (A). Graphs display frequencies (middle) and cell numbers (right) of CD4+Foxp3−CD69hi activated T cells present in the mLN and PP of indicated offspring. (C) Colony forming units (cfu) recovered from the mLN of the indicated offspring d21 post birth. Dashed line indicates lower limit of detection. (D) Frequencies of CD4+Foxp3−CD44hi Teff cells present in the mLN and PP of mice cross-fostered with WT or µMT−/− dams at d1 post birth. Indicated offspring were analyzed at d25 of age. Bars on graphs indicate medians; symbols represent individual mice. With the exception of (C), data are representative of 2–10 independent experiments with four to six mice per group and statistical significance was determined using two-way ANOVA. Bonferroni post-tests were used for pairwise comparisons. For (C), data are compiled from 4 independent experiments and statistical significance was determined using a Mann-Whitney test. See also Figure S5.
This prompted us to test whether the absence of maternal antibodies allowed translocation of commensal microbes that would otherwise be restricted to the lumen or rapidly cleared upon entry. At the time of weaning, bacteria were routinely present in the mLN of pups lacking maternal antibodies yet rarely detectable in WT born offspring (Figure 5C). To assess whether the expanded CD4 T cells were activated in an antigen-specific fashion by these bacteria, we restricted the specificity of T cells to the non-microbial antigen ovalbumin using OT-II TCR-transgenic mice. We bred B cell sufficient and deficient females to hemizygous OT-II TCR transgenic sires and analyzed the resulting offspring at d26 post birth. Approximately half of each litter has a polyclonal T cell repertoire, while the others inherit the OT-II transgene resulting in a restricted T cell repertoire. As previously, we observed a significant increase in the frequency of polyclonal Teff in pups of B cell deficient dams as compared to mice with WT mothers (Figure S5B). Strikingly, the enhanced Teff response was abrogated in offspring of µMT−/− dams containing a restricted T cell repertoire (Figure S5B). This suggests that Th cells from maternal antibody deficient mice are inappropriately activated in response to antigens derived from commensal microbes. It is also possible that other constituents of the microbiota, such as endogenous viruses or fungi, contribute to this response (Pfeiffer and Virgin, 2016).
To confirm the accumulation of Teff cells in pups lacking maternal antibodies resulted from differences in maternal antibody concentrations rather than variation in the composition of the inherited microbiota (Ubeda et al., 2012), we co-housed wild-type and µMT−/− female mice for five weeks prior to breeding to equilibrate the microbial composition of the two strains. Secondly, we generated and analyzed the progeny from littermate µMT+/− and µMT−/− dams. In the latter experiment, dams inherited identical microbiota from B-cell sufficient mothers and were cohoused until approximately four days prior to parturition. In both experiments, we again observed an expansion in the frequency of CD4 Teff and activated CD69+Foxp3− Th cells within the mLN and PP of mice lacking maternal antibodies (Figure S5C, S5D). Finally, we cross-fostered a portion of progeny born to antibody sufficient or deficient dams one day after birth. Pups reared by µMT−/− females had reduced titers of serum IgG2b and IgG3 antibodies, correlating with similar increases in Teff and activated CD69+Foxp3− Th cells in the mLN and PP compared with pups reared by WT dams, regardless of genotype (Figure 5D, S5E, S5F). Thus, enhanced T cell activation results from a lack of maternal antibodies rather than differences in the composition of the inherited microbiota. Further, acquisition of maternal antibodies post birth, rather than in utero, is required to dampen mucosal T cell activation in young mice.
Maternal IgG and IgA Cooperate to Limit Mucosal T cell Responses
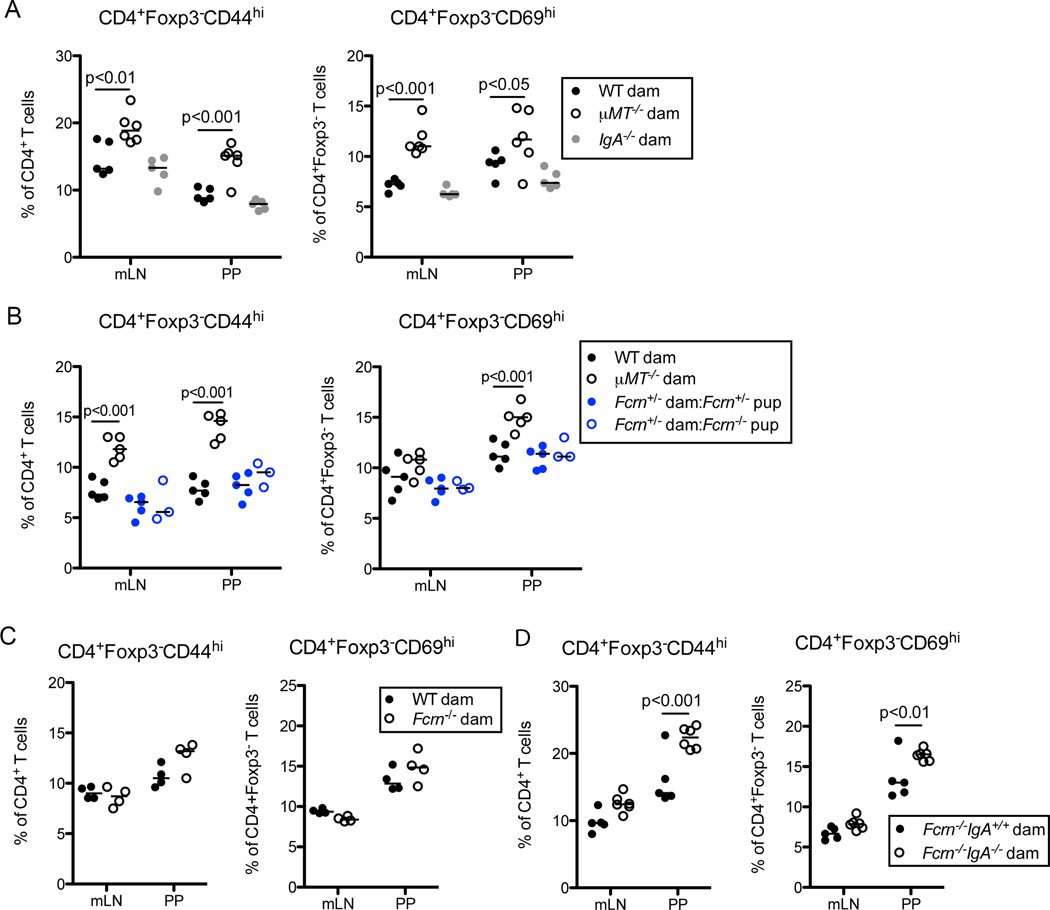
Within mammary tissue, the polymeric Ig receptor transports IgA and, to a lesser extent, IgM into breast milk. IgA has been proposed to promote barrier function by limiting bacterial interaction with the intestinal epithelium and preventing microbial translocation to underlying tissues (Rogier et al., 2014). To ascertain if maternal IgA was required to dampen mucosal Th cell responses, we compared offspring of IgA-deficient dams with progeny of WT or µMT−/− dams at d25 post birth. In contrast to pups lacking all maternal antibodies, we observed equivalent accumulation of Teff and activated CD4+Foxp3−CD69+ T cells in the mLN and PP of mice born to either WT or IgA-deficient dams (Figure 6A). Thus, surprisingly, maternal IgA is dispensable for the initial regulation of gut Th cell responses.
Figure 6. Maternal IgG and IgA Cooperate to Limit Mucosal T cell Responses.
(A) Frequencies of CD4+Foxp3−CD44hi Teff cells (left) or CD4+Foxp3−CD69hi activated T cells (right) present in the mLN and PP of mice born to WT, µMT−/− or IgA−/− dams at d25 of age. (B) Same as (A), comparing Fcrn+/− or Fcrn−/− mice born to Fcrn+/− dams with offspring of WT or µMT−/− dams as indicated. (C) Same as (A) comparing mice born to WT or Fcrn−/− dams. (D) Same as (A) comparing offspring of Fcrn−/− or Fcrn−/−IgA−/− dams. Bars on graphs indicate medians; symbols represent individual mice. Data are representative 2–10 independent experiments with 4–6 mice per group. Statistical significance was determined using two-way ANOVA. Bonferroni post-tests were used for pairwise comparisons. See also Figure S6.
Considering our findings that anti-commensal IgG2b and IgG3 are acquired maternally and bind the microbiota of young mice, we tested whether lack of these antibodies underlies the expansion of commensal specific T cells. FcRN is required for acquisition of maternal IgG antibodies in utero and transports lumenal IgG across the intestinal epithelium of neonates (Roopenian and Akilesh, 2007); however, our analyses of FcRN-deficient and FcRN-sufficient pups born to FcRN+/− dams revealed no alterations of T cell responses (Figure 6B). This agrees with the cross-fostering experiment (Figure 5D, S5E), indicating that maternal antibodies acquired from the breast milk, rather than in utero, are necessary and sufficient to prevent mucosal T cell responses. While FcRN-deficient pups had significantly reduced serum titers of maternal IgG2b and IgG3, both FcRN-deficient and FcRN-sufficient animals have similar levels of maternal IgG in their lumen (Figure S6A, S6B). FcRN has also been implicated in transporting IgG into breastmilk (Hurley and Theil, 2011; Roopenian et al., 2003), so we tested levels of maternally acquired IgG2b and IgG3 in pups born to FcRN−/− dams. Concentrations of IgG2b and IgG3 were reduced in the intestinal lumen and blood of pups born to FcRN-deficient dams, but the defect was incomplete when compared to offspring of µMT−/− dams (Figure S6C and data not shown). In addition, similar to our results of maternal IgA-deficient mice, we saw no increase in CD4 Teff and CD69+Foxp3− activated Th cells in mucosal tissues of pups of FcRN-deficient dams compared to control offspring (Figure 6C, S6D).
Finally, we considered that maternal IgA and FcRN-dependent IgG antibodies might coordinate to regulate mucosal T cell responses and mask any effect in pups born to FcRN or IgA singly deficient mice, especially considering the incomplete block in IgG acquisition in offspring of FcRN-deficient dams. To test this, we generated dams lacking both FcRN and IgA and bred these to WT males. We observed significant increases in the frequencies and numbers of CD4 Teff and CD69+Foxp3− activated Th cells in mLN and PP of mice lacking maternal IgA and FcRN-dependent IgG compared to pups of FcRN-deficient mothers at 25 days of age (Figure 6D, S6E). Importantly, this rules out any role for maternally acquired IgM and demonstrates that maternal IgA and IgG coordinate to limit Th responses to mucosal antigens.
Compensatory Germinal Center Responses Restore Homeostasis in Mice Lacking Maternal Antibodies
We next sought to ascertain the consequences, if any, of this immune dysregulation. Mice born to µMT−/− dams consistently weighed 10–15% less than pups born to µMT+/− dams at d25 post-birth, but this difference resolved in older mice (Figure 7A, S7A). Similarly, young mice lacking maternal antibodies showed slightly elevated levels of inflammatory serum cytokines and fecal lipocalin-2 when challenged with dextran sodium sulfate, but no difference was observed in older mice (Figure S7B, C) (Chassaing et al., 2012). This suggested that the dysregulation in young animals lacking maternal antibodies may be transient and that mechanisms may exist to restore homeostasis in older mice. Consistent with this possibility, we observed expansion of activated CD4 T cells only in young mice (Figure 7B).
Figure 7. Compensatory Germinal Center Responses in Mice Lacking Maternal Antibodies.
(A) Weights of offspring of µMT+/− and µMT−/− littermate dams at d25 post birth. (B) Frequencies of CD4+Foxp3−CD44hi Teff cells in the mLN (left) and PP (right) of mice sufficient or deficient in maternal antibodies at the indicated days post birth. (C) Frequencies of CD4+Foxp3−CD44hiT-bet+ (Th1), CD4+Foxp3−CD44hiRORgt+ (Th17) or CD4+Foxp3−CD44hiPD-1+CXCR5+ (Tfh) present in the mLN and PP of mice sufficient or deficient in maternal antibodies at d25 of age. Representative flow cytometric plots (right) of CXCR5 and PD-1 expression by gated live CD4+Foxp3−CD44hi Teff cells in the PP of the indicated offspring. (D) Representative flow cytometric analysis (left) of PNA and GL-7 expression by gated live CD19+IgDlo B cells present in the mLN of mice as in (C). Graphs display frequencies (middle) and numbers (right) of GC B cells present in the mLN and PP of indicated offspring. (E) Frequencies out of CD19+ cells of CD19+IgDloPNA+GL-7+ GC B cells present in the mLN and PP of mice with or without maternal antibodies at the indicated days post birth. (F) Frequencies out of CD19+ cells of CD19+IgDloPNA+GL-7+ GC B cells present in the mLN and PP of mice born to WT, µMT−/− or IgA−/− dams at d25 of age. Bars on graphs indicate medians; symbols represent individual mice. With the exception of (A), data are representative of 2–8 independent experiments with 4–6 mice per group, statistical significance was determined using two-way ANOVA, and Bonferroni post-tests were used for pairwise comparisons. For (A), data are compiled from 4 independent experiments, and statistical significance was determined using an unpaired student’s t-test. n.s. = not significant. See also Figure S7.
To better characterize the Th cell response in mice lacking maternal antibodies and examine how homeostasis may be restored, we profiled expression of transcription factors and trafficking receptors on CD4 Teff cells isolated from offspring of maternal antibody sufficient or deficient dams. We observed no reproducible difference in the proportion of T-bet+ or RORγT+ Teff cells in mice born to antibody-deficient dams as compared to controls (Figure 7C). Instead we found a marked expansion of CD4 Teff cells expressing the Tfh markers PD-1 and CXCR5 in pups devoid of maternal antibodies (Figure 7C). Tfh cells reside in the follicles of secondary lymphoid organs such as the mLN and PP where they provide help to differentiating B cells within germinal centers (GCs), thereby facilitating the production of high affinity, class switched antibodies (Crotty, 2014). Correlating with increased Tfh cells, we observed more GC B cells within the mLN and PP of maternal antibody-deficient mice as compared to control offspring (Figure 7D). Expectedly, the activation of CD4 T cells preceded the expansion of GC B cells (Figure 7E). Notably, offspring of dams deficient solely in IgA did not exhibit increased frequencies or numbers of GC B cells in the mLN and PP (Figure 7F, S7D), consistent with the lack of Teff cells expansion in these mice (Figure 6A).
Discussion
Here, we characterize a mechanism that limits immune responses to newly acquired commensal antigens in neonates. We report a broad, anti-commensal, TI IgG response, composed primarily of IgG2b and IgG3 isotypes, that is partially dependent on TLR2 and TLR4. Maternally acquired IgG and IgA limit mucosal T-cell activation and subsequent T-dependent B cell responses during early life. At the time of weaning, commensal microbes localize close to the intestinal epithelium (Rogier et al., 2014), and maternal antibodies limit bacterial translocation to the mLN (Figure 5C). In the absence of maternal antibodies, new commensal antigens induce a compensatory T-dependent antibody response that appears to restore homeostasis. Whether such aberrant T and B cell responses lead to other long-term consequences remains unclear and is an important topic for further study. Nevertheless, our work shows that control of T-dependent immunity to gut microbes requires instructional cues from TI antibodies and the innate immune system.
Commensal-specific IgA and IgG may work by distinct mechanisms and in discrete anatomical compartments to regulate intestinal immunity. IgA may entrap microbes in the lumen, while IgG2b and IgG3 antibodies regulate responses to translocated microbes. Importantly, lumenal IgG can be transported into the systemic circulation, and maternal IgG antibodies present on both sides of the intestinal epithelium may cooperate to limit mucosal responses. Coating of bacteria by breast milk-derived IgG may function similarly to IgA by discouraging bacterial association with the intestinal epithelium; IgG could also enhance bacterial sampling by FcRN-expressing intestinal epithelial cells. Additionally, maternal-derived IgG antibodies may coat naked microbes sampled at inductive sites to facilitate microbial clearance. These two compartments of maternal IgG cooperate with IgA to ensure intestinal homeostasis such that, in the absence of either IgA or IgG, antibodies of the other isotype are sufficient to quell aberrant immune responses. Dual recognition of microbes by both IgA and IgG highlight the cooperative nature of these antibodies (Figure 2A, 2B). While our study demonstrates the importance of commensal-specific IgG antibodies when acquired maternally, it is certainly possible that they serve important functions in adults.
Notably, IgG can engage immune effector mechanisms that IgA cannot. IgG2b and IgG3 may target whole microbes to intestinal macrophages for rapid, non-inflammatory clearance. A recent paper proposed that maternal IgG delivers microbial molecules to offspring, which increases the number of intestinal type 3 innate lymphoid cells and reinforces barrier integrity (Gomez de Aguero et al., 2016). Similar mechanisms may underlie the immune activation we observe. Alternatively, ligation of Fc receptors or complement receptors on mucosal dendritic cells by IgG2b and IgG3 antibodies may modulate the immunostimulatory capacity of commensal microbes or microbial components, perhaps by preventing production of polarizing cytokines, thereby influencing T cell differentiation. Mouse IgG2b binds both activating and inhibitory Fc receptors, whereas mouse IgG3 binds to complement receptor 2 and FcγR1 (Bruhns, 2012; Diaz de Stahl et al., 2003). Interestingly, a unique receptor for mouse IgG3 has been proposed but remains undiscovered (Saylor et al., 2010).
IgG3 and IgG2b producing B cells are considerably less frequent than their IgA-secreting counterparts, yet the breadth of IgG-mediated recognition of the microbiota exceeded that of IgA. Anti-commensal IgG antibodies may recognize common features of commensal microbes. In support of this, we generated a hybridoma that produced IgG3 capable of binding multiple Lactobacillus isolates, including L. salivarius, L. brevis and L. murinus (data not shown). As anti-commensal IgG responses were largely dependent on B cell intrinsic TLR2 and TLR4 stimulation by microbial ligands (Figure 4), it is possible that these antibodies target TLR ligands, namely bacterial lipopeptides and LPS, although our preliminary analyses suggest this possibility is unlikely. While our study was under review, another group described T-dependent, anti-commensal IgG antibodies that cross-react with gram-negative pathogens such as S. typhimurium (Zeng et al., 2016). We did not observe binding of S. enterica serovar Typhimurium or C. rodentium by IgG3 or IgG2b from sera of healthy mice from our colony or Jackson Labs (Figure S1B). These conflicting results might result from differences in the flora of mice used for the two studies. Interestingly, vaccination of mice with a glycan present on an E. coli isolate elicited IgG2b, IgG3 and IgM antibodies that cross-reacted with P. berghei and could restrict parasite burden following intradermal Plasmodium infection (Yilmaz et al., 2014). Thus, IgG antibodies elicited by commensal species may help confer protection to pathogens containing related epitopes.
Considering the overlap in microbes bound by IgG3 and IgA, it is conceivable that these two isotypes share the same specificities. Indeed, B cells induced to produce IgG3 in vitro undergo further class switching to secrete IgA upon culture with TGF-β (Deenick et al., 1999). Binding by IgA can induce downregulation of antigens on bacteria (Cullender et al., 2013). As IgG3 and IgG2b antibodies are not secreted into the intestinal lumen and are not in constant interaction with microbes, we speculate that anti-commensal IgG antibodies may target unique antigens to facilitate recognition of gut microbes that avoid detection by IgA.
Similar to the majority of commensal-specific IgA, T cells were dispensable for the production of IgG2b and IgG3 antibodies targeting the microbiota (Figure 3)(Bunker et al., 2015; Macpherson et al., 2000). A recent study demonstrated that a phenotypically and functionally distinct population of B cells, termed B-1 cells, was sufficient for production of TI commensal-specific IgA (Bunker et al., 2015). Following activation by cytokines or microbial products, such as LPS, B-1 cells from the peritoneal and pleural cavities migrate to the gut LP and secrete IgA (Ha et al., 2006; Kroese et al., 1989; Moon et al., 2004). As IgG2b+ and IgG3+ B cells in the mLN and PP expressed increased levels of the B-1 marker CD43, it is possible that these B cells belong to the B-1 subset. Precisely how microbiota-derived TLR2 and TLR4 ligands activate B cells to produce IgG antibodies remains unclear. B cells may be stimulated by whole microbes sampled from the lumen (Macpherson and Uhr, 2004), by bacteria colonizing the PP (Obata et al., 2010) or by dissociated microbial ligands that access host tissues (Henao-Mejia et al., 2012).
Our characterization of anti-commensal IgG responses helps reconcile several observations regarding the function of antibodies in maintaining intestinal homeostasis. Despite the abundance of IgA within the gut, humans lacking this isotype display only subtle perturbations in intestinal immunity (Yel, 2010). In contrast, a proportion of human subjects with low levels of all circulating antibodies due to common variable immunodeficiency syndrome display pronounced intestinal defects including frequent gastrointestinal infections accompanied by a lymphoproliferative disorder of the small intestine (Washington et al., 1996). Some animals (e.g., cows, sheep, and horses) lack trans-placental transport of maternal antibodies. Notably, newborn calves fail to thrive without antibody-rich colostrum (Smith and Little, 1922), which, in light of our results, may be attributed to dysregulated immunity to commensal antigens in the absence of maternal antibodies. This study expands our understanding of how appropriate mucosal immune responses are established following microbial colonization and reveals how immune dysregulation during development can influence intestinal homeostasis throughout life. The importance of maternal IgG has traditionally been ascribed to provision of passive immunity against a variety of pathogens, thereby protecting offspring from potentially fatal infections (Niewiesk, 2014). By demonstrating that mice deficient in maternal antibodies exhibit dysregulated mucosal responses, our data reveal a broader function for maternal IgG in helping to establish immune homeostasis early in life.
Experimental Procedures
Animals
Mice strains were maintained under SPF conditions at UC Berkeley to normalize the microbiota as much as possible, except for mice shown in Figure 1F, where mice were analyzed upon receipt from the indicated vendor. For cohousing experiments, mice were combined at 3–4 weeks of age until breeding age. For breeding of littermate or cohoused dams, females were separated into individual cages and bred for 2–3 nights, dams were then “re-cohoused” for approximately 18 days. Pregnant females were separated and housed individually until parturition. All experiments with mice were performed in accordance with the guidelines of the Animal Care and Use Committee at UC Berkeley. See Supplemental Experimental Procedures for details.
Bacterial Strains
Commensal bacterial strains were isolated from wild-type C57Bl/6 mice from UC Berkeley. See Supplemental Experimental Procedures for details.
Flow Cytometry and Cell Sorting
For bacterial flow cytometry, bacteria were washed in sterile-filtered PBS with 1% bovine serum albumin (BSA; Fisher) and 0.05% Azide (Fisher) and resuspended at approximately 5×106 bacteria/mL. Mouse serum was diluted 1:25 in PBS/BSA/Azide buffer and 25µl of this solution was mixed with 25µl diluted microbes in a v-bottom plate. Staining was performed with flourochrome conjugated or biotinylated antibodies followed by streptavidin-PECy7 (eBioscience). Cells were washed and resuspended in PBS with SYBR Green (Invitrogen) and analyzed by FACS. Pathogens were resuspended in 2% PFA. Unless otherwise stated, feces and serum samples for mFLOW were taken from the same mouse. For most experiments, we included both SYBRhi and SYBRmid events in our analyses (referred to as SYBR+). In some experiments in Figure 1, analysis was restricted to SYBRhi events.
For flow cytometry on mouse cells, dead cells were excluded using a fixable live/dead stain (Life Technologies) and all stains were carried out in PBS containing 1%BSA (w/v) and 0.1% Azide (w/v) including anti-CD16/32 blocking antibody (2.4G2) and normal rat serum (Sigma). Cells were stained for 20min at 4°C with surface antibodies. For transcription factor staining, surface stained cells were permeabilized with Fix/Perm buffer (eBioscience) for 20min at 4°C. Cells were washed and stained for 20min at 4°C with antibodies to transcription factors. Data were acquired on LSR II or X20 (BD Biosciences). See Table S2 for antibody details.
Statistics
Statistical significance was determined as indicated in the figure legends with Prism 5 (GraphPad Software Inc).
16S rRNA Gene Sequencing and Microbial Community Analysis
16S rRNA sequencing was performed by the Alkek Center for Metagenomics and Microbiome Research at Baylor College of Medicine using an Illumina MiSeq sequencer. Sequence processing for antibody-bound sorted populations of bacteria was performed using a custom pipeline described in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank N. Arpaia, A. Rudensky and J. Sonnenberg for providing samples from Tcrb−/−Tcrd−/− and GF animals, respectively, H. Nolla and A. Valeros (Berkeley Cancer Research Laboratory Flow Cytometry Facility) for assistance with cell sorting, T. Huynh, J. Yap, A. Lin La, S. Chu, J. Nguyen, C. Purba, A. Ahn, E. Lau, E. Wu, K. Shamardani and the Life Sciences Addition Vivarium Staff for mouse colony maintenance, K. Ching and B. Russell for technical assistance, and Aduro Biotech for use of their ImmunoSpot C.T.L. Analyzer. We thank R. Vance, M. Headley, E. Roberts and members of the Barton and Vance labs for critical reading of the manuscript and helpful discussions. This work was supported by the NIH (AI063302, AI095587, AI104914 to G.M.B.). G.M.B. is a John P. Stock Faculty Fellow and the recipient of a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease award. M.A.K. was supported by postdoctoral fellowship #252507 from the Crohn’s and Colitis Foundation of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atarashi K, Tanoue T, Ando M, Kamada N, Nagano Y, Narushima S, Suda W, Imaoka A, Setoyama H, Nagamori T, et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell. 2015;163:367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos NA, Kimura H, Meeuwsen CG, De Visser H, Hazenberg MP, Wostmann BS, Pleasants JR, Benner R, Marcus DM. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol. 1989;19:2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- Bruhns P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood. 2012;119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- Bunker JJ, Flynn TM, Koval JC, Shaw DG, Meisel M, McDonald BD, Ishizuka IE, Dent AL, Wilson PC, Jabri B, et al. Innate and Adaptive Humoral Responses Coat Distinct Commensal Bacteria with Immunoglobulin A. Immunity. 2015;43:541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Meth. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassaing B, Srinivasan G, Delgado MA, Young AN, Gewirtz AT, Vijay-Kumar M. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, Werner JJ, Angenent LT, Bell ME, Hay AG, Peterson DA, et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe. 2013;14:571–581. doi: 10.1016/j.chom.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deenick EK, Hasbold J, Hodgkin PD. Switching to IgG3, IgG2b, and IgA is division linked and independent, revealing a stochastic framework for describing differentiation. J Immunol. 1999;163:4707–4714. [PubMed] [Google Scholar]
- Diaz de Stahl T, Dahlstrom J, Carroll MC, Heyman B. A role for complement in feedback enhancement of antibody responses by IgG3. J Exp Med. 2003;197:1183–1190. doi: 10.1084/jem.20022232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
- Fransen F, Zagato E, Mazzini E, Fosso B, Manzari C, El Aidy S, Chiavelli A, D’Erchia AM, Sethi MK, Pabst O, et al. BALB/c and C57BL/6 Mice Differ in Polyreactive IgA Abundance, which Impacts the Generation of Antigen-Specific IgA and Microbiota Diversity. Immunity. 2015;43:527–540. doi: 10.1016/j.immuni.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Garofalo R, Chheda S, Mei F, Palkowetz KH, Rudloff HE, Schmalstieg FC, Rassin DK, Goldman AS. Interleukin-10 in human milk. Pediatr Res. 1995;37:444–449. doi: 10.1203/00006450-199504000-00010. [DOI] [PubMed] [Google Scholar]
- Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, et al. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand TW, Dos Santos LM, Bouladoux N, Molloy MJ, Pagan AJ, Pepper M, Maynard CL, Elson CO, 3rd, Belkaid Y. Acute gastrointestinal infection induces long-lived microbiota-specific T cell responses. Science. 2012;337:1553–1556. doi: 10.1126/science.1220961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen HJ, Pouwels SD, Funke A, Bos NA, Dijkstra G. Crohn’s disease patients have more IgG-binding fecal bacteria than controls. Clin Vaccine Immunol. 2012;19:515–521. doi: 10.1128/CVI.05517-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442–474. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane SV, Acquah LA. Placental transport of immunoglobulins: a clinical review for gastroenterologists who prescribe therapeutic monoclonal antibodies to women during conception and pregnancy. Am J Gastroenterol. 2009;104:228–233. doi: 10.1038/ajg.2008.71. [DOI] [PubMed] [Google Scholar]
- Kroese FG, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol. 1989;1:75–84. doi: 10.1093/intimm/1.1.75. [DOI] [PubMed] [Google Scholar]
- Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukens D, Brinkman BM, Raes J, De Vos M, Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2016;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BL, Moon JE, Shu JH, Yuan L, Newman ZR, Schekman R, Barton GM. UNC93B1 mediates differential trafficking of endosomal TLRs. Elife. 2013;2:e00291. doi: 10.7554/eLife.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science. 1994;264:1936–1938. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a New Phylogenetic Method for Comparing Microbial Communities. Applied and Environmental Microbiology. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5:169–172. doi: 10.1038/ismej.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and Qualitative β Diversity Measures Lead to Different Insights into Factors That Structure Microbial Communities. Applied and Environmental Microbiology. 2007;73:1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Rogers PR, Grusby MJ, Parker DC, Glimcher LH. B lymphocyte development and activation independent of MHC class II expression. J Immunol. 1993;150:1223–1233. [PubMed] [Google Scholar]
- Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39–50. doi: 10.1016/j.cell.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol. 2004;172:6020–6029. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- Moon C, Baldridge MT, Wallace MA, Burnham CA, Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521:90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol. 2014;5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Goto Y, Kunisawa J, Sato S, Sakamoto M, Setoyama H, Matsuki T, Nonaka K, Shibata N, Gohda M, et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc Natl Acad Sci U S A. 2010;107:7419–7424. doi: 10.1073/pnas.1001061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- Palm NW, de Zoete MR, Cullen TW, Barry NA, Stefanowski J, Hao L, Degnan PH, Hu J, Peter I, Zhang W, et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell. 2014;158:1000–1010. doi: 10.1016/j.cell.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- Pfeiffer JK, Virgin HW. Viral immunity. Transkingdom control of viral infection and immunity in the mammalian intestine. Science. 2016:351. doi: 10.1126/science.aad5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci U S A. 2014;111:3074–3079. doi: 10.1073/pnas.1315792111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY, Shaffer DJ, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- Saylor CA, Dadachova E, Casadevall A. Murine IgG1 and IgG3 isotype switch variants promote phagocytosis of Cryptococcus neoformans through different receptors. J Immunol. 2010;184:336–343. doi: 10.4049/jimmunol.0902752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack E, Hapfelmeier S, Stecher B, Velykoredko Y, Stoel M, Lawson MAE, Geuking MB, Beutler B, Tedder TF, Hardt W-D, et al. Innate and adaptive immunity cooperate flexibly to maintain host-microbiota mutualism. Science. 2009;325:617–620. doi: 10.1126/science.1172747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Little RB. The Significance of Colostrum to the New-Born Calf. J Exp Med. 1922;36:181–198. doi: 10.1084/jem.36.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Hoebe K, Janssen EM, Du X, Georgel P, Crozat K, Mudd S, Mann N, Sovath S, Goode J, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Lipuma L, Gobourne A, Viale A, Leiner I, Equinda M, Khanin R, Pamer EG. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J Exp Med. 2012;209:1445–1456. doi: 10.1084/jem.20120504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij LA, Mesander G, Limburg PC, van der Waaij D. Direct flow cytometry of anaerobic bacteria in human feces. Cytometry. 1994;16:270–279. doi: 10.1002/cyto.990160312. [DOI] [PubMed] [Google Scholar]
- Washington K, Stenzel TT, Buckley RH, Gottfried MR. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol. 1996;20:1240–1252. doi: 10.1097/00000478-199610000-00010. [DOI] [PubMed] [Google Scholar]
- Weisz-Carrington P, Roux ME, Lamm ME. Plasma cells and epithelial immunoglobulins in the mouse mammary gland during pregnancy and lactation. J Immunol. 1977;119:1306–1307. [PubMed] [Google Scholar]
- Yel L. Selective IgA deficiency. J Clin Immunol. 2010;30:10–16. doi: 10.1007/s10875-009-9357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, Regalado A, Cowan PJ, d’Apice AJ, Chong AS, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell. 2014;159:1277–1289. doi: 10.1016/j.cell.2014.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng MY, Cisalpino D, Varadarajan S, Hellman J, Warren HS, Cascalho M, Inohara N, Nunez G. Gut Microbiota-Induced Immunoglobulin G Controls Systemic Infection by Symbiotic Bacteria and Pathogens. Immunity. 2016;44:647–658. doi: 10.1016/j.immuni.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.