Abstract
The testis-specific serine/threonine kinase 2 (TSSK2) has been proposed as a candidate male contraceptive target. Development of a selective inhibitor for this kinase first necessitates the production of highly purified, soluble human TSSK2 and its substrate, TSKS, with high yields and retention of biological activity for crystallography and compound screening. Strategies to produce full-length, soluble, biologically active hTSSK2 in baculovirus expression systems were tested and refined. Soluble preparations of TSSK2 were purified by immobilized-metal affinity chromatography (IMAC) followed by gel filtration chromatography. The biological activities of rec.hTSSK2 were verified by in vitro kinase and mobility shift assays using bacterially produced hTSKS (isoform 2), casein, glycogen synthase peptide (GS peptide) and various TSKS peptides as target substrates. Purified recombinant hTSSK2 showed robust kinase activity in the in vitro kinase assay by phosphorylating hTSKS isoform 2 and casein. The ATP Km values were similar for highly and partially purified fractions of hTSSK2 (2.2 and 2.7 μM, respectively). The broad spectrum kinase inhibitor staurosporine was a potent inhibitor of rec.hTSSK2 (IC50 = 20 nM). In vitro phosphorylation experiments carried out with TSKS fragments revealed particularly strong phosphorylation of a recombinant N-terminal region representing aa 1–150 of TSKS, indicating that the N-terminus of human TSKS is phosphorylated by human TSSK2. Production of full-length enzymatically active recombinant TSSK2 kinase represents the achievement of a key benchmark for future discovery of TSSK inhibitors as male contraceptive agents.
Keywords: TSSK2, male contraceptive drug targets, recombinant kinases, mobility shift assay, TSKS
1. Introduction
To offer an alternative to vasectomy, condoms and withdrawal methods, a pharmacological approach to male contraception has been a long-standing goal in reproductive medicine. Various steroid-based formulations, including testosterone and testosterone analogues, alone and combined with various progestagens, have entered clinical trials in recent years as both oral and topical formulations [1–5]. To date, however, no drug has been approved in any country specifically for male contraception, although anabolic steroids used by some athletes to enhance athletic performance have been shown to reduce sperm counts and to result in infertility [6–8].
Small molecule kinase inhibitors are a theoretically attractive strategy for male contraception, provided testis selectivity of drug action can be achieved. One means to attain testis-selective drug action would be to target kinases that are unique to spermatogenesis and known to be essential for both spermatogenesis and fertility in mammals. The testis-specific serine/threonine kinaseTSSK2 is one such candidate contraceptive target. TSSK2 belongs to a five member testis-specific serine/threonine kinase family within the calcium/calmodulin-dependent protein kinase (CaMK) superfamily [9]. TSSK2 is part of a branch in the human kinome that contains the five TSSKs: TSSK1, TSSK2 [10–15], TSSK3 [16, 17], TSSK4 (also known as TSSK5) [18–20] and TSSK6 [21, 22]. TSSK2 has generated particular interest as a contraceptive target because of its pattern of expression in the testis and the testis-specific expression of its substrate TSKS. Moreover, targeted deletions in mice employing TSSK1 and TSSK2 constructs that bridged both genes resulted in infertility as shown in two independent studies [13, 14]. In situ hybridization localized the mRNAs for TSSK2 in spermatids, and protein localizations showed that the TSSK2 kinase and the TSKS substrate are first detected in spermatids within the testis and persist in ejaculated sperm [12, 23]. These findings support a TSSK targeting model that posits restricted contraceptive drug action on spermiogenesis, the post-meiotic step of spermatogenesis, provided that a selective kinase inhibitor can be identified that does not produce off-target effects on related kinases involved in non-gonadal pathways found more widely in other tissues.
TSKS has been localized near the base of the spermatid nucleus in association with the developing sperm flagellum in an organelle that has been interpreted to be the centriole [12] or the chromatoid body [14]. A second protein substrate of TSSK2, SPAG16 [24], is a component of the central apparatus in the flagellar axoneme, which is essential to sperm motility. Deletion of the SPAG16L protein resulted in sperm motility defects and infertility [25]. Thus, the two known substrates of TSSK2, TSKS and SPAG16L, both associate with organelles within the sperm flagellum. Furthermore, Zhang et al. [26] recently provided evidence that single nucleotide polymorphisms of the TSSK2 gene are associated with impairment of spermatogenesis in idiopathically infertile males. These findings establish an indispensable role for the TSSK pathway in spermiogenesis and validate the TSSK2 enzyme as a candidate male contraceptive target. Inhibitors of TSSK2 are predicted to interrupt sperm flagellar function, including sperm motility [27], flagellogenesis or other activities of spermiogenesis involving centriolar function [28], and/or intraflagellar transport [29].
One of the critical initial challenges to discovering inhibitors targeting a specific kinase is the production of soluble, biologically active recombinant enzyme and substrate in adequate quantities for high throughput screening and drug target interaction studies. Approaches are described here to obtain full-length soluble recombinant hTSSK2, to develop methods to validate the biological activity of this kinase, and to screen for TSSK2 inhibitors. Recombinant, soluble versions of hTSKS were also engineered and were used to test the biological activity of the full-length TSSK2. Additionally, analysis of the protein domains within the full-length (Isoform 1) form of hTSKS identified a discrete TSKS fragment containing the primary residues phosphorylated by TSSK2.
2. Materials and Methods
2.1. Design of constructs and expression of hTSKS in E. coli
Human TSKS is known to have two main splice variants. The full-length human TSKS (isoform 1, accession number: NM_021733) contains 592 aa (11), whereas isoform 2 lacks the first 5 exons while retaining exons 6–10 and adding a unique first exon, yielding a predicted 43 kDa protein of 386 aa [13]. The cDNA for hTSKS-isoform 2 (accession number: AAL60464) was PCR amplified and subcloned into the pET 28b vector with a His tag at the N-terminus of the protein. The primers were: forward, 5′AGT CAT ATG GCT AGC GAC TCT TGC CCT GCA; and reverse, 5′TG CTC GAG AGC ATG AGA GGC CAT TTA TTG TTC AGG. To express the protein, induction was performed at 37 °C with 0.5 mM isopropyl-beta-D-thiogalactopyranoside (IPTG).
2.2. Cloning and Expression of hTSSK2 in insect cells
The ORF of full-length hTSSK2 (358 aa) was amplified by PCR from human testis cDNA. The primers were: forward, AGT CCA TGG ACG ATG CCA CAG TCC TAA; and reverse, GAG CGG CCG CCT AGG TGC TTG CTT TCC CCAC. The DNA fragment was cloned into a pFastBac- HTb vector (Life Technologies, Carlsbad, USA) with a His-tag at the N-terminus of the protein under the control of the polyhedrin promoter. The recombinant plasmid was transformed into competent E. coli DH10Bac cells (Life Technologies) for transposition into the bacmid. Sf9 cells were transfected with recombinant bacmid using Cellfectin II transfection reagent (Life Technologies) followed by 94 h of growth at 27 °C. After several rounds of virus amplification, recombinant protein was expressed by infecting Sf9 cells for 94 h in suspension culture or in adherent culture. Cell pellets were either used directly for protein purification or flash-frozen in liquid nitrogen and stored at −80 °C.
2.3. Generation of recombinant TSKS peptides
The full-length hTSKS (592 aa, Acc. No. NP_068379) was divided into 4 peptide segments sequentially named TSKS-pep1 to TSKS-pep4, containing 150, 150, 150 and 142 aa, respectively. Primer sets were: TSKS-Pep1-F, ATC ATA TGT AAA TGG CGA GCG TGG TGG TGA AGA CG; TSKS-Pep1-R, GTC TCG AGC AAG CTG GTG ATG GAG TCT T TG GC; TSKS-Pep2-F, ATC ATA TGT AAA TGA AGG AAA AGA CCA ACC GGG TT; TSKS-Pep2-R, GTC TCG AGG GGG ACC AGG CCG TGT GGC CGC G; TSKS-Pep3-F, ATC ATA TGT AAA TGG CAG GCT GGG GAA TGG GGC CT; TSKS-Pep3-R, GTC TCG AGG GCA CAG TTG CCT TGG TGG GAC CG; TSKS-Pep4-F, ATC ATA TGT AAA TGC GCT GTG CCA GCC AGG GGT CG; and TSKS-Pep4-R, GTC TCG AGT TGT TCA GG G GCT GAG CCC CCC TG. All the forward primers carried an NdeI restriction site and the reverse primers carried an XhoI site. The cDNA encoding each TSKS peptide segment was PCR amplified by using the full-length TSKS plasmid DNA as the template. The PCR amplified DNA was subcloned into the pET 28b vector in such a way that expressed polypeptides included a C-terminal His tag. The plasmids were transformed into BL21 DE3 codon plus strains of E. coli and expression of TSKS peptides were performed by inducing with IPTG at 37 °C.
2.4. Purification of hTSSK2 and TSKS
For purification of bacterially produced soluble hTSKS, the induced cells were suspended in a binding buffer: 50mM Tris-HCl buffer (pH 7.9) containing 0.5 M NaCl, 5% glycerol, 5 mM imidazole and protease inhibitor cocktail (Sigma). The suspended cells were sonicated and centrifuged at 15,000 x g, and the supernatants containing the soluble recombinant proteins were collected. The Sf9 cells producing hTSSK2 were lysed in 50 mM HEPES buffer (pH7.5) containing 0.5 M NaCl, 5% glycerol, 5 mM imidazole and EDTA free protease inhibitors (Clontech). Lysis was enhanced by homogenization of the suspended cells using a dounce homogenizer. The homogenate was then centrifuged at 15,000 x g for 1 h to obtain the clear supernatant containing soluble hTSSK2. The rec-hTSKS and -hTSSK2 from both bacterial and insect cell preparations were purified from the soluble fraction by immobilized metal affinity chromatography (IMAC) on a His binding Ni-NTA column (Novagen) under non-denaturing conditions. Ni-NTA purified, concentrated hTSSK2 was loaded onto a Superdex 200 gel filtration column (GE Healthcare, Uppsala, Sweden). Aggregated and monomeric peaks of hTSSK2 were collected and analyzed by SDS-PAGE and by the in vitro kinase assay described below.
2.5. Densitometry
To quantify fraction purity after two-step purification with Ni-NTA and gel filtration, densitometry of Ponceau stained blots was performed using the NIH ImageJ software. The tiff images of the blots were scanned using the ImageJ processing program by measuring the intensities of TSSK2 specific bands and other co-purified bands. The densitometric values were expressed as integrals after subtracting the background. The percentage purity of a recombinant TSSK2 was obtained by comparing its densitometric value to that of other bands.
2.6. In vitro kinase assay
In vitro kinase assays were performed as described previously [30]. Briefly, partially purified or homogeneously purified rec.hTSSK2 was incubated at 30°C for 10 min in a buffer containing 10 mM Tris–HCl (pH 7.4), 10 mM MgCl2, 10 mM MnCl2, 10 μM aprotinin, 10 μM leupeptin, 100 μM Na3VO4, 5 mM p-nitrophenyl phosphate, 0.2 mg/ml of BSA, 40 mM β-glycerol phosphate and 5 μCi [32P] γATP. To evaluate the ability of TSSK2 to phosphorylate TSKS, bacterially produced purified hTSKS-isoform 2 or purified peptide fragments of hTSKS-isoform1 were added to the reaction. Casein was used as a generic substrate and 10 units of casein kinase II (CKII, New England Biolabs) was used as the positive control. The phosphorylation assay was stopped by boiling in sample buffer and the 32P incorporation was analyzed using 12% SDS-PAGE and autoradiography.
2.7. SDS-PAGE and Western blotting
Protein electrophoresis was performed on discontinuous polyacrylamide gels on a Bio-Rad Criterion II cell apparatus or on 16 × 16 × 0.15 cm slab gels. Gels were stained with Coomassie Blue or blotted. For Western blots, proteins were transferred to nitrocellulose membrane and processed as described previously [31].
2.8. MS/MS Protein Microsequencing
Coomassie stained protein spots were isolated and removed from SDS-PAGE gels using finely milled coring tools [32]. The gel pieces were destained in methanol, reduced in 10 mM DTT, and alkylated with 50 mM iodoacetamide in 0.1 M ammonium bicarbonate. The gel pieces were then incubated with 12.5 ng/ml trypsin in 50 mM ammonium bicarbonate overnight at 37 °C. They were dehydrated in acetonitrile and rehydrated in trypsin, chymotrypsin or Glu-C in ammonium bicarbonate. The samples were digested overnight at 37 °C and the peptides w ere extracted and microsequenced by tandem mass spectrometry at the Biomolecular Research Facility of the University of Virginia.
2.9. Microfluidic mobility shift assay (MSA)
The concentrations of rec.hTSSK2 produced in insect cells and ATP were optimized using a 10 aa fluorescently labeled glycogen synthase (5-FAM-GS) peptide (5-FAM-PLSRTLSVSS-NH2; Anaspec) substrate. A 5 μL volume of reaction buffer (10 mM MgCl2, 0.015% Brij-35, 100 mM HEPES, pH 7.5) supplemented with 4mM DTT and 2-fold serially diluted purified or partially purified hTSSK2 preparations, or a commercially sourced hTSSK2 (Carna Biosciences), ranging from 0.84 μg to 20 ng per well, was dispensed into low-volume microplates (Corning #3677). The reaction was then initiated with the addition of 5 μL of reaction buffer containing 1.5 μM of 5-FAM-GS substrate and 100 μM ATP. The total reaction volume was 10 μL. Subsequently, the ATP KM was determined in a similar manner employing 2-fold serially diluted ATP over a range of 12 concentrations (100 μM to 50 nM) in reaction buffer, with the kinase reaction initiated by the addition of 0.84 μg hTSSK2 per well. A kinetic study with reaction sampling intervals of one minute was conducted employing a Caliper LC3000 (Perkin Elmer) system using optimized separation parameters to evaluate reaction velocities resulting from each hTSSK2 preparation. Separations were performed using an EZ Reader 12-sipper® microchip. Fluorescent substrate (2 μM) was introduced before and after each run as a reference marker. The separation buffer consisted of 100 mM HEPES (pH 7.5), 0.015% Brij-35 solution, 0.1% (w/v) Coating Reagent #3 (Perkin Elmer) and 10 mM EDTA for all analyses.
The broad-spectrum kinase inhibitor staurosporine was selected for dose-response analyses and was tested using a 5-fold serial dilution over eight concentrations in duplicate, ranging from 0.25 nM to 20 μM. Staurosporine was added to the plate using an Echo 550 contactless acoustic dispenser (Labcyte). A 5 μL volume of each of the reaction and substrate cocktails containing 5 μM ATP were dispensed into the plate, the plate was sealed under vacuum, mixed on a plate shaker for 1 min, and the reaction allowed to proceed for 1 h at 30 °C. The reaction was terminated with the addition of 1 μL of 250 mM EDTA in 100 mM HEPES, pH 7.5. An end-point determination of the analyses was conducted using the LC3000. HTS Well Analyzer® (Perkin Elmer) was used to evaluate the TSSK2 progress curves and Prism (GraphPad) was used to calculate ATP Km and IC50 values from three independent experiments.
3. Results
3.1 Expression and purification of soluble full-length hTSKS-isoform 2 in E. coli
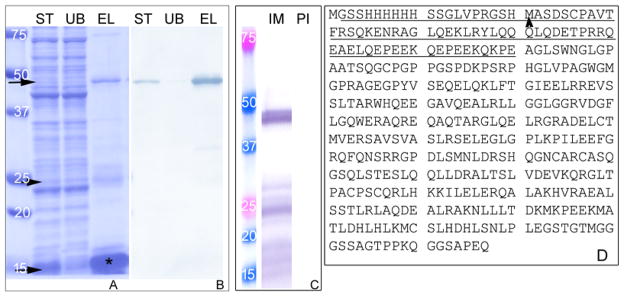
A partially soluble hTSKS isoform 2 (387 aa) was produced with a plasmid construct expressing a His-tag at the N-terminus of the protein using the pET28b expression vector. hTSKS was purified under non-denaturing conditions by one-step Ni-NTA affinity chromatography (Fig. 1A, B). In addition to the predicted 46 kDa form of the fusion protein, several low molecular weight bands (see arrows in Fig. 1A) were negative or weakly positive with an anti-His antibody and reactive with an anti-hTSKS antibody (Fig. 1C), indicating that they were peptide fragments of hTSKS. The very low molecular weight TSKS peptides ranged in mass from ~12–17 kDa. MS/MS microsequencing of a 12–17 kDa band recovered TSKS peptides from a continuous stretch of 80 amino acids located at the N-terminus including the -His tag (underlined in Fig. 1D; starting methionine of TSKS indicated by arrowhead) confirming that these bands represent breakdown products of hTSKS. This one-step Ni-NTA purified recombinant hTSKS isoform 2 was used subsequently for in vitro kinase assays. The non-denaturing method of purification produced relatively good yield of soluble hTSKS-isoform 2 at the range of 6–8 milligrams per liter of culture (Table 1).
Figure 1.
SDS-PAGE and Western blot analysis of recombinant hTSKS isoform 2 expressed in E. coli and purified by non-denaturing Ni-NTA chromatography. (A) The purified hTSKS showed several bands in the Coomassie stained gel (see arrows) in addition to the predicted band at a molecular weight of ~46 kDa. (B) 46 kDa band immunoreacted with anti-His antibody. (C) Low molecular weight bands at approximately 25kDa and 12–17 kDa were negative or very weakly positive to anti-His antibody, but were positive with anti-TSKS antibody suggesting that they are breakdown products of TSKS. Microsequencing by mass spectrometry of the low molecular weight band at ~12–17 kDa (asterisk in A) recovered peptides from a continuous stretch of 80 amino acids on the N-terminus including the His tag (underlined in D; starting methionine of TSKS designated with arrow head). These results indicated that these low molecular weight bands were authentic TSKS. IM: Immune, PI: Pre-immune, ST: Starting material, UB: Unbound, EL:Elute.
Table 1.
Yield and recovery of purified soluble recombinant hTSKS-isoform 2 and hTSSK2
| Rec. Protein | Expression host | Culture conditions | Purification Step | Yield/L | % Recovery |
|---|---|---|---|---|---|
| hTSKS-Isoform 2 | E.–coli | 37°C | IMAC | 8mg/L | |
| hTSSK2-Full length | Baculovirus-Sf9 cells | Suspension Cells (2×106 cells/ml) | IMAC | 1 mg/L | 100 |
| Gel filtration | 0.1mg/L | 10 | |||
| Adherent Cells | IMAC | 1.4mg/L | 100 | ||
| Gel Filtration | 0.85mg/L | 60 |
3.2. Expression and purification of full-length hTSSK2 in insect cells
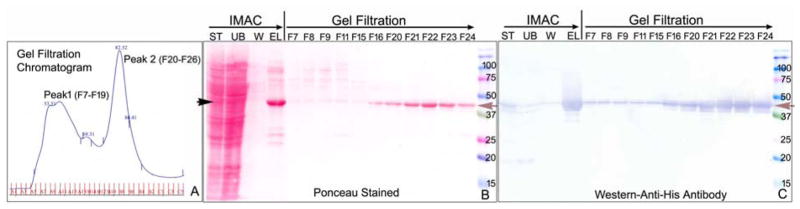
Full-length hTSSK2 was expressed in Sf9 cells. Partially soluble hTSSK2 with a His tag at the N-terminus was produced. Initial purification of the soluble TSSK2 was achieved by IMAC under non-denaturing conditions. Fig. 2 shows a representative purification profile of Coomassie stained and western blotted protein extracts illustrating the enrichment of soluble TSSK2 by IMAC. Gel filtration chromatography was used for the second step purification of TSSK2 to obtain pure monomeric hTSSK2 (Fig. 3). Two major peaks were noted by gel filtration (Fig. 3A). Peak 1 fractions eluted with the exclusion volume at approximately 600 kDa mass, and contained the bulk of the hTSSK2, which showed aggregation with other proteins when the fractions were analyzed. Peak 2 fractions eluted at approximately 40–50 kDa (Fig. 3 A, B and C, fractions 20–22) and contained a highly pure monomeric form of hTSSK2 as revealed after SDS-PAGE analysis and Western blotting (Fig. 3 B, C). The TSSK2 band represented 99% of this fraction pool.
Figure 2.

SDS-PAGE and Western blot analysis of full-length recombinant hTSSK2 expressed in baculovirus/Sf9 cells and purified by non-denaturing Ni-NTA chromatography. (A) Ni-NTA purification enabled the enrichment and partial purification of soluble TSSK2 at a predicted molecular weight of 41kDa (arrowhead) as shown in the Coomassie stained gel. (B) The expressed rec.TSSK2 containing an N-terminal histidine tag, migrated at 41 kDa and immunoreacted with the anti-His antibody, verifying the authenticity of the purified protein.
Figure 3.
Analysis of soluble recombinant hTSSK2 from Sf9 cells maintained in suspension culture. (A) Soluble hTSSK2 was purified under non-denaturing conditions by Ni-NTA chromatography followed by Superdex 200 gel filtration. (B) SDS-PAGE of Peak 1 fractions (F 4–10) revealed a prominent ~41 kDa TSSK2 band (arrow) which was immunoreactive with anti-His antibody (C, Western blot) along with several higher molecular weight non-immunoreactive protein bands (B, Ponceau stain). Highly pure homogeneous hTSSK2 eluted in Peak 2 at ~41 kDa (F 20–22) and was confirmed as authentic recombinant hTSSK2 by anti-His immunoreactivity.
Initial attempts using the two-step purification produced a relatively low yield of ~100 μg of monomeric TSSK2 per litter of suspension culture (Table 1). The recovery in the gel filtration step was about 10% of the IMAC purified protein. However, maintenance of Sf9 cells in an adherent state rather than in suspension without changing any other purification strategies increased the yield several-fold to ~850μg/L. A recovery of more than 60% of IMAC purified protein was achieved in the gel filtration step (Table 1). Moreover, a much higher ratio of Peak 2 to Peak1 in the gel filtration chromatogram indicates that adherent cells predominantly expressed hTSSK2 in the monomeric form (Figure 4A). These results suggest that the adherent Sf9 cells were more efficient in the manufacture of fully-folded TSSK2 in a non-aggregated form.
Figure 4.
Analysis of recombinant hTSSK2 from Sf9 cells maintained in adherent culture conditions. (A) Gel filtration of Ni-NTA purified TSSK2 provided 2 peaks. (B) IMAC purification enriched 41 kDa recombinant hTSSK2 (arrow) as shown by SDS-PAGE analysis (see lane EL in B, Ponceau stained) which was also immunoreactive to anti-His antibody (see lane EL in C). SDS-PAGE of Peak 1 fractions (F 7–16) contained a faint ~41 kDa TSSK2 band (B, arrow) which was immunoreactive with anti-His antibody (C, Western blot) along with several higher molecular weight non-immunoreactive protein bands (B, Ponceau stain). Homogeneous hTSSK2 that eluted in Peak 2 at ~41 kDa (F 20–24) separated by SDS-PAGE as very prominent bands that were confirmed to be authentic recombinant hTSSK2 by anti-His immunoreactivity. ST: Starting material, UB:Unbound, W: Wash, EL: Elute, IMAC: Immobilized Metal-Affinity Chromatography.
3.3. Enzymatic activity of hTSSK2
An in vitro kinase assay (Fig. 5) was performed using the purified hTSSK2 shown in Fig. 3 including the aggregated form of hTSSK2 (peak 1, fraction 4, 1 μg/20 μl reaction) and the highly pure monomeric hTSSK2 (peak 2, fraction 21, 0.2 μg/20 μl reaction). Autoradiography of the separated products of this reaction revealed robust autophosphorylation of hTSSK2 (Fig. 5, arrowhead 1) in both heterogeneous and pure enzyme preparations. Abbreviated forms of hTSKS at ~12–15 kDa (Fig. 5, arrowhead 3) and the generic substrate casein (Fig. 5, arrowhead 2) both were phosphorylated by the fraction 4 aggregated hTSSK2 and by fraction 21 monomeric hTSSK2. Although loaded at ~5 fold less protein than fraction 4, fraction 21 TSSK2 showed greater phosphorylation of both substrates with phosphorylation of casein exceeding that of the positive control (10 units of casein kinase II). However, it should be noted that the specific activity of casein kinase II used in the assay was very high (859 units/μg) and the conversion of the unit measure to weight shows that 10 ng of CKII was used as compared to 200 ng of hTSSK2 in the 20 μl reaction, implying that casein is a specific substrate for CKII as expected. Taken together, these results, combined with earlier observations of the purification profile of the preparations, indicated that the highly purified monomeric hTSSK2 preparation (peak 2) consists of biologically active, properly folded, homogeneous hTSSK2.
Figure 5.

Enzymatic activity of recombinant hTSSK2 purified from Sf9 cells (maintained in suspension) in the in vitro kinase assay. Autoradiograph of SDS-PAGE gel from an in vitro kinase assay using 1 μg of partially pure hTSSK2 (F 4) or 0.2 μg of homogeneous hTSSK2 (F 21) incubated with or without substrates: truncated hTSKS (TSKS-Tr) (1 μg) or casein (10 units= 10 ng) in the presence of [32P]γATP. A strong signal at ~41 kDa (arrow 1), the molecular weight of recombinant hTSSK2, indicated that TSSK2 was autophosphorylated. Casein (arrow 2) and low molecular weight fragments of hTSKS (arrow 3) were also phosphorylated. F 21 hTSSK2 showed more activity than the partially pure F 4 hTSSK2 although the concentration of homogeneous hTSSK2 used in the reactions was ~5 fold less. CKII showed phosphorylation of casein, while CKII phosphorylation of TSKS was strikingly less than that achieved by F 21 TSSK2.
3.4. Enzyme kinetics and development of an inhibitor screening assay for hTSSK2
Partially purified and homogeneously purified hTSSK2 produced in the baculovirus system were analyzed using MSA, a non-radioactive and non-antibody-based technique [33–34]. Fluorescently labeled peptide PLSRTLSVSS derived from the N-terminus of glycogen synthase (5-FAM-GS) was utilized as a substrate. Phosphorylation of a serine residue in the 5-FAM-GS substrate via hTSSK2 alters the electrophoretic mobility of the substrate, allowing a ratiometric determination of product and substrate. The partially purified hTSSK2 preparation at a concentration of 840ng/well generated 25–30% conversion of the substrate within 1 h (Fig. 6A, left panel). By comparison, homogeneous hTSSK2 generated about the same level of conversion at the much lower level of 22ng/well (Fig. 6A, right panel). An ATP KM of 2.7± 0.6 μM (Fig. 6B) and a specific activity of 0.24 ± 0.03 nmol·min−1·mg E−1 was determined for the partially purified hTSSK2 preparation, whereas homogeneous hTSSK2 had an ATP KM of 2.17 ± 0.04 μM and a specific activity of 9.7 ± 0.2 nmol·min−1·mg E−1. The latter was 40-fold higher than that of the partially pure preparation, and >50-fold higher than commercially obtained hTSSK2, which yielded a specific activity of 0.17 ± 0.01 nmol·min−1·mg E−1. Staurosporine, a broad spectrum kinase inhibitor, inhibited the partially pure hTSSK2 with an IC50 of 20 ± 1 nM (Fig. 6C).
Figure 6.
Enzymatic activity of recombinant hTSSK2 purified from Sf9 cells in the mobility shift assay. (A) Progress curves for partially pure (F 10–19, left panel) and homogeneously pure (F 21, right panel) hTSSK2 preparations expressed by Sf9 cells in suspension. Two-fold serial dilutions of each hTSSK2 preparation in reaction buffer supplemented with 1.5 μM 5-FAM-GS substrate and 5 μM ATP were sampled over 45 min. (B) Determination of ATP KM for the partially pure hTSSK2. Two-fold serial dilutions of 100 μM ATP in reaction buffer supplemented with 1.5 μM substrate and partially pure hTSSK2 were analyzed kinetically for 1 h. (C) Staurosporine inhibition of partially pure hTSSK2 activity. Staurosporine was incubated in reaction buffer supplemented with 1.5 μM substrate, partially pure hTSSK2 and 5 μM ATP for 1 h at 30 °C. All data represent the means of three independent experiments.
3.5. Identification of small hTSKS peptide substrates for hTSSK2
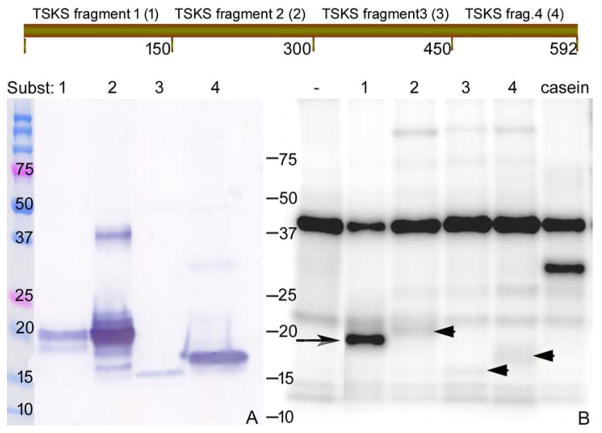
hTSKS isoform 1 was dissected in an effort to identify a portion which would constitute a small natural peptide substrate suitable for kinase assays. Full-length hTSKS (isoform 1, 592aa) was divided into four segments containing 150, 150, 150 and 142 amino acids, respectively, beginning from the N-terminus. Each of the four fragments was expressed in E. coli and purified in soluble form as shown in Fig. 7A. An in vitro kinase assay using each fragment (Fig. 7B) revealed maximum phosphorylation of TSKS fragment 1 (aa 1–150) (Fig. 7B; arrow) and weak phosphorylation of fragments 2, 3 and 4 (Fig. 7B; arrow heads) suggesting that major residues phosphorylated by TSSK2 are present in the first 150 amino acids of TSKS isoform 1.
Figure 7.
Identification of a TSKS domain involved in phosphorylation by hTSSK2. hTSKS isoform1 was divided into 4 fragments of ~150 amino acids as shown and recombinant fragments 1–4 were generated with a His-tag at each C-terminus. (A) Soluble TSKS peptides were purified by Ni-NTA and evaluated by SDS-PAGE followed by Western blot using anti-His antibody. 1 μg of each purified recombinant peptide was used in the in vitro kinase assay using highly pure hTSSK2 prepared from Sf9 cells expressing TSSK2 in adherent culture. The autoradiogram (panel B) shows intense phosphorylation of TSKS fragment 1 (long arrow) and much lower phosphorylation of TSKS fragments 2, 3 and 4 (arrow heads).
4. Discussion
Recombinant expression of soluble, active human hTSSK2 with subsequent purification will permit the screening of compound collections to identify inhibitors of this candidate male contraceptive target. Whereas other kinase studies frequently use truncated constructs expressing only the kinase domain [35–37], and the available commercial source offers a truncated form of the TSSK2 molecule, the present studies achieved an important benchmark: the production of soluble full-length recombinant TSSK2 in baculovirus, which was purified in a two-step process of nickel affinity chromatography and gel filtration to achieve a 99% pure active enzyme preparation suitable for inhibitor screening and crystallization studies. No previous studies or commercial sources have achieved this full-length soluble form of TSSK2 which is predicted to be more suitable for drug screens, particularly allosteric inhibitors, and for determining the structure in a form closer to the native state.
Initial purification experiments of TSSK2 were performed using Sf9 cells that were maintained in suspension culture in spinner flasks. The yield of the purified monomeric TSSK2 in these cases was only about 100μg/L, even though about 1 mg of partially pure TSSK2 was obtained following Ni-NTA purification step. Various strategies including increasing the salt concentration, addition of non-ionic detergents (Triton X-100 and IGEPAL), variation of pH and buffers (Tris- HCl, HEPES, phosphate), and more stringent washing conditions did not achieve improvements in the final protein yield. However, switching to adherent cell culture dramatically improved the yield of the pure monomeric TSSK2 about 6-fold. The explanation could be that adherent culture conditions favor the synthesis of properly folded non-aggregated enzyme TSSK2.
It is well known that protein expression in insect cells may permit proper folding, post-translational modification and oligomerization in a manner that more closely resembles those processes that occur in mammalian cells as opposed to bacteria [38]. Indeed, post-translational processing identical to that of mammalian cells has been reported in the baculovirus/insect cell system for a range of proteins including active kinases such as MAP kinase [39], insulin receptor protein kinase [40] cyclin–dependent kinase 2 [41], p38 mitogen-activated protein kinase [42] and aurora kinase [43].
Several strategies have been applied to the production of TSSKs and related recombinant kinases. In previous studies, efforts to produce TSSK1 and TSSK2 in E. coli with a His tag resulted in the aggregation of the proteins into insoluble inclusion bodies [11, 12] necessitating their purification by denaturing methods. Among other TSSKs, biologically active recombinant human and mouse TSSK3 were made in a soluble form as GST fusion proteins [16]. Wei et al. [20] utilized a mammalian expression system for activity and interaction studies of TSSK4. Recombinant bacterial production of functionally active calcium/calmodulin-dependent kinases such as AMPK [44] and cam kinase II [45].have been reported previously. To summarize, different TSSKs and closely related serine/threonine kinases behave differently in each recombinant expression method, requiring tailoring of the expression system to each member of the family.
Both the full length (isoform 1) and truncated (isoform 2) forms of human TSKS contain the potential homologous phosphorylation site that corresponds to the ser281 site that was previously identified by in vivo microsequencing experiments in mice [12]. Soluble, truncated isoform-2 hTSKS with an N-terminus His tag was successfully expressed in E. coli. Attempts to produce soluble, full-length hTSKS (isoform 1) were not successful in either E coli or in the baculovirus system. Thus, current experiments employed recombinant isoform 2 hTSKS for in vitro kinase assays. In vitro phosphorylation of the whole, 46 kDa form of recombinant hTSKS (isoform 2) was not observed with recombinant hTSSK2 in these experiments. However, low molecular weight fragments of the recombinant TSKS were clearly phosphorylated. One explanation for this observation is that the phosphorylation sites of the whole recombinant protein might be inaccessible because of its aggregation and folding pattern. Our inability to purify the 46 kDa form to homogeneity by gel filtration (data not shown) is in accord with this interpretation. Production of properly folded TSKS utilizing the baculovirus-insect cell system and/or by addition of solubility tags remain future strategies to demonstrate phosphorylation and to validate this hypothesis. Even though the low molecular weight peptide fragments were mapped to the N-terminus of TSKS isoform 2, further investigations on the specific residues undergoing phosphorylation were inconclusive. Nevertheless, these TSKS peptides may be useful as novel substrates for in vitro kinase assays for TSSK2 activity in gel assays.
A natural peptide substrate from TSKS may provide more specificity than generic substrates for compound screening and inhibitor characterization. Efforts to identify a natural substrate focused on TSKS isoform 1 (592 aa), a full-length form which contains an additional 221 residues at the N-terminus compared to the truncated TSKS isoform 2. Expressing hTSKS isoform 1 as smaller fragments facilitated the recombinant production of soluble regions of this substrate. This truncation strategy allowed further phosphorylation analysis to identify the specific domain(s) of hTSKS isoform 1 involved in TSSK phosphorylation, and showed that aa 1–150 at the N-terminus of hTSKS harbors the residue(s) that are strongly phosphorylated by hTSSK2. This finding corroborates the observations of Xu et al. [12], which demonstrated that the interacting region of TSKS lies within aa 1–147 in the N-terminal domain of hTSKS. This N-terminal recombinant fragment is unique to hTSKS isoform 1 as the region is lacking in isoform 2. The fragment will be studied further to identify the specific phosphorylated residues in order to synthesize a natural peptide substrate for compound screening.
A robust mobility shift assay using recombinant full-length hTSSK2 enzyme obtained from the baculovirus/insect cell system was developed, utilizing mainly the partially purified hTSSK2 because of its availability in larger amounts. The kinetic studies demonstrate that this recombinant preparation of hTSSK2 is suitable for the initiation of large-scale screening of compounds to identify inhibitors of this kinase. The methods to characterize the biological activity of a full-length hTSSK2 and screen for inhibitors using a high throughput mobility shift assay together with the identification of the TSKS domain specifically phosphorylated provides an important benchmark on the pathway to identifying male contraceptive drug candidates directed to TSSK2.
Soluble full-length hTSSK2 was produced in a baculovirus expression system and purified.
The biological activity of the recombinant hTSSK2 was studied by in vitro kinase and mobility shift assays.
Recombinant hTSSK2 showed robust kinase activity in the in vitro kinase assay.
The ATP Km values were similar for the highly and partially purified fractions of hTSSK2.
In vitro phosphorylation experiments revealed that the N-terminus of human testis specific kinase substrate [TSKS] is phosphorylated by human TSSK2.
Acknowledgments
Funding: This work was funded by NIH-1U01 HD060491, 1U01HD076542 and the contract HHSN275201300017C from the Contraceptive Development Branch of NICHD and by Schering AG, Berlin.
The authors wish to thank Dr. Nicholas E. Sherman at the Biomolecular Research Facility at the University of Virginia for protein sequencing by mass spectrometry. The authors would like to acknowledge the support and encouragement of Drs. Diana Blithe and Min Lee of NIH and Drs. Alfred Pauls and Ursula Habenicht of Schering AG (Bayer).
Abbreviations used
- TSSK
Testis-specific serine threonine kinase
- TSKS
Testis-specific serine threonine kinase substrate
- IMAC
Immobilized-metal affinity chromatography
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- MSA
Mobility shift assay
- EDTA
Ethylenediaminetetraacetic acid
- Ni-NTA
Nickel-Nitrilotriacetic acid
- E. coli
Escherichia coli
Footnotes
Competing interests
The authors have declared no competing interests.
Authors’ roles:
Each author’s contribution towards this article is as follows: J.S: first author, experimental design, performed most of the experiments, writing and revision of manuscript. R.S: experimental design, performed experiments on TSSK activity by mobility shift assays (MSA), writing MSA-related sections and revision of manuscript. I.A.S: experimental design, purification of the TSSKs by gel filtration and revision of manuscript. W.M: experimental design and revision of manuscript. J.Z: experimental design and revision of manuscript. J.E.H: experimental design for activity assay and revision of manuscript. G.I.G: experimental design, revision of manuscript. C.J.F: experimental design, revision of manuscript. J.C.H: corresponding author, experimental design, directing and performing experiments, obtaining and providing funding, writing and revising manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, Page ST, Bremner WJ, Sitruk-Ware R, Kumar N, Blithe DL, Wang C. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab. 2012;97:3476–3486. doi: 10.1210/jc.2012-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth MY. Male hormonal contraception. Virtual Mentor. 2012;14:126–132. doi: 10.1001/virtualmentor.2012.14.2.stas1-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisberg E. Developments in contraception. Expert Opin Pharmacother. 2014;15:203–210. doi: 10.1517/14656566.2014.862234. [DOI] [PubMed] [Google Scholar]
- 4.Kogan P, Wald M. Male contraception: history and development. Urol Clin North Am. 2014;41:145–161. doi: 10.1016/j.ucl.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Murdoch FE, Goldberg E. Male contraception: Another holy grail. Bioorg Med Chem Lett. 2014;24:419–424. doi: 10.1016/j.bmcl.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Shokri S, Aitken RJ, Abdolvahhabi M, Abolhasani F, Ghasemi FM, Kashani I, Ejtemaeimehr S, Ahmadian S, Minaei B, Naraghi MA, Barbarestani M. Exercise and supraphysiological dose of nandrolone decanoate increase apoptosis in spermatogenic cells. Basic Clin Pharmacol Toxicol. 2010;106:324–330. doi: 10.1111/j.1742-7843.2009.00495.x. [DOI] [PubMed] [Google Scholar]
- 7.Shokri S, Hemadi M, Bayat G, Bahmanzadeh M, Jafari-Anarkooli I, Mashkani B. Combination of running exercise and high dose of anabolic androgenic steroid, androlone decanoate, increases protamine deficiency and DNA damage in rat spermatozoa. Andrologia. 2014;46:184–190. doi: 10.1111/and.12061. [DOI] [PubMed] [Google Scholar]
- 8.de Souza GL, Hallak J. Anabolic steroids and male infertility: a comprehensive Review. BJU In. 2011;108:1860–1865. doi: 10.1111/j.1464-410X.2011.10131.x. [DOI] [PubMed] [Google Scholar]
- 9.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 10.Kueng P, Nikolova Z, Djonov V, Hemphill A, Rohrbach V, Boehlen D, Zuercher G, Andres AC, Ziemiecki A. A novel family of serine/threonine kinases participating in spermiogenesis. J Cell Biol. 1977;139:1851–1859. doi: 10.1083/jcb.139.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Z, Jha KN, Kim YH, Vemuganti S, Westbrook VA, Chertihin O, Markgraf K, Flickinger CJ, Coppola M, Herr JC, Visconti PE. Expression analysis of the human testis-specific serine/threonine kinase (TSSK) homologues. A TSSK member is present in the equatorial segment of human sperm. Mol Hum Reprod. 2004;10:433–444. doi: 10.1093/molehr/gah052. [DOI] [PubMed] [Google Scholar]
- 12.Xu B, Hao Z, Jha KN, Zhang Z, Urekar C, Digilio L, Pulido S, Strauss JF, 3rd, Flickinger CJ, Herr JC. TSKS concentrates in spermatid centrioles during flagellogenesis. Dev Biol. 2008;319:201–210. doi: 10.1016/j.ydbio.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu B, Hao Z, Jha KN, Zhang Z, Urekar C, Digilio L, Pulido S, Strauss JF, 3rd, Flickinger CJ, Herr JC. Targeted deletion of Tssk1 and 2 causes male infertility due to haploinsufficiency. Dev Biol. 2008;319:211–222. doi: 10.1016/j.ydbio.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shang P, Baarends WM, Hoogerbrugge J, Ooms MP, van Cappellen WA, de Jong AA, Dohle GR, van Eenennaam H, Gossen JA, Grootegoed JA. Functional transformation of the chromatoid body in mouse spermatids requires testis-specific serine/threonine kinases. J Cell Sci. 2010;123:331–339. doi: 10.1242/jcs.059949. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Sosnik J, Brassard L, Reese M, Spiridonov NA, Bates TC, Johnson GR, Anguita J, Visconti PE, Salicioni AM. Expression and localization of five members of the testis- specific serine kinase (Tssk) family in mouse and human sperm and testis. Mol Hum Reprod. 2011;17:42–56. doi: 10.1093/molehr/gaq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucko-Justyna M, Lipinski L, Burgering BM, Trzeciak L. Characterization of testis- specific serine-threonine kinase 3 and its activation by phosphoinositide- dependent kinase- dependent signalling. FEBS J. 2005;272:6310–6323. doi: 10.1111/j.1742-4658.2005.05018.x. [DOI] [PubMed] [Google Scholar]
- 17.Zuercher G, Rohrbach V, Andres AC, Ziemiecki A. A novel member of the testis specific serine kinase family, tssk-3, expressed in the Leydig cells of sexually mature mice. Mech Dev. 2000;93:175–177. doi: 10.1016/s0925-4773(00)00255-0. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Lin G, Wei Y, Hexige S, Niu Y, Liu L, Yang C, Yu L. TSSK5, a novel member of the testis-specific serine/threonine kinase family, phosphorylates CREB at Ser-133, and stimulates the CRE/CREB responsive pathway. Biochem Biophys Res Commun. 2005;333:742–749. doi: 10.1016/j.bbrc.2005.05.157. [DOI] [PubMed] [Google Scholar]
- 19.Su D, Zhang W, Yang Y, Deng Y, Ma Y, Song H, Zhang S. Mutation screening and association study of the TSSK4 Gene in Chinese infertile men with impaired spermatogenesis. J Androl. 2008;29:374–378. doi: 10.2164/jandrol.107.004598. [DOI] [PubMed] [Google Scholar]
- 20.Wei Y, Wang X, Fu G, Yu L. Testis specific serine/threonine kinase 4 (Tssk4) maintains its kinase activity by phosphorylating itself at Thr-197. Mol Biol Rep. 2013;40:439–447. doi: 10.1007/s11033-012-2078-x. [DOI] [PubMed] [Google Scholar]
- 21.Spiridonov NA, Wong L, Zerfas PM, Starost MF, Pack SD, Paweletz CP, Johnson GR. Identification and characterization of SSTK, a serine/threonine protein kinase essential for male fertility. Mol Cell Biol. 2005;25:4250–4261. doi: 10.1128/MCB.25.10.4250-4261.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sosnik J, Miranda PV, Spiridonov NV, Yoon SY, Fissore RA, Johnson GR, Visconti PE. Tssk6 is required for Izumo relocalization and gamete fusion in the mouse. J Cell Sci. 2009;122:2741–2749. doi: 10.1242/jcs.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu B, Hao Z, Jha KN, Digilio L, Urekar C, Kim YH, Pulido S, Flickinger CJ, Herr JC. Validation of a testis specific serine/threonine kinase [TSSK] family and the substrate of TSSK1 & 2, TSKS, as contraceptive targets. Soc Reprod Fertil Suppl. 2007;63:87–101. [PubMed] [Google Scholar]
- 24.Zhang Z, Shen X, Jones BH, Xu B, Herr JC, Strauss JF., 3rd Phosphorylation of mouse sperm axoneme central apparatus protein SPAG16L by a testis-specific kinase TSSK2. Biol Reprod. 2008;79:75–83. doi: 10.1095/biolreprod.107.066308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, Bennett J, Gerton GL, Moss SB, Radice GL, Strauss JF., 3rd Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod. 2006;74:751–759. doi: 10.1095/biolreprod.105.049254. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Su D, Yang Y, Zhang W, Liu Y, Bai G, Ma M, Ma Y, Zhang S. Some single-nucleotide polymorphisms of the TSSK2 gene may be associated with human spermatogenesis impairment. J Androl. 2010;31:388–392. doi: 10.2164/jandrol.109.008466. [DOI] [PubMed] [Google Scholar]
- 27.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palermo GD, Colombero LT, Rosenwaks Z. The human sperm centrosome is Responsible for normal syngamy and early embryonic development. Rev Reprod. 1997;2:19–27. doi: 10.1530/ror.0.0020019. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- 30.Visconti PE, Johnson LR, Oyaski M, Fornés M, Moss SB, Gerton GL, Kopf GS. Regulation, localization, and anchoring of protein kinase A subunits during mouse sperm capacitation. Dev Biol. 1997;192:351–363. doi: 10.1006/dbio.1997.8768. [DOI] [PubMed] [Google Scholar]
- 31.Shetty J, Diekman AB, Jayes FC, Sherman NE, Naaby-Hansen S, Flickinger CJ, Herr JC. Differential extraction and enrichment of human sperm surface proteins in a proteome: identification of immunocontraceptive candidates. Electrophoresis. 2001;22:3053–66. doi: 10.1002/1522-2683(200108)22:14<3053::AID-ELPS3053>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Shetty J, Herr JC. Methods of Analysis of sperm antigens related to infertility. In: Krause WKH, Naz RK, editors. Immune Infertility. Springer; 2009. pp. 13–31. [Google Scholar]
- 33.Perrin D, Fremaux C, Shutes A. Capillary microfluidic electrophoretic mobility shift assays: Application to enzymatic assays in drug discovery. Expert Opin Drug Discovery. 2009;51:1–3. doi: 10.1517/17460440903493431. [DOI] [PubMed] [Google Scholar]
- 34.Yagi Y, Abe K, Ikebukuro K, Sode K. Kinetic Mechanism and Inhibition Characterization of WNK1 Kinase. Biochemistry. 2009;48:10255–10266. doi: 10.1021/bi900666n. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Rudge DG, Koos JD, Vaidialingam B, Yang HJ, Pavletich NP. mTOR kinase structure, mechanism and regulation. Nature. 2013;497:217– 223. doi: 10.1038/nature12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fischmann TO, Smith CK, Mayhood TW, Myers JE, Reichert P, Mannarino A, Carr D, Zhu H, Wong J, Yang RS, Le HV, Madison VS. Crystal structures of MEK1 binary and ternary complexes with nucleotides and inhibitors. Biochemistry. 2009;48:2661–2674. doi: 10.1021/bi801898e. [DOI] [PubMed] [Google Scholar]
- 37.Panneerselvam S, Marx A, Mandelkow EM, Mandelkow E. Structure of the catalytic and ubiquitin-associated domains of the protein kinase MARK/Par-1. Structure. 2006;14:173–183. doi: 10.1016/j.str.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 38.Jarvis DL. Baculovirus-insect cell expression systems. Methods Enzymol. 2009;463:191–222. doi: 10.1016/S0076-6879(09)63014-7. [DOI] [PubMed] [Google Scholar]
- 39.Lee SJ, Zhou T, Goldsmith EJ. Crystallization of MAP kinases. Methods. 2006;40:224–33. doi: 10.1016/j.ymeth.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Wei L, Hubbard SR, Hendrickson WA, Ellis L. Expression, characterization, and crystallization of the catalytic core of the human insulin receptor protein- tyrosine kinase domain. J Biol Chem. 1995;270:8122–8130. doi: 10.1074/jbc.270.14.8122. [DOI] [PubMed] [Google Scholar]
- 41.Rosenblatt J, De Bondt H, Jancarik J, Morgan DO, Kim SH. Purification and crystallization of human cyclin-dependent kinase 2. J Mol Biol. 1993;230:1317–1319. doi: 10.1006/jmbi.1993.1248. [DOI] [PubMed] [Google Scholar]
- 42.Wilson KP, Fitzgibbon MJ, Caron PR, Griffith JP, Chen W, McCaffrey PG, Chambers SP, Su MS. Crystal structure of p38 mitogen-activated protein kinase. J Biol Chem. 1996;271:27696–27700. doi: 10.1074/jbc.271.44.27696. [DOI] [PubMed] [Google Scholar]
- 43.Cheetham M, Knegtel RM, Coll JT, Renwick SB, Swenson L, Weber P, Lippke JA, Austen DA. Crystal structure of aurora-2, an oncogenic serine/threonine kinase. J Biol Chem. 2002;27745:42419–424122. doi: 10.1074/jbc.C200426200. [DOI] [PubMed] [Google Scholar]
- 44.Neumann D, Woods A, Carling D, Wallimann T, Schlattner U. Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr Purif. 2003;30:230–237. doi: 10.1016/s1046-5928(03)00126-8. [DOI] [PubMed] [Google Scholar]
- 45.Shoju H, Sueyoshi N, Ishida A, Kameshita I. High level expression and preparation of autonomous Ca2+/calmodulin-dependent protein kinase II in Escherichia coli. J Biochem. 2005;138:605–611. doi: 10.1093/jb/mvi161. [DOI] [PubMed] [Google Scholar]